ABSTRACT
Besides mass drug administration, successful elimination of human lymphatic filariasis, a mosquito-borne tropical parasitic disease, requires developing other suitable prophylactic agents such as vaccines. The Brugia malayi abundant larval transcript-2 (BmALT-2) protein has been identified as one possible candidate. So far, it (E-ALT-2) has been expressed as a 24-kDa non-glycosylated protein in Escherichia coli. Easy and low-cost downstream purification of secreted BmALT-2 in Pichia pastoris may be a vital option to inexpensive large-scale production. This study focused on expression and molecular characterization of BmALT-2 (P-ALT-2) in P. pastoris. Sodium dodecyl sulphate polyacrylamide gel electrophoresis analysis showed that some P. pastoris colonies produced 27 and 24 kDa bands and few colonies produced a 24-kDa band. Preliminary studies confirmed glycosylation of 27 kDa P-ALT-2. The ratio of glycosylated and non-glycosylated P-ALT-2 was 20:80–70:30 in various colonies. The maximum yield of glycosylated and non-glycosylated P-ALT-2 was measured as 7.99 ± 1.12 and 9.18 ± 1.35 mg L−1 compared to 6.5 ± 1.2 mg L−1 of E-ALT-2 in shake flasks. Their overall expression was about 25% and 35% higher than that in E. coli. The glycosylated P-ALT-2 exhibited about 15% higher immunoreactivity with human endemic normal sera. The enhanced secreted production by P. pastoris may lead to cost-effective large-scale production of BmALT-2.
Introduction
Lymphatic filariasis is a neglected tropical parasitic disease caused mostly by mosquito-borne Wuchereria bancrofti and Brugia malayi. In 2000, the World Health Organization initiated the ‘Global Program to Eliminate Lymphatic Filariasis’, which focuses on mass drug administration using albendazole, diethylcarbamazine or ivermectin [Citation1–7]. These drugs pose problems due to their undesirable side effects. Thus, there is a need to develop a suitable agent such as vaccine for the successful elimination of human lymphatic filariasis [Citation8–11]. According to various research reports, Brugia malayi abundant larval transcript-2 (BmALT-2) is the most abundant of the L3-expressed, stage-specific novel proteins [Citation12–13]. Being an L3-specific antigen, the ALT-2 protein is prevalent in endemic normal (EN) sera followed by chronic pathology (CP) and microfilariae (MF). BmALT-2 contains multiple B- and T-cell epitopes, one being in its putative signal sequence [Citation14]. ALT-2 modulates cytokine-induced signalling mechanism and alt-2 transfected parasites are more resistant to interferon gamma (IFN-γ) induced killing by macrophages. ALT-2 plays a role in the evasion strategy of the filarial nematodes by amplifying Th2 responses along with interfering signals necessary for the development of pro-inflammatory Th1 populations [Citation12,Citation15]. Earlier studies proposed BmALT-2 of B. malayi as a promising vaccine candidate with about 76% protection in animal models [Citation12,Citation15–22]. Therefore, ALT-2 is considered the most potential vaccine candidate for lymphatic filariasis.
Escherichia coli can produce large amounts of heterologous protein. However, it makes misfolded proteins inactive or insoluble due to the difference in protein-folding environment and inability to perform post-translational modifications. So far, filarial proteins have been expressed in E. coli, but this system does not maintain the proteins’ native properties of post-translational modification. The purification of intracellular recombinant proteins from large-scale culture of E. coli is labour intensive and expensive for commercial vaccine production. An attempt has been made earlier to express BmALT-2 in a eukaryotic expression system such as tobacco plants without large-scale process optimization [Citation23]. The methylotrophic yeast, Pichia pastoris has also been developed for the commercial production of heterologous proteins [Citation24–26]. P. pastoris has become important for the industrial production of therapeutic proteins owing to the availability of new strains with mammalian-type glycosylation capabilities [Citation27]. Multiple copy integration of recombinant genes in Pichia increases the expression of desired proteins in Pichia strains [Citation28–29]. Progressive increase in the expression of proteins such as glucoamylase, lipase, green fluorescence protein, influenza hemagglutinin and human serum albumin was achieved with up to seven gene copies [Citation30]. Proteins such as cattle tick vaccine [Citation31], endo-β-1,4-mannase [Citation32] and hepatitis B surface antigen [Citation33–34] have been successfully expressed in P. pastoris.
In the present study, we cloned Bmalt-2 in P. pastoris under an AOX1 promoter with an α-signal sequence for secretory expression. The expression of BmALT-2 was optimized in flask culture. This was a new attempt to express this vaccine candidate in a eukaryotic expression system such as P. pastoris. BmALT-2 was expressed in E. coli under a T7 promoter for intracellular expression. We compared the yield of BmALT-2 in both systems along with its glycosylation followed by their reactivity with human clinical sera samples.
