?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this study a strain of Streptomyces sp. was isolated from soil and identified by 16S rRNA gene sequencing technology. The strain was screened for antibiotics production effective against biocontrol of Rhizoctonia solani AG-3 to cure the target spot disease in tobacco. For enhance production of secondary metabolites, central composite design of response surface methodology (RSM) was applied in submerged fermentation. The maximum metabolite production was using medium volume of 55 mL in 250 mL flask, agitation speed of 165 rpm, incubation temperature 30 °C, initial medium pH of 6.8 and inoculum size of 7%. Solvent extraction method was used to extract the secondary metabolites and active compounds were purified by silica gel column chromatography. The purified fractions were further investigated by gas chromatography-mass spectrophotometer (GC-MS). GC-MS analysis showed 48 compounds, among them 12 were active against pathogen. These findings indicated that the strain Streptomyces TA 1123 was a potential antagonist against R. solani AG-3.
Introduction
In 2006 it was identified that Rhizoctonia solani Kühn is the causal pathogen of target spot and causes heavy economic loss in the production of tobacco in Liaoning province of China. Target spot was caused by Rhizoctonia solani AG [Citation1]. Root rot and stem rot caused by AG-1, AG-2-2, and AG-4 anastomosis groups of R. solani and the target spot was caused by the AG-3 [Citation2]. Streptomyces spices are the source of 75% of useful antibiotics [Citation3]. Several species of actinomycetes from the genera of Streptomyces are good antagonistic agents against the fungal phytopathogens [Citation4]. Nonetheless, the culture yield for the production of antibiotics has been found unstable, often yielding inconsistent amounts of the target active ingredients either through the physical or chemical parameters viz. pH value, temperature, and time period that critically impact yield of such substances [Citation5,6]. Activity of important enzymes that are crucial for the production of antibiotics can be affected by pH values [Citation7]. In vitro and in vivo biocontrol bacterial fermentation is affected by the composition of nutrition media and other physical parameters. In the process of fermentation, statistical experimental approaches enhance the quality and quantity of desirability goal and also time and cost saving with less inconsistency. The conventional method, single factor optimization, has two drawbacks -- unreliability and time consumption. Both of these can be eliminated by using response surface methodology. It can be used to estimate numerous comparative impacts and composite interfaces [Citation8,9]. There are a few benefits of using response surface methodology, such as reducing the number of experiments and increased reliability for numerous factor trails, and finding optimal conditions and predictions. For the optimization of fermentation processes this method is usually used [Citation10–12]. Several Streptomyces strains were tested against the Rhizoctonia solanai. Strain TA 1123 showed good activity against the pathogen. In this study, we find the best fermentation conditions for the production of Streptomyces Strain TA 1123 and determine the best combination of the conditions to improve the yield and reduce the cost and time. Chemical screening was done by the gas chromatography-mass spectrometer (GC-MS), 12 compounds were identified to control the R. solani AG-3 and cure target spot in tobacco.
Materials and methods
Isolation, screening, and identification of strain
About 24 soil samples were collected from different regions of Liaoning Province, China and strains were isolated by soil dilution plate technique. Test organisms were Bacillus cereus, Escherichia coli ATCC8739, and Fusarium sp. Isolated strains were maintained on nutrient medium and gausses medium. About six isolated strains were screened against the R. solani AG-3. Identification of the strain was done on the basis of phenotypic, chemotaxonomic, and physiological tests [Citation13]. PCR amplification of 16S rRNA gene sequence of Strain TA 1123 was performed using the primer: 27f (5′AGTTTGTCMTGGCTCAG-3) and 1492r (5'-GGTTACCTTACGACTT-3) (verity TM 96-well PCR, Applied Biosystems, Singapore). The PCR products were sent to Sangon Biotech (Shanghai, China) Co., Ltd for sequence determination. Phylogenetic analysis was conducted using Mega version 6. Antifungal activity was measured by an Oxford cup plate assay medium [Citation14]. Potato dextrose agar medium (30 mL) was heated until it was completely melted and cooled to 50 °C, and then mixed with the 10 mL of a spore suspension of R. solani before being poured into plate and allowed it solidify at room temperature .The supernatant (0.2 mL) was added into each Oxford cup on the plate incubated at 28 °C for 48 h in darkness.
