ABSTRACT
The emergence of multi-drug-resistant (MDR) pathogenic bacteria is considered as a global problem. The aim of this study was to evaluate the antimicrobial inhibitory effects of the oily aqueous extract of Moringa peregrina Forssk against MDR clinical Salmonella enterica isolates. Four MDR S. enterica isolates were proved to have a gene mutation in amino acids codon 83 and 87 of gyrA and 67, 76 and 80 of parC gene by polymerase chain reaction (PCR) amplification and sequencing. The active components of M. pregrina extract were purified using GLC and TLC techniques and by using IR, NMR and mass spectra. The M. peregrina Forssk extract effect on bacterial cells was determined using scanning and transmission electron microscopies. Results demonstrated that M. peregrina Forssk have an excellent inhibitory effect against 34 MDR S. enterica isolates with different minimum inhibitory concentration (MIC) (109.37–437.5 mg/mL). The active component was identified as oleic acid-3 hydroxy propyl ester. The main abnormalities of Salmonella cells were observed – destruction in the cell wall that led to a reduction of protoplast besides, disruption of cytoplasmic membranes and, consequently, loss in their metabolic functions and death. This is the first report that deeply highlights the antimicrobial activity of M. peregrina Forssk against MDR clinical S. enterica isolates.
Introduction
Salmonella enterica is one of the major and leading enteric pathogens in animals and humans. Non-typhoid Salmonella serotype is the causative agent in the incidence of salmonellosis. Salmonellosis is considered a self-limited infection, but sometimes Salmonella causes severe infection, such as bacteremia, due to spread to the bloodstream, which can result in complication as osteomyelitis and endocarditis, which occur more frequently in the elderly and immunocompromised patients [Citation1]. Antimicrobial resistance has evolved in non-typhoid Salmonella serotypes and the resistance rate varies with different serotypes and different antibiotics. The overuse of antibiotics in the animal feeds causes the emergence of antibiotic resistance that is considered as a potential risk to public health [Citation2], especially in developing countries. Earlier studies have reported that fluoroquinolone resistance rate in Salmonella was higher in adult [Citation3]. Resistance to flouroquinolones in S. enterica sp. was mainly owing to (1) variations in the quinolone-resistance-determining regions (QRDRs) of the target genes gyrA, gyrB, and parC, parE, which encode DNA gyrase and topoisomerase IV, respectively, or/and (2) low growth of the antimicrobial activity within the cell, as a result of over-expression of the AcrABTolC efflux pump [Citation4] and/or alteration in cell wall permeability, due to the loss of one or more of the outer membrane proteins. Consequently, there is an argument need for new sources of antimicrobial agents in order to fight the multi-drug-resistant (MDR) strain infection [Citation5]. One of the most promising sources of antimicrobial activities is plants that were used in accordance with the reply to World Health Organization directions in clinical and preclinical studies [Citation6].
Moringa peregrina Forssk belonged to Moringaceae family and was utilized by the ancient Egyptians, Romans and Greeks. It is a small and fast-growing tree with greyish-green bark, long leaves, and yellowish-white to pink, fragrant flowers [Citation7], cultivated in the tropic and subtropic zones of Asia and Africa [Citation8]. It has a medicinal value where it possesses antioxidant, antibacterial and antifungal activities [Citation9]. Several investigations reported that parts of Moringa tress are rich in antimicrobial agents, as are seeds due to their content of dimer cationic protein (13kd) [Citation10], as well as roots that contain 4-α-L-rhamnosyloxy benzyl isothiocyanate that attributed to their antimicrobial power [Citation11,Citation12]. identified aglycone of deoxyniazimicine (N-benzyl, S ethyl thiofor- mate) that have an antimicrobial effect from ethanol extract of the root bark. Furthermore, fresh leaf and stem bark juice have antibacterial and fungicidal activities against Staphylococcus aureus and Pseudomonas aeruginosa [Citation13]. The goal of the study was the use of M. peregrina Forssk seeds extract as a natural source of antimicrobial agent against MDR Salmonella isolates.