Materials and methods
Yeast strain, vector selection and cloning
P. pastoris GS115 was used for the expression of pPIC9K vector (Invitrogen, San Diego, CA, USA) [Citation35]. Bmalt-2 of B. malayi (accession number U84723) was a kind gift from Dr Thomas B. Nutman, National Institutes of Health, Bethesda, MD, USA. Bmalt-2 was cloned in pRSETB vector as described by Ramachandran et al. [Citation21]. Bmalt-2 was amplified from pRSETB/Bmalt-2 plasmid construct by polymerase chain reaction (PCR) (Applied Biosystems, Foster City, CA, USA) with PR DNA polymerase. Two primers, 31-bp Bmalt-2 forward primer 5′-CCGGAATTCCGGATGAATAAACTTTTAATAG-3′ and 25-bp Bmalt-2 reverse primer 5′-TAAAGCGGCCGCAAATCTATGCGCA-3', were designed to amplify the gene (Eurofins, Bangalore, India). EcoRI and NotI restriction sites were incorporated at 5′ of the forward and reverse primers. A 405-bp band of Bmalt-2 was identified under ultraviolet transluminator (Life Technologies, Carlsbad, CA, USA) and purified by Qiagen gel extraction kit (Hilden, Germany). Purified Bmalt-2 gene and pPIC9K vector were subjected to restriction digestion with high-fidelity EcoRI-HF® and NotI HF® enzymes (New England Biologicals, Herts, UK) followed by ligation with T4 DNA ligase (New England Biologicals). Integration of Bmalt-2 in pPIC9K (Invitrogen) was confirmed by restriction digestion and sequencing at Xcelris Lab Ltd. (Ahmedabad, India). The result was cross-checked with the NCBI (National Center for Biotechnology Information) website [Citation15].
Transformation and screening of His+Mut+ and His+Muts colonies
Pichia-competent cells were prepared from P. pastoris GS115 culture (optical density at OD600 = 1.5) in yeast peptone dextrose (YPD) using ice-cold 1 mol L−1 sorbitol (Sigma Aldrich, St. Louis, MO, USA) to make the final concentration of 7.2 at OD600. SacI-HF®-restricted and purified pPIC9K-Bmalt-2 was mixed with Pichia competent cells (17.85 × 108) and transformed using 0.22 mm electroporation cuvette (BTX, Holliston, MA, USA) in Gene Pulser system (Bio-Rad, Gurugram, India, conditions used: 1.5 kV, 200 R, 25 F and 4.8 ms). The transformed colonies were screened for His+ on regeneration dextrose medium (RDB) plates followed by YPD plates containing 0, 0.5, 1.0, 2.0 and 4.0 mg mL−1 geneticin (Himedia, Mumbai, India) [Citation36]. Mut+ and Muts phenotypes of the transformants were evaluated by spotting them on minimal dextrose (MD) plates and minimal methanol (MM) plates. Genomic DNA was isolated from 48 h culture of screened colonies using the phenol--chloroform method with glass beads (0.5 mm) in 20 mmol L−1 tris-acetate buffer, pH 8.
Screening for expression of P. pastoris/Bmalt-2 in shake flasks
Screening was performed by culturing different colonies in 10 mL of buffered glycerol-complex medium for 48 h at 28 °C and 200 r min−1. Then cells were cultured in buffered minimal methanol yeast medium for three days at 24 °C and 200 r min−1. The expression of P-ALT-2 protein was induced by adding 100% methanol to a final concentration of 1.0% v/v methanol after each 24 h interval for three days (Invitrogen). Then, the culture broth was centrifuged at 10,000 g for 10 min and the supernatant was subjected to dot-blot method. Nitrocellulose membrane was incubated with mouse anti-BmALT-2 primary antibody (SPAN Diagnostic, Surat, India) for 2 h followed by alkaline--phosphatase-conjugated goat anti-mouse secondary antibody (Sigma) for 45 min as 1:2000 and 1:5000 dilution, respectively.
Determination of gene copy number
The number of Bmalt-2 gene incorporated into P. pastoris/Bmalt-2 genome was determined by real-time PCR (Applied Biosystems) as described by Ingham et al. [Citation37]. The reaction mixture contained SYBR Green dye (Roche, Indianapolis, IN, USA), specially designed AOX1 forward (5′-GAAGCTGCCCTGTCTTAAACCTT-3′) and reverse (5′-CAAAAGCTTGTCAATTGGAACCA-3′) primers and genomic DNA (5–10 ng µL−1). ARG4 forward (5′-TCCTCCGGTGGCAGTTCTT-3′) and reverse (5′-TCCATTGACTCCCGTTTTGAG-3′) primers were used to amplify the ARG4 gene as reference gene copy number. DNA from P. pastoris GS115 was used as control. More than 100 colonies were screened for the determination of gene copy number incorporated in various colonies. The gene copy number was calculated by determining 2−∆∆CT as described in various articles [Citation28,Citation29].