Fermentation process
Fermentation was performed in two stages, seed growth and production of active antifungal substance. Strain TA 1123 was grown on plates of gausses medium at 28 °C for 5 days after spore production in liquid fermentation medium. Two spore cakes (5 mm) of Strain TA 1123 were used to inoculate a 250 mL flask volume was kept at 40 mL and then the flasks were incubated on 160 rpm for 48 h. Then, 5% (v/v) seed culture were inoculated aseptically into 250 mL flask containing 40 mL of fermentation medium and incubated at 28 °C in rotatory shaker (HZQ-F16 Harbin Dong Lian Electronic Technology Production. Co., Ltd., China) at the speed of 160 rpm for 96 h. After that the fermented culture were centrifuged the supernatant was stored at −4 for further work [Citation7]. The antifungal activity was determined by measuring the diameter of inhibition zones.
Experimental design and optimization
A five-factor-three-level central composite design (CCD) was employed in this study, requiring 32 experiments. The fractional factorial design consisted of 9 factorial points, 14 centre points, and 9 axial points with five parameters. The parameters and their levels used for the optimization of fermentation broth for the production of secondary metabolites were: X1: medium volume (30–80 mL), X2: rotary speed (120–200), X3: pH (3–10), X4: molar temperature (24–2 °C), and X5: inoculation volume (2–12) (). Contour plots were generated to illustrate the main and interactive effects of the independent variables on the dependent ones. The optimum combination of parameters can be determined on the basis of the ridge maximum analysis and the canonical analysis using the optimization function of the MINITAB 14 software. Optimum value of any variable for maximum antimicrobial activity was determined by the response optimizer tool in the software.
Table 1. Experiment design and results of optimization of Strain TA 1123 fermentation conditions by the central composite design observed value and predicted values of fermentation conditions.
Statistics analysis
Response surface regression procedure was used for the fitted experimental results of response surface methodology (RSM). The variables given coded values according to the equation:(1)
(1) where Xi is an independent variable coded value, Xi is the independent variable's real value, X is the independent variable's real mean, and Xi is the step change value. The second-order polynomial model was fitted a response curve fitting the equation:
(2)
(2) where Y is the measured response; b0 is the intercept term; bi, bij, and bii are measures of the effects of variables xi, xixj, and xi, respectively. The variable xixj represents the first-order interaction between xi and xj (i < j). Statistical analysis of the model was performed in the form of analysis of variance (ANOVA), including the Fisher's F-test, associated probability P (F), determination coefficient R2, and correlation coefficient R that measures the goodness of fit regression model. The analysis also included Student's t-value for the estimated coefficients and associated probabilities, P (t). For each variable, the quadratic models were represented as contour plots.
Extraction of the fermentation broth
The cultural filtrate (1 L) was extracted with diethyl ether 1:1(v/v). Diethyl ether layer that contained active substance was concentrated by evaporating to dryness at 50 °C and crude extract was obtained. Antifungal activity was measured by an Oxford cup plate method.
Purification and identification of the compound
Extracted substance was purified by silica gel column chromatography. Methanol used as eluent, and purified fractions were bioassay against fungal pathogen. The antifungal compounds were identified by using GC-MS. Agilent technologies 6890-5973 N with capillary column Tr-Fame ((30 m × 250 μm × 0.25 μm) system were used. Mass detector used in split mode, and helium gas with flow rate of 0.9 mL/min was used as a carrier. Injector was operated at 230 °C and oven temperature for initial setup was 80 °C for 1 min, ramp 4/min to 220 °C for 10 min. For the analysis, NIST/EPA/NIH MASS SPECTRAL LIBRARY (NIST 05) and NIST MASS SPECTRAL SEARCH PROGRAM version 2.0d were used [Citation15].
Results and discussion
Isolation and screening
Six strains of Streptomyces were screened against the R. solani AG-3. Streptomyces TA 1123 showed to be highly active against the pathogen (). The distinctively larger inhibition zone seen in this study strongly implied liberation of higher amount of antibiotic that led to the observed inhibition. This is one of the many key criteria considered by scientists, when bioprospecting for potent biological control agent [Citation16]. Over 60 compounds are used in over 9000 bioactive components that were isolated from actinomycetes in antibiotics, agro-antibiotics, and research. About 80% extracted from the Streptomyces [Citation17]. Physical and chemical parameters affect in biological synthesis of secondary metabolites. Aerial hyphae trigger the synthesis of secondary metabolites when the development bacterial filaments tic and sporulation start. Several studies demonstrated that starvation of nutrient is the cause of secondary metabolites especially phosphate and the triggering mechanism over secondary metabolite regulation were recently reviewed [Citation18,19]. In this study, Streptomyces were isolated from the cultivation soils of Liaoning province of China. Soil Streptomyces are very active antagonist against fungal phytopathogens. Polyene macrolide antibiotics, one of the most important subgroup of polyketides, possess a typical polyene structure ranging from three to seven double bonds in length. To date, more than 200 known polyene macrolide antibiotics (e.g. rapamycin, nystatins, filipins, and amphotericin B) have been isolated and characterized, most of which are produced by the genus Streptomyces [Citation20].