Materials and methods
Clinical sitting
Clinical bacterial isolates were obtained from blood and stool specimens of patients with acute gastroenteritis and bacterimia in the period from July 2012 to October 2013 from the Department of Clinical Microbiology, Kasr el-Eini Hospital, Cairo, Egypt. There were 35 isolates of S. enterica; 19 S. typhimurium, 11 S. enteritidis and 5 others. The isolates were stored at −20 °C in tryptic soya broth with 20% glycerol. This study was clinically approved by the Ethics Committee of Cairo University Hospitals.
Susceptibility testing and MIC determination
Antimicrobial susceptibility testing was performed against 35 S. enterica isolates by disc diffusion method using cefepime, aztreonam, amikacin, ceftazidime, ofloxaciln, levofloxacin, ciprofloxacin, nalidixic acid, meropenem, ampicillin/sulbactam, sulfamethoxazole/trimethoprim and piperacillin/tazobactam. Minimum inhibitory concentration (MIC) values of ciprofloxacin, levofloxacillin and ofloxacin were determined by the microdilution method using 96-well plates. Results were analysed as described by the Clinical and Laboratory Standards Institute guidelines [Citation14].
Detection of target gene mutation of QRDS in resistant Salmonella isolates
Total genomic DNA of four MDR S. enterica isolates were extracted using polymerase chain reaction (PCR) colony method. PCR was performed to screen for variations in the QRDRs of the gyrA, and parC genes using primers CGTTGGTGACGTAATCGGT Rv CCGTACCGTCATAGTTATC and Fw CTATGCGATGTCAGAGCTGG Rv TAACAGCAGCTCGGCGTATT, respectively designed by [Citation15,Citation16] (Sigma Aldrich, USA). Bands of the correct size were removed and purified by adding the DNA extraction kit (Montage, Milipore) then sequenced by Jena Gen GmbH Biotechnologie-Gentechnik-Diagnostik (Jena, Germany) by applying the BigDye™ Cycle Sequencing Kit (Applied Biosystems, Weiterstadt, Germany) on a 3130 sequencer (Applied Biosystems). Final results were investigated using bioedit softwere v.7 and by direct comparison with the gene sequences for the appropriate serotype using BLAST under reference accession no. X78977 and AE008878 for gyrA and parC gene, respectively.
Plant material
M. peregrina Forssk fresh seeds were collected from El-Orman Garden, Cairo, Egypt. The selected samples were transported to the laboratory. To remove any debris and dust particles, they were washed with running tap water before rinsing in distilled water for five minutes. To reach constant weight, they were air-dried at room temperature.
Moringa peregrina Forssk seed extract
One hundred grams of powdered seeds of M. peregrina Forssk were extracted with n-hexan, n-butanol and cooled in distilled water as follows: fresh seeds of M. peregrina Forssk were crushed straightly by grinder after weighing, dipped into 400 mL cold distilled water into a conical flask and left for one day. Using sterile filter paper (Whattman no. 1), the extract was filtered into a clean conical flask and applied to water bath evaporation where the water was evaporated at its boiling temperature of 100 °C. The obtained extracts were then stored in a refrigerator at 4 °C for antibiogram activity [Citation17]. In the case of n-hexan and n-butanol, the same steps were taken for cold water, except evaporation was at 40 °C.
Antibacterial assay of Moringa peregrina Forssk seed extracts against MDR Salmonella enterica
Different extracts of M. peregrina Forssk seeds were screened for their antimicrobial activity against MDR S. enterica isolates using disc diffusion and agar well method. For disc diffusion method, the antimicrobial activity of the M. peregrina Forssk extract was evaluated using modified Kirby–Bauer disk diffusion method [Citation18]. Briefly, the strains were inoculated in tryptic soy agar [Citation10] and incubated at 35 °C for 24 h. Then cultures were adjusted to a concentration of 108 CFU/mL by producing a suspension in 0.85% saline solution and matched with the 0.5 McFarland turbidity standards. By using a sterilized swab, aliquots from each tube were spread on Muller–Hinton agar (Difco), discs soaked with extract were added and incubated at 35 °C for 24 h. DMSO was used as a negative control. The agar well method was carried out using the procedure previously described in Ref. [Citation19]. Briefly, 1 mL of bacterial suspension was inoculated into agar plates after which about 0.01 mL of the extract was added, and then incubated for 24 h at 37 °C after which the plates were inspected for zones of inhibition. Each of the extract solutions was repeated three times.