Downstream processing and purification of Pichia expressed BmALT-2 (P-ALT-2)
Proteins were isolated from whole cell and culture supernatant for analytical purpose. The washed cell pellet was incubated with breaking buffer (50 µmol L−1 Na3PO4, pH 7.4, 1 µmol L−1 ethylenediaminetetraacetic acid (EDTA) and 50 mL L−1 glycerol) and 100 µmol L−1 phenylmethylsulfonyl fluoride An equal volume of acid-washed glass beads (0.5 mm, Sigma) was also added to it, followed by eight cycles of vortexing (Invitrogen) for 30 s and incubation on ice during intervals. The mixture was centrifuged at 12,000 r min−1 for 10 min at 4 °C. Clear supernatant was transferred to a fresh microcentrifuge tube and stored at −20 °C. For isolating protein from the culture medium, trichloroacetic acid was added to Pichia culture supernatant at a final concentration of 10% and incubated at 4 °C overnight. Then, the sample was centrifuged at 12,000 r min−1 for 10 min at 4 °C. White pellet was washed three times with 80% ice-cold acetone. Protein pellet was dissolved in buffer of 20 mmol L−1 Tris and 5 mmol L−1 EDTA, pH 8.
For large-scale protein isolation from culture medium, P-ALT-2 was precipitated from Pichia culture broth with 70% ammonium sulphate at 4 °C overnight on a magnetic stirrer. Then, dialysis was performed using 10-kDa dialysis membrane (HiMedia) overnight. P-ALT-2 was purified by ion exchange chromatography as described by Amersham Biosciences. The column packed with the matrix Q-Sepharose ™ fast flow (GE Healthcare, Danderyd, Sweden) was used for isolation of P-ALT-2 from the dialysed protein sample using 20–50 mmol L−1 NaCl in elution buffer (20 mmol L−1 Tris and 5 mmol L−1 EDTA, pH 8.0). Then, the semi-purified protein was purified by size exclusion chromatography using Superdex™ 75 matrix (GE Healthcare) with 150 mmol L−1 NaCl in 20 mmol L−1 Tris buffer, pH 8.0. The protein was concentrated using 10 kDa Amicon membrane filter (Millipore, Billerica, MA, USA). The purity of the protein was checked by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) and the samples were stored at −20 °C until further use.
Expression and purification of BmALT-2 (E-ALT-2) in E. coli
The E-ALT-2 was expressed in E. coli BL21 (DE3) pLysS under T7 promoter of pRSETB vector using Luria–Bertani broth induced with 1 µmol L−1 isopropyl β-D-1-thiogalactopyranoside for 3 h at 37 °C and 150 r min−1 in a shaker (Sciegenics Biotech, Chennai, India) was expressed as an intracellular product with six histidine residues as an N-terminal fusion peptide. Cells were disrupted by sonication followed by centrifugation at 10,000 r min−1 for 10 min. The metal-binding domain in the fusion peptide allows simple one-step purification of recombinant protein by immobilized metal ion affinity chromatography (IMAC) [Citation38]. Samples were applied to the NiCl2-charged Ni-NTA column (Amersham Pharmacia Biotech, Hong Kong) at a rate of 5 mg mL−1 of recombinant protein and were allowed to bind. The column was washed with elution buffer (0.1 mol L−1 phosphate buffer, pH 6.5) to remove all contaminating proteins, followed by elution with increasing concentration of imidazole (25–250 mmol L−1 in elution buffer).
Estimation and quantification of P-ALT-2 and E-ALT-2
Protein concentration in Pichia and E. coli was determined using Bradford's reagent [Citation39]. Absorbance was recorded at 595 nm by a plate reader (Biotek Synergy HT, Winooski, VT, USA). Purified P-ALT-2 was estimated as a fraction of crude P-ALT-2 concentration using indirect enzyme-linked immunosorbent assay (ELISA) by the protocol described by Pasamontes et al. [Citation40]. P-ALT-2 and E-ALT-2 were coated onto each microtiter plate well (Nunc, Rochester, NY, USA) separately at 0.1 mg mL−1 in 0.1 mol L−1 carbonate buffer (pH 9.6). Mouse anti-BmALT-2 antibody (SPAN, Surat, India) as 1:100 dilution and alkaline phosphatase (ALP)-conjugated goat anti-mouse secondary antibody (Sigma) as 1:1000 dilution were used. The colour was measured at 405 nm using a micro-plate reader (Biotek Synergy HT).