Identification of the strain
The alignment of the 16S rRNA gene sequence (1412 nucleotide) of Strain TA 1123 was deposited in GenBank under accession number KX852460 and nearest closed match range 98% with the Streptomyces diastatochromogenes EF37141. From the morphology, colour of both substrate mycelium and aerial mycelium, presence of spores, growth on International Streptomyces Project (ISP) mediums, and growth on other mediums (Bennet agar medium, nutrient medium, and sabouraud medium, meso-diaminopimelic acid isomer present in the cell wall, detection of glucose, galactose, ribose and mannose, utilization of nitrogen and carbon sources indicated that this strain belongs to Streptomyces, 16S rRNA analysis and neighbour joining tree indicated close resemblance to Streptomyces strain diastatochromogenes EF37141. Soil bacteria that are similar to the Streptomyces are the main basis of production of biologically active compounds and used as antibiotics at a large scale. Streptomyces produced 75% of important antimicrobial agents [Citation21]. Morphological, chemotaxonomy, and physiological test revealed that Strain TA 1123 belongs to Streptomyces.
Fermentation conditions
CCD was used by the response surface methodology. Analysis helped to find out the cultural condition for the highest production of antibiotic. Volume in flask, rotary speed, pH, temperature, and inoculum volume were cultural conditions. Most fermentation processes need oxygen and dissolved oxygen has the main impact. Availability of oxygen is the most crucial parameter in cell growth, in the production of secondary metabolites and during the fermentation process [Citation14,22,23]. To find the optimum combination of the cultural conditions, 32 trials were conducted in triplicate according to the CCD, data shown in . Experimental data arranged through multiple regression analysis. CCD results were fixed with second-order polynomial equation. Results of regression analysis are shown in . T-test and p-values of the Student's test were used to determine the significance of every coefficient that showed the interaction among independent variables larger than the t values and smaller than the p-values [Citation9]. All the linear term regression coefficients showed great impact on the antibiotic activity according to the significance of corresponding p-values (pb1 = 0.000, pb2 = (0.000), pb3 = (0.000), pb4 = (0.000), and pb5 = (0.000). Volume in flask, speed, pH, temperature, and inoculum volume % showed their great effect during the production of the antibiotic. Quadric coefficients of b11, b22, b33, b44, and b55 were significant and negative effect of these quadrants determined that amplifying the production of the antibiotic as the parameter's values enlarged and decreased as the parameter values increased above from definite. Interactive terms coefficients of b13, b15, b23, and b35 were significant. To determine the antibiotic activity, second-order polynomial equation by the multiple regression analysis as follows:where Y is the response (the antibiotic activity units); and X1, X2, X3, X4, and X5 are the coded values of the independent factors, viz., temperature, rotary speed, initial pH, medium volume in the flask and inoculation volume, respectively. ANOVA () for second-order response surface model is given as coefficient of determination, R2 showed the appropriateness of the adequate model. R2 values also determined the trail parameters, their interaction, and showed unpredictability in the response. The positive and negative signs in front of term indicate the synergistic and antagonistic effects, respectively, suggesting the influence of independent variables on the esterification process. It has been indicated that the large F-value coupled with a very small P-value would demonstrate the significance of the corresponding coefficient. Therefore, the results of this study suggest that the factor of b3 (pH value) being one with the largest effect on the Y (antibiotic activity). Prediction of response and strength of the model is more in case of R2 value is very near to 1.00 [Citation24]. In this study, coefficient determination R2 = 0.9851 or 98.51% indicated that about 1% variations were not determined by the model. The adjusted determination coefficient R2 = 0.9821 or 98.21% also showed that the model is highly significant. To find from the results that polynomial model's precision and capability was great, and analysis of the response was reliable. Effects of independent variables and interactive influence of each variable were determined to designed surface plots by regression models. The shape of each surface plot directed that the reciprocal interactions among the independent variables are significant or not significant. (A–J) shows the activity for each pair of variables and kept the others constant at their middle values. The increase in pH values from the lowest of pH 3 to pH 7 resulted in the amplification of antibiotic activities and started to reduce on further increase in pH value. pH value near 7 was the optimum value ((A–F)). In the same way, indicated from the analysis that pH, temperature, and inoculum volume had significant roles in the production of antibiotic. High pH value and low pH values resulted in decreased antibiotic production ((G,J). In the phenomenon of production of secondary metabolites and their nature, pH plays a key role [Citation25].