Minimum inhibitory concentration of Moringa peregrina Forssk aqueous extract
The MIC of the M. peregrina Forssk seeds extract was prepared as described by Chandrasekaran and Venkatesalu [Citation20] using a twofold serial dilution method. Briefly, 0.1 mL of seeds extract were added to 9 mL of suspension of testing bacteria (108 CFU/mL), the mixture was incubated for 24 h at 37 °C. Controls were used by the test organisms, using distilled water as an alternative of the plant extract. Minimum concentration of the samples with no visible growth was taken as the MIC.
Extraction, purification and identification of Moringa peregrina Forssk seeds components
The oily aqueous extract of M. peregrina Forssk was analysed by gas–liquid chromatography (GLC) according to Ackman and Sipos [Citation21] using GLC Hewlett packard 5840A, USA. Each component separated using thin-layer chromatography (TLC) by elution with ethyl acetate:methanol:water (50:45:5) and the active spot was identified by measuring Infrared (IR) (Opus 19810/28/2.204, Germany), nuclear magnetic resonance spectroscopy (NMR) (EM-390 90MH2, Germany) and gas chromatography mass spectra [Citation11] (Hp model Ms-5988, USA).
Interaction of Salmonella cells with Moringa peregrina Forssk seeds extract using scanning and transmission electron microscopes
The S. enterica Typhimurium 57 and S. enterica Entritidies 22 suspensions were prepared as described previously in susceptibility test. 1.0 mL of the 24-h-old bacterial suspension was inoculated in a 50.0-mL conical flask containing 30.0 mL of sterilized Tryptic soya broth, then incubated in a shaker at 37 °C, 150 rpm for 18 h. The bacterial suspension was then added to the extract stock solution (the final concentration in each flask was at the MIC value) and incubated at the required incubation time (6 and 24 h). By centrifugation, the treated and untreated cells were harvested and were prefixed with a solution of 2.5% glutaraldehyde overnight at 4 °C. Subsequently, by centrifugation, the cells were again collected and washed three times with 0.1 mol/L sodium phosphate buffer solution (pH 7.2). The sample preparation for scanning (SEM) [Citation22] and transmission electron microscopies (TEM) followed the method described by Yogalatha et al. [Citation23]. The prepared samples were then viewed under a scanning (Jeol) and transmission (Jeol) at a voltage of 120 kV.
Ethics committee
This study was submitted to the research ethical committee of the Kasr el-Eini Hospital, Cairo University. The ethical approval was permitted before specimens collection under the number 120-33-15.
Results and discussion
Antimicrobial susceptibility testing of 35 clinical S. enterica isolates revealed that (four) isolates were MDR to fluoroquinolones antibiotics that were chosen as the last defense line against non-typhoid infection. The ciprofloxacin showed 82.85% resistance of Salmonella isolates, 97.14% of Salmonella isolates that were resistant to nalidixic acid. Of the 11 isolates of S. enteritidis, 10 isolates were nalidixic acid resistant; whereas all 19 S. typhimurium isolates were resistant. The residual five Salmonella serotypes were nalidixic acid resistant and three were ciprofloxacin resistant.
The results of MIC for the isolated S. enterica are summarized in . MIC values of ciprofloxacin (MICs: 64 to >512 μg/mL) have been reported in MDR S. enterica isolates, as shown in , where this antibiotic may be a limited clinical utility for the treatment of Salmonella infection.
Table 1. Antimicrobial susceptibility pattern of 35 clinical isolates of S. enterica against 12 antibiotics.
Table 2. Characteristics of the studied S. enterica strains.