SDS-PAGE and western blot analysis of P-ALT-2 and E-ALT-2
The P-ALT-2 and E-ALT-2 were analysed by 12% SDS-PAGE [Citation41]. After electrophoresis, protein bands were transferred from SDS-PAGE to nitrocellulose membrane (HyBond, Amersham Pharmacia Biotech, Little Chalfont, UK) as described by Towbin et al. [Citation42]. Electrophoretic transfer was carried out at 20 V and 120 mA for 90 min using Hoefer TE 70 semi-dry electroblotting apparatus (Amersham Pharmacia Biotech). After the transfer, nitrocellulose (NC) membrane was stained with 0.2% Ponceau S (Sigma). The molecular weight marker lane was cut and stained with Amido black. The rest of the NC membrane was subjected to incubation with mouse anti-BmALT-2 primary antibody (1:2000) dilution followed by ALP-conjugated goat anti-mouse secondary antibody (Sigma) as 1:5000 dilution. Colour was developed using 5-bromo-4-chloro-3-indolyl phosphate (BCIP; USB, Amersham Pharmacia) and nitroblue tetrazolium (NBT; USB, Amersham Pharmacia).
Periodic acidic and Schiff's staining and PNGase assay for glycosylation study
Deglycosylation of P. pastoris and E. coli expressed BmALT-2 was done with PNGase F enzyme (New England Biolabs, Herts, UK). Ten micrograms of purified BmALT-2 were used for analysis according to the manufacturer's protocol to study the post-translational modifications like glycosylation. An equivalent amount of treated and untreated samples was analysed by 12% SDS-PAGE. Periodic acid and Schiff's staining (PAS staining) was performed as described by Fairbanks et al. [Citation43] to confirm the deglycosylation by PNGase F. Glycopred (University of Nottingham, UK) and NetOGlyc-3.1 [Citation44] were used as tools for glycosylation prediction.
Ethical statement for human blood samples
We used the EN, MF and CP sera from human individuals provided by our lab with the permission (Ref No-26433/VCII/SI/2012) of the Department of Public Health and Preventive Medicine, Government of Tamil Nadu. MF samples (from asymptomatic microfilaremics; n = 10) were collected from endemic regions of Tamil Nadu (India) after the patients were identified with the traditional thick smear microscopic data provided by the Department of Public Health and Preventive Medicine and Zonal Entomology Team, Vellore. EN samples (n = 10) were collected from individuals from the endemic villages having no MF present in thick smear microscopic test. The CP samples (n = 10) with visible clinical symptoms of lymphedema were collected from Vellore and Tiruvannamalai districts of Tamil Nadu, India. The control non-endemic normal (NEN) sera were provided by Professor Murray Selkrirk, Imperial College London, UK.
Reactivity of BmALT-2 with human clinical sera
Purified P-ALT-2 and E-ALT-2 along with L3 extract were coated onto each microtiter plate well (Maxisorp, Nunc, Rochester, NY, USA) separately at a concentration of 100 ng and 1 µg per well in 0.1 mol L−1 carbonate buffer (pH 9.6), respectively, and were incubated at 4 ˚C overnight. After washing three times with phosphate-buffered saline/Tween (PBST), the plates were blocked with 5% skimmed milk in PBST at 37 °C for 1 h. Skimmed milk (5% in PBST) was discarded and the plate was washed three times with PBST. After washing, 100 uL of human clinical sera from each of 10 patients with NEN, EN, MF and CP were added as 1:100 dilution and incubated for 1 h at 37 °C. Again, after washing with PBST, 100 µL of ALP-conjugated mouse anti-human IgG antibody was added as 1:1000 dilution and was incubated for 1 h at 37 °C. The plates were washed thrice with PBST followed by three PBS washes. The substrate para-nitrophenylphosphate was added to wells and subsequent development of colour was measured at 405 nm using a microplate reader (BIOTEK Instruments Inc., Winooski, VT, USA).
Statistical analysis
All data were analysed using Graphpad Prism 7 with at least three independent experiments and replicates for each experiment. The differences among groups were calculated using one-way analysis of variance (ANOVA). Statistical significance was calculated by Student's test; where P < 0.05 was considered significant.
Results and discussion
Human lymphatic filariasis is the most debilitating and disfiguring neglected mosquito-borne parasitic tropical disease. The pathologic progression of this disease is intriguing and gruesome making it a difficult task to develop a vaccine against this multicellular parasite. Of the different vaccine candidates identified for lymphatic filariasis, the ALT-2 protein stands out as a remarkable one with no known homologue in mammalian hosts, imparting about 74% protection in jirds [Citation13–22]. This study will lead to the development of commercial large-scale production of BmALT-2 as an inexpensive vaccine candidate.
P. pastoris as an expression system offers the advantage of secreting the protein of interest into the culture medium. The regulation of heterologous gene expression by a mixed carbon source has been proved to be simple and cost effective for industrial protein production. We cloned and expressed filarial Bmalt-2 gene in Pichia as a secretory product for the first time. BmALT-2 was expressed in P. pastoris as a secretory protein under AOX1 promoter, containing the α-secretory signal. One advantage of being an extracellular protein is that it is convenient to be easily purified, making it cost effective.