Table 2. Regression results from the data of central composite designed experiments.
Table 3. Analysis of variance.
Figure 2. Surface plot of antibiotic activity of Streptomyces TA 1123 diameter of inhibition zone (mm): (a) the effect of pH and medium volume on the inhibition zone, (b) the effect agitation speed and medium volume on inhibition zone, (c) the effect of medium volume and inoculum volume on inhibition zone, (d) the effect of temperature and medium volume on inhibition zone, (e) the effect of agitation speed and temperature on inhibition zone, (f) the effect of pH and agitation speed on inhibition zone, (g) the effect of temperature and pH on the inhibition zone, (h) inoculum volume and agitation speed on the inhibition zone, (i) the effect of temperature and inoculum volume on inhibition zone, and (j) the effect of pH and inoculum volume on inhibition zone.
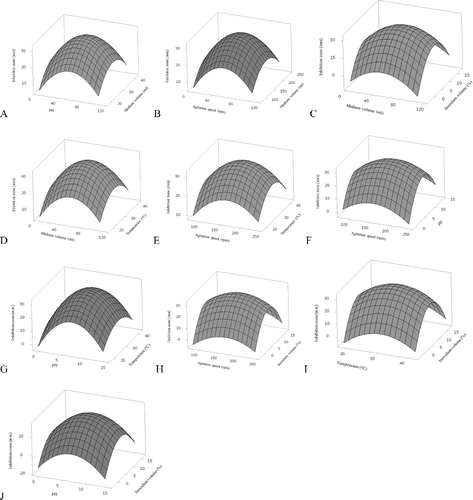
Medium volume and rotary speed had great influence on antibiotic activity. Volume in flask and rotary speed, directly linked to dissolve oxygen temperature, also had significant effect on antibiotic activity -- with increase in temperature from 24 to 30 there was an increase in antibiotic activity and on further increase started to decrease; optimum temperature was determined to be 30 ((D, E, and G)). At moderate initial pH and temperature, raised volume in flask resulted in a decrease in antibiotic activity, and raised rotary speed in amplified antibiotic activity ((B)). Inoculum volume and volume in flask had interaction and influence on antibiotic activity, from low (2%) of inoculum volume towards high volume (7%) led to increased antibiotic activity and further raise of the amount of inoculum caused a decrease in activity. In shaken flask oxygen, availability was directly linked to the medium volume and rotary speed. shows that the values of both factors were significant. Rotary speed raised and reduced in the medium volume in the flask amplified the antibiotic activity. Several actinomycetes require high amount of oxygen during the process of fermentation. Cultural medium contained organic and inorganic elements that lead to decrease in the level of dissolved oxygen; to meet this requirement there should be proper ventilation [Citation26]. Temperature had great impact on production, during low temperature environmental pressure affected the secondary metabolites and resulted in amplified production [Citation27]. From (I) we see that high and low temperatures are not favourable conditions for antibiotic production. The temperature affects the growth of actinomycetes. Favourable growth temperature is 23–37 °C. Growth of the bacteria affected by the temperature and aeration that resulted influenced on the production of the antibiotics [Citation28,29]. Another important factor that influences the production of the antibiotic is inoculum volume. Moderate inoculum volume has a positive effect on yield, as well as on the potency of the antibiotic product. If the volume was increased the result was less supply of oxygen and space and if the inoculum volume was decreased then extra material turned poising and made the fermentation product unfit. To optimization of fermentation broth CCD and response surface methodology were used in shake flask. The optimum predicted cultural conditions were as follows: temperature = 30 °C, rotary speed = 165 rpm, initial pH = 6.8, medium volume = 57 mL/250 mL, and inoculation volume = 7%. These values predict a 34 mm diameter inhibition zone. Verification of the results using the optimized cultural conditions was accomplished by carrying out shake-flask experiments. The maximum antibiotic activity obtained experimentally was a 32.32 mm diameter of inhibition. This is in agreement with the model prediction. As a result, the model developed was considered to be accurate and reliable for predicting the production of antibiotic by Strain TA 1123.