Data about the antimicrobial susceptibility of different extracts of M. peregrina Forssk seeds () demonstrate that the oily aqueous extract was the most effective extract against almost all tested S. enterica isolates, followed by n-hexan and n-butanol extract. Nineteen (54.28%) of S. enterica were inhibited by n-hexane and n-butanol extract. Our results showed that there is a variation in sensitivity between isolates, but four isolates of S. Typhimurium (nos. 14, 29, 16, 49) were the most sensitive isolates to M. peregrina Forssk oily aqueous extract. Those MDR S. enterica isolates were inhibited by oily aqueous extract, therefore used for further experiments.
Table 3. MIC of aqueous extract of Moringa peregrina Forssk seeds against 10 MDR clinical isolates of S. enterica.
The MIC of most sensitive 10 resistant S. enterica isolates was determined. Higher MIC was recorded for S. enterica Typhimurium no. 54, which recorded 437.5 mg/mL; while lower MIC was noticed for S. enterica Typhimurium no. 8, 57, 17 and 14 with 109.37 mg/mL.
One of the most important mechanisms producing resistance fluoroquinolones was pointing to mutations in the gyrA and parC gene. According to amplification results, four resistant isolates were identified as Salmonella serovar based on the presence of two genes, followed by sequencing of two fluoroquinolone-resistant isolates for gene mutation. As proved, both isolates have gyrA gene mutation (Figure SI1) (between amino acid codons 67 and 106) and only one isolate has parC gene mutation as shown in . There is another mutation inside QRDR, alanine substituted by serine at 119 codons in S. typhimurium no. 57 (Figure SI2); whereas in S. enteritidis no. 22, there are seven mutations within QRDR from codon 115–122.
Two tested Salmonella isolates proved to have a main target mechanism of fluoroquinolone resistance that is caused by gene coding mutation for DNA gyrase (gyrA) and topoisomerase (parC) and this result in line with Chen et al. and Rushdy et al. [Citation24,Citation25] found that amino acid changes at Ser-83 to (Phe, Tyr or Ala) or at Asp 87 to (Gly, Asn or Tyr) are the most mutation detected in nalidixic-acid-resistant strains. Similarly, Guerra et al. [Citation26] identified double mutation at both codons in clinical S. Typhimurium isolates that showed high-level fluoroquinolones resistance. Results showed that only one Salmonella isolates (S. Enteritidis no. 22) had a mutation in parC gene, which could be explained as being not necessary to produce high-level resistance to ciprofloxacin and this substitution was likely to be a polymorphism that was not common in resistant Salmonella isolates as represented by Wu et al. [Citation27].
In the present investigation, oily aqueous, n-hexane and n-butanol extracts of M. peregrina Forssk seeds had potent antimicrobial activity against tested MDR S. enterica isolates and this result was in line with Eilert et al. [Citation28,Citation29], who recorded that oily aqueous extract, ethanol and petroleum ether extracts of M. peregrina Forssk have high antimicrobial activity. Furthermore, these results were coincided with those of Walter et al. [Citation30] who showed antimicrobial properties of M. olievera leaf within the cotyledons of the seeds.
Different studies proved that M. peregrina Forssk antimicrobial activity was due to the presence of a number of phytochemicals and the short polypeptide and benzyl isothiocyanate compounds, as explained by Sultana et al. [Citation11]. Our findings of physochemical properties of the active component of oily extract M. peregrina Forssk seeds when compared with data obtained by Tsaknis [Citation10], showed that it was rich in oleic acid which was similar to oleic acid-3 hydroxxy propyl ester. Gharibzahedi et al. [Citation31] recorded that M. peregrina Forssk was rich in oleic acid (77.9%) and may have antimicrobial activity.
Herein, the phisochemical studies proved the presence of carboxylic acid as a functional group that was considered as secondary metabolite and showed antibacterial action. Similarly, Gharibzahedi et al. [Citation11] found that olean-27-carboxylic acid of triterprine type and ursolic acid that had potent antibacterial activity from Aceriphyllum rossii and Hedyotis corymbosa, respectively, and [Citation12] reported that Sesame radiatum had carboxylic acid that showed antimicrobial activity. Furthermore, our analysis reported the involvement of hydroxyl groups that was related to phenols which was connected to the toxicity towards microorganisms and with increasing hydroxylation, the inhibitory action increased. Earlier studies concluded that M. oeifera seeds contained polypeptides which were used as disinfecting agents, a water clarification agent and led to growth inhibition of bacteria, as in antibiotic resistant human pathogens, instead of traditional clarifying agents [Citation13].