Screening and confirmation of Bmalt-2 integration in P. pastoris
PCR amplification of Bmalt-2 using gene-specific forward and reverse primers was visualized in 1.7% agarose gel. This showed a band of near 405 bp of the Bmalt-2 gene (387 bp) along with 5′ and 3′ restriction sites. PCR amplification and double digestion confirmed the recombinant pPIC9K-Bmalt-2 because of a pop-out of 405 bp. Sequencing also confirmed the successful integration of the Bmalt-2 gene in pPIC9K.
Recombinant P. pastoris/pPIC9K-Bmalt-2 colonies with His+ were grown on RDB plate followed by screening for multiple gene integration on YPD plate containing 2 mg mL−1 geneticin. His+Mut+ and His+MutS colonies were identified on MD and MM agar plate by growing His+ colonies from YPD plates. Colonies grown on MD plate, but not on MM plate are Muts and colonies grown on both plates are Mut+. Bmalt-2 gene integration in His+Mut+ P. pastoris/Bmalt-2 colonies was confirmed by lysate PCR. The copy number of the gene integrated into the Pichia genome was 1–23 as determined by Real-Time PCR. P. pastoris/Bmalt-2 transformants were screened for expression by the dot blot method.
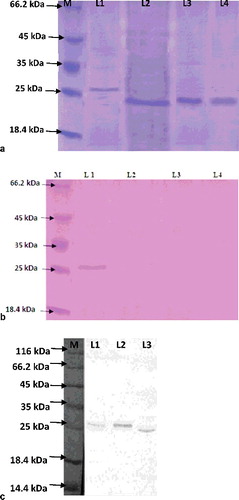
His+Mut+ with 11 copies of the Bmalt-2 gene was the best expressed colony for P-ALT-2 expression (Figure S1 in the Online Supplementary Appendix). SDS-PAGE analysis showed that some P. pastoris colonies produced 27-kDa and 24-kDa bands and few colonies produced a 24-kDa band of P-ALT-2 (Figure S2a in the Online Supplementary Appendix).
Expression and purification of BmALT-2 in P. pastoris and E. coli
E. coli/Bmalt-2 expressed 6.5 ± 1.2 mg L−1 of non-glycosylated E-ALT-2 in shake flask (Figure S2b in the Online Supplementary Appendix). The ratio of glycosylated and non-glycosylated P-ALT-2 was 20:80–70:30 in various colonies of P. pastoris at various times (data not shown). The maximum glycosylated P-ALT-2 expression was measured as 7.99 ± 1.12 mg L−1 compared to 9.18 ± 1.35 mg L−1 of non-glycosylated P-ALT-2 in flask culture (Figure S2a in the Online Supplementary Appendix). The E-ALT-2 protein was estimated to be 11%–14% of the total protein in flask culture. Finally, high cell density culture of P. pastoris/Bmalt-2 led to 25%–35% more production of P-ALT-2 in flask culture. Codon optimization of the Bmalt-2 gene for the Pichia expression system may lead to enhanced production of this noble protein. Expressed E-ALT-2 showed a 24-kDa band in E. coli and, 27-and 24-kDa P-ALT-2 bands in P. pastoris. The P-ALT-2 of 27 kDa was glycosylated with 3 kDa more molecular weight than the 24-kDa non-glycosylated protein. The purification efficacy of 24-kDa E-ALT-2 from E. coli culture with IMAC was 85%. It was 72% and 59% for 24-and 27-kDa P-ALT-2 from Pichia culture broth, respectively, with 70% ammonium sulphate precipitation followed by ion exchange chromatography and size exclusion chromatography.
Pichia expressed BmALT-2 as a glycosylated protein
Asparagines at the 2nd, 87th, 110th and 1117th position of BmALT-2 were predicted to be N-glycosylated. O-glycosylation sites were predicted to be the 35th, 52nd, 91st and 109th threonine and the 23rd, 30th, 38th, 44th and 117th serine. P. pastoris has a majority of N-linked glycosylation of the high-mannose class (8–14 mannose residues per side chain) and very little O-linked glycosylation [Citation45]. The deglycosylation of 27-kDa P-ALT-2 showed a 24-kDa band in P. pastoris, but no change of the 24-kDa band of E-ALT-2 in E. coli ((a)). This suggests glycosylation of BmALT-2 in P. pastoris. PAS staining also validated glycosylation of BmALT-2 in the Pichia system, as the 27-kDa protein showed a band before PNGase F treatment, but no band after deglycosylation in PAS-stained gel ((b)). Excessive glycosylation is a common problem for proteins produced in fungal hosts [Citation26,Citation46]. However, BmALT-2 was not hyperglycosylated. Moreover, glycosylation was not strong, as all colonies were not producing the glycosylated form of ALT-2. Even the ratio of the glycosylated and non-glycosylated form in the same colony was different at various times (data not shown). The degree, type and position of glycosylation may further be confirmed by liquid chromatography–tandem mass spectrometry. Purified BmALT-2 was confirmed by 12% SDS-PAGE and western blot ((c)). Glycosylated BmALT-2 may have enhanced or reduced immunological response and it should be examined in mice and human clinical samples before performing large-scale production.