Extraction and purification
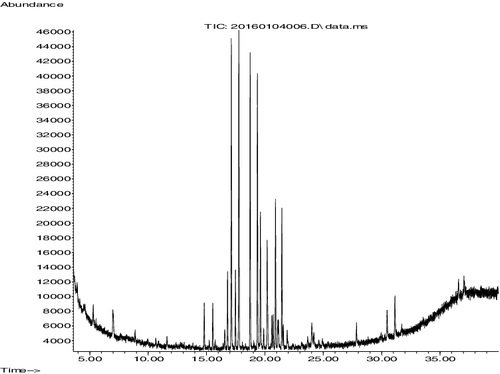
Fermentation broth was extracted with diethyl ether 1:1 (v/v). The lower phase of diethyl ether contains bioactive component. After drying about 1 g brown crude extract obtained and activity against the R. solani AG-3 was checked. Silica gel column chromatography was used to purify the crude extract and methanol was used for eluent. Fifteen fractions of 10 mL were obtained and showed activity against the pathogen. GC-MS analysis showed the chemical composition of the purified extract. About 48 compounds were identified and 12 compounds were identified as bioactive against the pathogens (). Peak of these compounds were shown in . Extracted brown compound showed inhibition spectrum against the pathogen R. solani AG-3. Most of the Streptomyces secondary metabolites are active antagonist against the fungal pathogens. Recently, GC-MS analyses are applied for the identification of secondary metabolites used to synthesis medicine [Citation30], antifungal study [Citation15], and antibacterial [Citation31]. This study concluded that Strain TA 1123 belongs to Streptomyces and is an active antagonist against the pathogen R. solani AG-3. It could be used to control the target spot.
Table 4. Compounds identified in the methanol bacterial crude extract GC-MS.
Conclusion
In this study Streptomyces TA 1123 strain (KX852460) was screened. Strain TA 1123 has great potency against the R. solani AG-3. The optimized fermentation conditions that enhanced the yield and quality of the antibiotic product from Strain TA 1123 were found. For chemical screening of the secondary metabolites, GC-MS was used and about 48 compounds were identified, 12 of them indicated active against the pathogen R. solani AG-3. On the base of these observations we find that, this strain will prove helpful for the biological control of target spot disease of tobacco in vivo.
Disclosure statement
All authors declared no conflict of interest.
Additional information
Funding
References
- Zhao YQ, Wu YH, Fu Y, et al. Characterization of Rhizoctonia solani AG-3 isolates causing target spot of flue-cured tobacco in China. Adv Mater Res. 2013;726–731:4321–4325.
- Johnk JS, Jones R, Shew H, et al. Characterization of populations of Rhizoctonia Solani AG-3 from potato and tobacco. Phytopathology. 1993;83:854–858.
- Bhavana M, Talluri VP, Kumar KS, et al. Optimization of culture conditions of Streptomyces carpaticus (mtcc-11062) for the production of antimicrobial compound. Int J Pharm Pharma Sci. 2014;6:281–285.
- Srividya S, Thapa A, Bhat DV, et al. Streptomyces sp. 9p as effective biocontrol against chilli soilborne fungal phytopathogens. Eur J Exp Biol. 2012;2:163–173.
- Waksman SA. The actinomycetes. Vol. II. classification, identification and descriptions of genera and species. The Actinomycetes vol II classification, identification and descriptions of genera and species. Baltimore (MD): The Williams and Wilkins Co.; 1961.
- Usha KM, Sudhakar P, Sreenivasulu K, et al. Optimization of culturing conditions for improved production of bioactive metabolites by Pseudonocardia sp. VUK-10. Mycobiology. 2011;39:174–181.
- Gao X, He Q, Jiang Y, et al. Optimization of nutrient and fermentation parameters for antifungal activity by Streptomyces lavendulae Xjy and its biocontrol efficacies against Fulvia fulva and Botryosphaeria dothidea. J Phytopathol. 2015;164(3):155–165.
- Rao KJ, Kim C-H, Rhee S-K. Statistical optimization of medium for the production of recombinant hirudin from Saccharomyces cerevisiae using response surface methodology. Process Biochem. 2000;35:639–647.
- Elibol M. Optimization of medium composition for actinorhodin production by Streptomyces coelicolor A3 (2) with response surface methodology. Process Biochem. 2004;39:1057–1062.
- Chang C-Y, Lee C-L, Pan T-M. Statistical optimization of medium components for the production of Antrodia cinnamomea AC0623 in submerged cultures. Appl Microbiol Biotechnol. 2006;72:654–661.