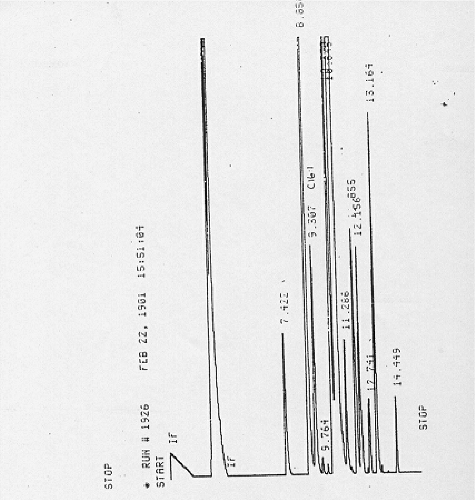
GLC analysis of M. peregrina Forssk oily aqueous extract indicated several peaks that reflect several individual components () that were further separated using TLC and by disk diffusion method against S. Typhimurium no 7 which gave one active spot. FTIR, NMR and MS spectroscopic studies of the active spot were carried to determine the antimicrobial agent that might be present by determining the characteristic bands as represented in
Table 4. Physicochemical properties of active spot of oily-aqueous extract of Moringa peregrina Forssk.
The morphological changes occurred due to the interaction of S. enterica Typhimurium 57 and S. Enteritidis 22 cells with aqueous extract of M. peregrina Forssk seeds were shown using SEM [Citation22] in . Salmonella cells (control cells) were regular in shape, but after 6 h with the addition of the oily extract, the cells had an irregular shape with holes in some parts of cell wall. Here, the cells became elongated with abnormal cell wall as shown in (B,C) after 24 h.. The ultrastructural changes that aqueous extract of M. peregrina Forssk brought about in this study were observed with transmission electron microscopes. (B,F) shows the main abnormalities noted in abnormally shaped hyphae, some pores found in the cell wall after 6 h of interaction with notable structural disorganization of the cytoplasm and wavy appearance of the cell membrane. After 24 h ((C,G)), the cell wall was disrupted and leakage of cellular components was observed, which consequently caused the cell death.
Figure 2. SEM of Salmonella enterica serovar Typhimurium and S. enterica srovar Enteritidis interact with aqueous seeds extract of Moringa perigrina. A: S. Typhimurium control; B: after 6 h; C: after 24 h. D: S. Enteritidis control; E: after 6 h; F: after 24 h.
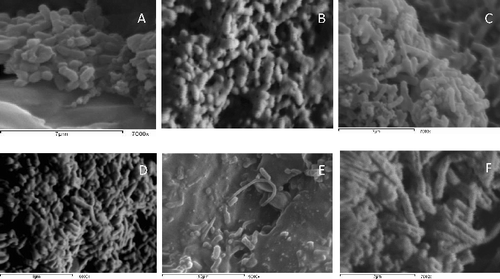
Figure 3. TEM of S. enterica serovar Typhimurium and S. enterica srovar Enteritidis interact with aqueous seeds extract of Moringa peregrina Forssk. A: S. Typhimurium control; B: after 6 h; C: after 24 h. D: S. Enteritidis control; E: after 6 h; F: after 24 h.
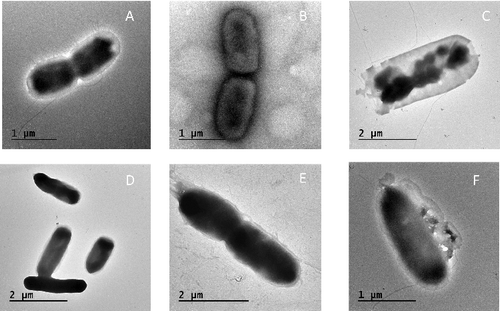
From SEM and TEM, the inhibition of S. Typhimurium no. 57 and S. Enteritidis no. 22 growth by M. peregrina Forssk extract was observed due to changes in cell wall integrity as that was deduced by Vijayarathna et al. [Citation14], who found that the E. guineensis leaf extract had a dramatic effect on yeast cell after 36-h treatment. Similarly, Anam et al. [Citation15] stated that a component of ethylacetate extract of Termnalia muelleri Benth leaves was found to inhibit S. aureus and methicillin-resistant S. aureus growth.