Immuno-reactivity with human clinical sera
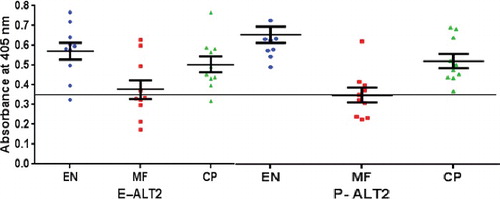
Identification of reactivity of ALT-2, recognized in natural infection of humans has practical implications in the understanding and prevention of disease. Many filarial proteins including secreted larval acidic protein 1 from Onchocerca volvulus, an ALT-2 homologue, have been characterized [Citation47]. A chimeric protein construct comprising thioredoxin, transglutaminase and abundant larval transcript-2 (ALT-2) showed enhanced immunological response [Citation48]. The hyper responsiveness of ALT-2 is also well documented with its T-cell and B-cell-specific responses [Citation49,Citation50]. The reactivity of ALT-2 protein with human clinical sera was tested by ELISA using EN, MF and CP sera (n = 10) from endemic regions. The level of antigenicity exhibited by the glycosylated P-ALT-2 with human EN, MF and CP sera (mean OD405 = 0.66 ± 0.027, 0.35 ± 0.038, 0.52 ± 0.022, respectively; P < 0.0001) was better than the non-glycosylated E-ALT-2 (mean OD405 = 0.57 ± 0.019, 0.37 ± 0.013, 0.50 ± 0.018, respectively; P < 0.0001) (), as shown in previous studies [Citation14]. Importantly, about 15% higher reactivity was observed in P-ALT-2 with EN sera (mean OD405 = 0.66 ± 0.027), indicating better reactivity of P-ALT-2 with anti-ALT-2 antibody in human sera. The immunoreactivity of P-ALT-2 with human clinical sera indicates its proper expression and, thereby, validates it as the Pichia expression system to achieve inexpensive large-scale production of BmALT-2 towards commercial vaccine development for lymphatic filariasis.
Conclusions
The P. pastoris expression system offers the advantage of simple and cost-effective industrial production by secreting proteins of interest into the culture medium. The cost-effective commercial production of the BmALT-2 candidate protein is indeed a future demand. The present study reported the secretory expression of BmALT-2 by P. pastoris leading to inexpensive large-scale production. It produced 24 kDa non-glycosylated ALT-2 alone or with glycosylated 27 kDa protein. The yield of glycosylated and non-glycosylated ALT-2 was 7.99 ± 1.12 and 9.18 ± 1.35 mg L−1, which were 25% and 35% higher than that in the E. coli system. Codon optimization may further enhance the expression. The P. pastoris-expressed glycosylated ALT-2 showed better immunoreactivity than the same expressed in E. coli with human clinical sera. Thus, P. pastoris can be used for inexpensive large-scale production of glycosylated or non-glycosylated BmALT-2 in its commercialization.
Acknowledgments
We are very much thankful to Centre for Biotechnology, Anna University, Chennai for providing instrumental facilities. We also acknowledge the hearty support from Dr K.B. Ramachandran, Indian Institute of Technology, Madras, for providing the electroporator. We give special thanks to Dr V. Rajagopal, Senior Entomologist, Zonal Entomological Research Laboratory, Vellore, Tamil Nadu, for his assistance in the sample collection. We thank Mr Vishal and Mr. Nazir of Centre for Biotechnology, Anna University, Chennai, for collecting the clinical blood samples. We also sincerely thank Dr Murray Selkirk, Professor of Biochemical Parasitology, Division of Cell and Molecular Biology, Imperial College, London, for kindly providing non-endemic sera samples.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- World Health Organization. Lymphatic filariasis fact sheet [Internet]. Geneva: World Health Organization; c2016 [cited 2016 Nov 16]. Available from: http://www.who.int/mediacentre/factsheets/fs102/en/
- Jayakody RL, De Silva CSS, Weerasinghe WMT. Treatment of bancroftian filariasis with albendazole: evaluation of efficacy and adverse reaction. Trop Biomed. 1993;10:19–24.
- Dreyer G, Addiss D, Noroes J, et al. Direct assessment of the adulticidal efficacy of repeat high-dose invermectin in bancrofitian filariasis. Trop Med Int Health. 1996;1:427–432.