- Grahovac J, Grahovac M, Dodić J, et al. Optimization of cultivation medium for enhanced production of antifungal metabolites by Streptomyces hygroscopicus. Crop Protect. 2014;65:143–152.
- Kong Y, Zou P, Miao L, et al. Medium optimization for the production of anti-cyanobacterial substances by Streptomyces sp. HJC-D1 using response surface methodology. Env Sci Poll Res. 2014;21:5983–5990.
- Smaoui S, Mathieu F, Fourati Ben Fguira L, et al. Taxonomy and antimicrobial activities of a new Streptomyces sp. TN17 isolated in the soil from an oasis in Tunis. Arch Biol Sci. 2011;63:1047–1056.
- Wang X, Huang L, Kang Z, et al. Optimization of the fermentation process of actinomycete strain Hhs. 015 T. BioMed Res Int. 2010;2010:1–10.
- Jalaluldeen AM, Sijam K, Othman R, et al. Growth characteristics and production of secondary metabolites from selected Streptomyces species isolated from the Rhizosphere of chili plant. Growth. 2015;4:1–8.
- Khalili E, Javed MA, Huyop F, et al. Evaluation of trichoderma isolates as potential biological control agent against soybean charcoal rot disease caused by Macrophomina phaseolina. Biotechnol Biotechnol Equ. 2016:30(3):1–10.
- Demain AL. Antibiotics: natural products essential to human health. Med Res Rev. 2009;29:821–842.
- Sola-Landa A, Moura R, Martin J. The two-component PhoR-PhoP system controls both primary metabolism and secondary metabolite biosynthesis in Streptomyces lividans. Proc Nat Acad Sci. 2003;100:6133–6138.
- Bibb MJ. Regulation of secondary metabolism in Streptomycetes. Curr Opin Microbiol. 2005;8:208–215.
- Stodůlková E, Kuzma M, Hench IB, et al. New polyene macrolide family produced by submerged culture of Streptomyces durmitorensis. J Antibiotics. 2011;64:717–722.
- Kumar PS, Al-Dhabi NA, Duraipandiyan V, et al. In vitro antimicrobial, antioxidant and cytotoxic properties of Streptomyces lavendulae strain SCA5. BMC Microbiol. 2014;14:291.
- Wang Y-H, Fang X-L, Li Y-P, et al. Effects of constant and shifting dissolved oxygen concentration on the growth and antibiotic activity of Xenorhabdus nematophila. Bioresour Technol. 2010;101:7529–7536.
- Dou Y, Xiao J-H, Xia X-X, et al. Effect of oxygen supply on biomass and helvolic acid production in submerged fermentation of Cordyceps taii. Biochem Eng J. 2013;81:73–79.
- Kaushik R, Saran S, Isar J, et al. Statistical optimization of medium components and growth conditions by response surface methodology to enhance lipase production by Aspergillus carneus. J Mol Catalysis B. 2006;40:121–126.
- Buckland B, Gbewonyo K, Hallada T, et al. Production of lovastatin, an inhibitor of cholesterol accumulation in humans. Amsterdam: Novel microbial products for medicine and agriculture Elsevier; 1989. p. 161–169.
- Song Q, Huang Y, Yang H. Optimization of fermentation conditions for antibiotic production by actinomycetes YJ1 strain against Sclerotinia sclerotiorum. J Agric Sci. 2012;4:95.
- Lai L-ST, Tsai T-H, Cheng T-Y. The influence of culturing environments on lovastatin production by Aspergillus terreus in submerged cultures. Enzyme Microb Technol. 2005;36:737–748.
- Akhurst R. Antibiotic activity of Xenorhabdus spp., bacteria symbiotically associated with insect pathogenic nematodes of the families Heterorhabditidae and Steinernematidae. Microbiology. 1982;128:3061–3065.
- Chen G, Maxwell P, Dunphy G, et al. Culture conditions for Xenorhabdus and Photorhabdus symbionts of entomopathogenic nematodes. Nematologica. 1996;42:124–127.
- Nandagopalan V, Gritto MJ, Doss A. GC-MS analysis of bioactive components of the methanol extract of Hibiscus tiliaceus Linn. Asian J Plant Sci Res. 2015;5:6–10.
- Khattab AI, Babiker EH, Saeed HA. Streptomyces: isolation, optimization of culture conditions and extraction of secondary metabolites. Int Curr Pharm J. 2016;5:27–32.