The inhibitory effect of M. peregrina Forssk oily extract on Salmonella isolates could be attributed to the existence of hydroxyl groups found on phenols that leads to enzyme inhibition by interaction with sulphahydryl group or proteins. However, phenolics have antimicrobial agents that result in adsorption and disruption of microbial membranes, non-specific interactions with enzymes and substrates, or metal ion deprivation as reported in [Citation16]. Suarez et al. [Citation17] explained that peptide disrupting cell membrane synthesis or synthesis of some essential enzymes results in growth in inhibition.
Conclusion
The study provided that M. peregrina Forssk tree is an excellent gift from nature that has showed powerful antimicrobial activity to fight the natural enemies of human as S. enterica sp. that proved gene mutation in deduced amino acid sequence against widely used fluoroquinolone antibiotics especially ciprofloxacin that was a drug of choice for salmonellosis infection treatment. Our study concluded that aqueous oily extract of seeds was better than other solvent extraction methods and anti-Salmonella activity with GLC analysis showed that the active ingredients proved to have powerful anti-Salmonella activity. In addition, this study proved that traditional medicines are still used as natural weapons against such MDR microorganisms.
Acknowledgment
The authors extend their appreciation to the Deanship of Scientific Research at King Saud University for funding this work through research group project No RGP-202.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Kumar V, Abbas AK, Fausto N, et al. The gastrointestinal tract. In: Schmitt W, Gruliow R, Sinclair J, Zanolle El, editors. Robbins and Cotran pathologic basis of disease. 7th ed. Philadelphia (PA): Elsvier Saunders; 2005. p. 859–868.
- McEwen SA, Fedorka-Cray PJ. Antimicrobial use and resistance in animals. Clin Infect Dis. 2002;34:S93–S106.
- Stoycheva M, Murdjeva M. Antimicrobial therapy of salmonelloses-current state and perspectives. Folia Medica. 2005;48:5–10.
- Fàbrega A, Madurga S, Giralt E, et al. Mechanism of action of and resistance to quinolones. Microb Biotechnol. 2009;2:40–61.
- Silver L, Bostian K. Discovery and development of new antibiotics: the problem of antibiotic resistance. Antimicrob Agents Chemother. 1993;37:377–381.
- Dilhuydy J. [Patients attraction to complementary and alternative medicine (CAM): a reality which physicians can neither ignore nor deny]. Bull Cancer. 2003;90:623–628.
- Olson ME. Intergeneric relationships within the Caricaceae‐Moringaceae Clade (Brassicales) and potential morphological synapomorphies of the clade and its families. Int J Plant Sci. 2002;163:51–65.
- Fahey JW. Moringa oleifera: a review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. part 1. Trees Life J. 2005;1:1–15.
- Jayawardana BC, Liyanage R, Lalantha N, et al. Antioxidant and antimicrobial activity of drumstick (Moringa oleifera) leaves in herbal chicken sausages. LWT-Food Sci Technol. 2015;64:1204–1208.
- Tsaknis J. Characterisation of Moringa peregrina Arabia seed oil. Grasas Aceites. 1998;49:170–176.
- Sultana T, Rashid MA, Ali MA, et al. Hepatoprotective and antibacterial activity of ursolic acid extracted from hedyotis corymbos a L. Bang J Sci Ind Res. 2010;45:27–34.
- Shittu L, Bankole M, Ahmed T, et al. Antibacterial and antifungal activities of essential oils of crude extracts of Sesame radiatum against some common pathogenic micro-organisms. Iran J Pharm Ther. 2008;6:165–1670.