- Noroes J, Dreyer G, Santos A, et al. Assessment of efficacy of diethylcarbamazine on adult Wuchereria bancrofti in vivo. Trans Roy Soc Trop Med. 1997;91:78–81.
- Molyneux DH, Taylor MJ. Current status and future prospects of the Global Lymphatic Filariasis Programme. Curr Opin Infect Dis. 2001;14(2):155–159.
- Gyapong JO, Kumaraswami V, Biswas G, et al. Treatment strategies underpinning the global programme to eliminate lymphatic filariasis. Expert Opin Pharmacother. 2005;6(2):179–200.
- Emilio P. Filariasis: diagnosis, treatment and prevention. Acta Biomed. 2008;79:106–109.
- Selkirk ME, Maizels RM, Yazdanbakhsh M. Immunity and the prospects for vaccination against filariasis. Immunobiol. 1992;184(2–3):263–281.
- Grieve RB, Wisnewski N, Frank GR, et al. Vaccine research and development for the prevention of filarial nematode infections. Pharm Biotechnol. 1995;6:737–768.
- Price VL, Kieny MP. Vaccines for parasitic diseases. Current Drug Targets Infect Disord. 2001;1(3):315–324.
- Vercruysse J, Schetters TPM, Knox DP, et al. Control of parasitic disease using vaccines: an answer to drug resistance? Rev Sci Tech Off Int Epiz. 2007;26:105–115.
- Gomez-Escobar N, Bennett C, Prieto-Lafuente L, et al. Heterologous expression of the filarial nematode Bmalt gene products reveals their potential to inhibit immune function. BMC Biol. [Internet]. 2005 [cited 2016 Jun 22];3:8. Available from: http://bmcbiol.biomedcentral.com/articles/10.1186/1741-7007-3-8
- Ben-Wen , Wang Z, Rush AC, et al. Transcription profiling reveals stage- and function-dependent expression patterns in the filarial nematode Brugia malayi. BMC Genomics. [Internet]. 2012 [cited 2016 Jun 22];13:184. Available from: http://bmcgenomics.biomedcentral.com/articles/10.1186/1471-2164-13-184
- Madhumathi J. Development of synthetic and recombinant multi-epitope peptide vaccines for human lymphatic filariasis [dissertation]. Chennai: Anna University; 2010. p. 163–165.
- Gregory WF, Atmadja AK, Allen JE, et al. The Abundant Larval Transcript-1 and -2 genes of Brugia malayi encode stage-specific candidate vaccine antigens for filariasis. Infect Immun. 2000;68:4174–4179.
- Murray J, Gregory WF, Gomez-Escobar N, et al. Expression and immune recognition of Brugia malayi VAL-1, a homologue of vespid venom allergens and Ancylostoma secreted proteins. Mol Biochem Parasitol. 2001;118(1):89–96.
- Anand SB, Kodumudi KN, Reddy MV, et al. A combination of two Brugia malayi filarial vaccine candidate antigens (BmALT-2 and BmVAH) enhances immune responses and protection in jirds. J Helminthol. 2011;4:1–11.
- Anand SB, Murugan V, Prabhu PR, et al. Comparison of immunogenicity, protective efficacy of single and cocktail DNA vaccine of Brugia malayi abundant larval transcript (BmALT-2) and thioredoxin peroxidase (TPX) in mice. Acta Tropica. 2008;107:106–112.
- Gnanasekar M, Rao KV, He YX, et al. Novel phage display-based subtractive screening to identify vaccine candidates of Brugia malayi. Infect Immun. 2004;72:4707–4715.
- Madhumathi J, Prince PR, Rao DN, et al. Dominant T-cell epitopes of filarial BmALT-2 and their cytokine profile in BALB/c mice. Parasite Immunol. 2010;32(11–12):760–763.
- Ramachandran S, Mishra PK, Reddy MVR, et al. The larval specific lymphatic filarial BMALT-2: induction of protection using protein or DNA vaccination. Microbiol Immunol. 2004;48:945–955.
- Thirugnanam S, Pandiaraja P, Ramaswamy K, et al. Brugia malayi: comparison of protective immune responses induced by Bmalt-2 DNA, recombinant BmALT-2 protein and prime-boost vaccine regimens in a jird model. Exp Parasitol. 2007;116(4):483–491.
- Ganapathy M, Perumal A, Mohan C, et al. Immunogenicity of Brugia malayi Abundant Larval Transcript-2, a potential filarial vaccine candidate expressed in tobacco. Plant Cell Rep. 2014;33(1):179–188.
- Buckholz FG, Gleeson MAG. Yeast systems for the commercial production of heterologous proteins. BioTechnol. 1991;9(11):1067–1072.
- Cregg JM, Cereghino JL, Shi J, et al. Recombinant protein expression in Pichia pastoris. Mol Biotechnol. 2000;16(1):23–52.
- Cereghino L, Sunga GP, Line AJ, et al. Expression of foreign genes in the yeast Pichia pastoris. Genet Engg. 2001;23:157–169.
- Hamilton SR, Davidson RC, Sethuraman N, et al. Humanization of yeast to produce complex terminally sialylated glycoproteins. Sci. 2006;313(5792):1441–1443.
- Thill GP, Davis GR, Stillman Cet at., Positive and negative effects of multicopy integrated expression vectors on protein expression in Pichia pastoris. In: Heslot H, Davies J, Florent Jet at., Proceedings of the 6th International Symposium on Genetics of Microorganisms, 2. Paris: Societe Francaise de Microbiologie; 1990. p. 477–490.
- Vassileva A, Chugh DA, Swaminathan S, et al. Effect of copy number on the expression levels of Hepatitis B surface antigen in the methylotrophic yeast Pichia pastoris. Protein Expr Purific. 2001;21(1):71–81.
- Shen Q, Wu M, Wang HB, et al. The effect of gene copy number and co-expression of chaperone on production of albumin fusion proteins in Pichia pastoris. Appl Microbiol Biotechnol. 2012;96(3):763–772.
- Canales M, Enríquez A, Ramos E, et al. Large-scale production in Pichia pastoris of the recombinant vaccine Gavac™ against cattle tick. Vaccine. 1997;15(4):414–422.
- Vu T, Thu H, Dinh T, et al. Cloning, high-level expression, purification, and properties of a novel endo-β;-1, 4-mannanase from Bacillus subtilis G1 in Pichia pastoris. J Microbiol Biotechnol. 2012;22(3):331–338.
- Cregg JM, Tschopp JF, Stillman C, et al. High-level expression and efficient assembly of hepatitis B surface antigen in the methylotrophic yeast Pichia pastoris. Biotechnol. 1987;5:479–485.
- Bo H, Minjian L, Guoqiang H, et al. Expression of Hepatitis B Virus S gene in Pichia pastoris and application of the product for detection of anti-HBs antibody. J Biochem Mol Biolog. 2005;38(6):683–689.
- EasySelectô Pichia expression kit: a manual of methods for expression of recombinant proteins using pPICZ and pPICZα in Pichia pastoris. Catalog no. K1740-01. Carlsbad (CA): Invitrogen Corporation.
- Lin-Cereghino J, Hashimoto M, Moy A, et al. Direct selection of Pichia pastoris expression strains using new G418 resistance vectors. Yeast. 2008;25(4):2934–299.
- Ingham DJ, Beer S, Money S, et al. Quantitative real-time PCR assay for determining transgene copy number in transformed plants. BioTechniques. 2001;31:132–140.
- Crowe J, Masone BS, Ribbe J. One-step purification of recombinant proteins with the 6xHis tag and Ni-NTA resin. Mol Biotechnol. 1995;4(3):247–258.
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72: 248–254.
- Pasamontes L, Denise E, Kurt B. Production of monoclonal and monospecific antibodies against non-capsid proteins of poliovirus. J Gen Viro. 1986;67:2415–2422.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nat. 1970;227(5259):680–685.
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Biotechnol. 1992;24:145–149.
- Fairbanks G, Steck TL, Wallach DFH. Electrophoretic analysis of the major polypeptides of the human erythrocyte membrane. Biochem. 1971;10:2606–2617.
- Steentoft C, Vakhrushev SY, Joshi HJ, et al. Precision mapping of the human O-GalNAc glycoproteome through simple cell technology. EMBO J. 2013;32(10):1478–1488.
- Tschopp JF, Sverlow G, Kosson R, et al. High level secretion of glycosylated invertase in the methylotrophic yeast Pichia pastoris. BioTechnol. 1987;5:1305–1308.
- Patrick MS, Fazenda ML, McNeil B, et al. Heterologous protein production using the Pichia pastoris expression system. Yeast. 2005;22:249–270.
- Mahalakshmi N, Aparnaa R, Kaliraj P. Evaluation of immune response elicited by inulin as an adjuvant with filarial antigens in mice model. Scandinavian J Immunol. 2014;80(4):261–270.
- Anugraha G, Madhumathi J, Prince PR, et al. Chimeric epitope vaccine from multistage antigens for lymphatic filariasis. Scandinavian J Immunol. 2015;82:380–389.
- Aparnaa R, Kaliraj P. Immunomodulation of ALT-2 and TLR may collude in antigen specific T cell hyporesponsiveness: proposed mechanism for elevated IL-10 levels in Balb/C mice. Acta Parasitol. 2014;59(1):25–30.
- Madhumathi J, Prince PR, Rao DNet al., Epitope mapping of Brugia malayi ALT-2 and the development of a multi-epitope vaccine for lymphatic filariasis. J Helminthol. 2016;19:1–12.