- Bukar A, Uba A, Oyeyi T. Phytochemical analysis and antimicrobial activity of Parkia biglobosa (Jacq.) Benth. extracts against some food-borne microorganisms. Adv Environ Biol. 2010;4:74–79.
- Vijayarathna S, Zakaria Z, Chen Y, et al. The antimicrobial efficacy of Elaeis guineensis: characterization, in vitro and in vivo studies. Molecules. 2012;17:4860–4877.
- Anam K, Suganda A, Sukandar E, et al. Antibacterial effect of component of Terminalia muelleri Benth. Against Staphylococcus aureus. Int J Pharm. 2010;6:407–412.
- Fattouch S, Caboni P, Coroneo V, et al. Antimicrobial activity of Tunisian quince (Cydonia oblonga Miller) pulp and peel polyphenolic extracts. J Agr Food Chem. 2007;55:963–969.
- Suarez M, Entenza J, Doerries C, et al. Expression of a plant‐derived peptide harboring water‐cleaning and antimicrobial activities. Biotech Bioeng. 2003;81:13–20.
- Kommanaboyina B, Rhodes C. Trends in stability testing, with emphasis on stability during distribution and storage. Drug Develop Ind Pharm. 1999;25:857–868.
- Ugbogu O, Akukwe A. The antimicrobial effect of oils from Pentaclethra macrophylla Bent, Chrysophyllum albidum G. Don and Persea gratissima Gaerth F on some local clinical bacteria isolates. Afr J Biotechnol. 2009;8:22–28.
- Chandrasekaran M, Venkatesalu V. Antibacterial and antifungal activity of Syzygium jambolanum seeds. J Ethnopharm. 2004;91:105–108.
- Ackman R, Sipos J. Application of specific response factors in the gas chromatographic analysis of methyl esters of fatty acids with flame ionization detectors. J Amer Oil Chem Soc. 1964;41:377–378.
- Semenov VV. Archeological and forensic science biological object search – by using ultraviolet radiation of prescribed energy density and recording phosphorescence signal on colour reversible film. Derwent Innovations Index. G01N-021/64 ed. Soviet Union Semenov, V V (SEME-Individual); 1989. p. 5.
- Latha LY, Darah I, Jain K, et al. Effects of Vernonia cinerea less methanol extract on growth and morphogenesis of Candida albicans. Europ Rev Med Pharm Sci. 2011;15:543–549.
- Rushdy AA, Mabrouk MI, Abu-Sef FA-H, et al. Contribution of different mechanisms to the resistance to fluoroquinolones in clinical isolates of Salmonella enterica. Braz J Inf Dis. 2013;17:431–437.
- Cloeckaert A, Chaslus-Dancla E. Mechanisms of quinolone resistance in Salmonella. Vet Res. 2001;32:291–300.
- Guerra B, Malorny B, Schroeter A, et al. Multiple resistance mechanisms in fluoroquinolone-resistant Salmonella isolates from Germany. Antimicrob Agents Chemother. 2003;47:2059–2063.
- Wu W, Wang H, Lu J, et al. Genetic diversity of Salmonella enteric serovar Typhi and Paratyphi in Shenzhen, China from 2002 through 2007. BMC Microbiol. 2010;10:32.
- Ellert U, Wolters B, Nahrstedt A. The antibiotic principle of seeds of Moringa oleifera and Moringa stenopetala Pl. Planta Medica. 1981;42:55–61.
- Tahany M, Hegazy A, Sayed AM, et al. Study on combined antimicrobial activity of some biologically active constituents from wild Moringa peregrina Forssk. J Yeast Fungal Res. 2010;1:15–24.
- Walter A, Samuel W, Peter A, et al. Antibacterial activity of Moringa oleifera and Moringa stenopetala methanol and n-hexane seed extracts on bacteria implicated in water borne diseases. Afr J Microbiol Res. 2011;5:153–157.
- Gharibzahedi S, Ansarifard I, Hasanabadi YS, et al. Physicochemical properties of Moringa peregrina seed and its oil. Quality Assur Safety Crops Foods. 2013;5:303–309.