ABSTRACT
Green synthesis is an attractive and eco-friendly approach to generate potent antibacterial silver nanoparticles (Ag-NPs). Such particles have long been used to fight bacteria and represent a promising tool to overcome the emergence of antibiotic-resistant bacteria. In this study, green synthesis of Ag-NPs was attempted using plant extracts of Aloe vera, Portulaca oleracea and Cynodon dactylon. The identity and size of Ag-NPs was characterized by ultraviolet–visible spectrophotometer and scanning electron microscopy. Monodispersed Ag-NPs were produced with a range of different sizes based on the plant extract used. The bactericidal activity of Ag-NPs against a number of human pathogenic bacteria was determined using the disc diffusion method. The results showed that Gram positive bacteria were more susceptible than Gram negative ones to these antibacterial agents. The minimum inhibitory concentration was determined using the 96-well plate method. Finally, the mechanism by which Ag-NPs affect bacteria was investigated by SEM analysis. Bacteria treated with Ag-NPs were seen to undergo shrinkage and to lose their viability. This study provides evidence for a cheap and effective method for synthesizing potent bactericidal Ag-NPs and demonstrates their effectiveness against human pathogenic bacteria.
Introduction
The emergence of antibiotic resistant bacteria is strongly linked to an increase in the use of antibiotics over the last 70 or so years. Such antibiotic resistant bacteria represent a continuous threat to humans, especially since more than 60% of bacteria causing nosocomial infections are resistant to at least one of the antibiotics most commonly used in their treatment [Citation1]. Individuals infected with multidrug resistant (MDR) bacteria are not easily treated and as a result, remain hospitalized for long periods [Citation2]. As a result, attempts to find alternative approaches to antibiotics in order to avoid the further development of antibiotic resistance are an essential research aim. Silver, and its derivatives, have long been used as antimicrobial agents against bacteria, fungi and viruses [Citation3–5]. The nano-form of silver has emerged as an alternative approach to antibiotics which need to be further evaluated to determine their effectiveness and safety in vivo. Silver nanoparticles (Ag-NPs) can be synthesized by several chemical, physical and biological methods [Citation6–9]. However, biological methods prove to be particularly cost-effective, nontoxic and eco-friendly methods for generating Ag-NPs [Citation10,Citation11]. Numerous ecofriendly biological sources can be used for biosynthesis of Ag-NPs which have potent antimicrobial activities [Citation12–15]. Lately, Ag-NPs have been synthesized by various extracts of plants such as leaves of Rosmarinus officinalis [Citation16], cowpea seeds (Vigna sp. L) [Citation17], root extracts of Diospyros paniculata [Citation18] and Terminaliachebula leaf extract [Citation19] in addition to microorganisms such as Aspergillus terreus [Citation20] and some products like monosaccharides [Citation21]. Plant extracts are particularly effective due to their rich contents of bio-reducing agents which include: terpenoids, flavones, ketones, aldehydes, amides, carboxylic acids, proteins, DNA and enzymes – all of which mediate the reduction process and Ag-NPs precipitation [Citation22]. The bactericidal activities of Ag-NPs against a wide array of MDR pathogenic bacteria, including Gram positive and Gram negative species, have been investigated in several studies which have shown that Ag-NPs are effective against Pseudomonas aeruginosa, ampicillin-resistant Escherichia coli, erythromycin-resistant Strepococcus pyogenes, methicillin-resistant Staphylococcus aureus (MRSA) and vancomycin-resistant Staphylococcus aureus (VRSA) [Citation23–28]. Ag-NPs are particularly useful because they can be effectively incorporated into dressings used to treat wounds and burns [Citation29–32]. In the present study, the biosynthesis of Ag-NPs was achieved by the reduction of silver nitrate using plant extracts as bio-reducing agents. Three plants were selected for the reduction process on the basis of their availability and ease of extraction. Synthesized Ag-NPs were analysed by ultraviolet (UV)–visible spectrophotometer and scanning electron microscopy (SEM). In addition, the bactericidal activity of Ag-NPs was tested against nine human pathogenic bacteria for determination of the minimum inhibitory concentration (MIC).
Materials and methods
Materials
Plants, chemicals and media: Three plants, Aloe vera, Portulaca oleracea and Cynodon dactylon; silver nitrate (Sigma–Aldrich, Munich, Germany), Muller-Hinton agar (MHA) (Sigma–Aldrich) and Nutrient broth (Sigma–Aldrich) were used. Bacterial strains: nine human pathogenic bacteria (obtained from the Botany and Microbiology Department, College of Science, King Saud University) were used, namely the Gram positive bacteria: Bacillus subtilis, Bacillus cereus, Staphylococcus aureus and Enterococcus faecalis, and Gram negative Salmonella typhi, Shigella sp., Escherichia coli, Pseudomonas aeruginosa and Acinetobacter baumannii.
Biosynthesis and characterization of silver nanoparticles
Broths from leaves of the three plants were prepared (A. vera, P. oleracea and C. dactylon). Fifty grams of leaves from each plant were rinsed several times with distilled water and cut into small pieces. The leaves were then boiled in three Erlenmeyer flasks (one for each plant) containing 200 mL of sterile, distilled water for 10 minutes. The extracts were allowed to cool and were then transferred to 50 mL falcon tubes and centrifuged at 15,000 rpm for 15 minutes (Universal 320R, Hettich, Germany). Supernatants containing the bio-reducing agents were then filtered using syringe filters into new sterile 50 mL falcon tubes. Plant extract (15 mL) was next added to an aqueous solution of silver nitrate (1 mmol/L). Upon mixing, a change in colour indicated the formation of monodispersed Ag-NPs. A further two flasks were used as controls where one flask contained only plant extract with water and the second contained only a Ag-solution. Biosynthesized Ag-NPs were separated from the solution by centrifugation (Universal 320R) at 15,000 rpm for 15 minutes, washed several times and then kept in the refrigerator for further studies. The biosynthesis process was performed for each plant giving three types of Ag-NPs. They were designated as A, B and C for, respectively, A. vera, P. oleracea and C. dactylon. The identity of Ag-NPs was characterized by UV–visible spectrophotometer (PerkinElmer LAMBDA, Waltham, USA). Spectra of surface plasmon resonance were obtained at different time intervals. The morphology and size of Ag-NPs were determined by SEM.
Bactericidal activities of Ag-nanoparticles
Bacterial strains were passaged three times in nutrient broth medium to restore their optimal physiological activities. In vitro bactericidal activity was assayed using MHA. Bacterial strains were tested against the three types of biosynthesized Ag-NPs (A, B and C). A range of concentrations of Ag-NPs (A, B and C) were tested, i.e. (50 µg/mL, 5 µg/mL and 0.5 µg/mL). Paper discs were soaked in each concentration. Each of the bacterial strain cell susupensions (100 µL) was inoculated onto the medium and spread (using a glass rod) across the medium and allowed to dry for 5 min. Ag-NPs-loaded discs were then placed on the surface of the medium and the compound was allowed to diffuse for 5 minutes. The plates were then incubated at 37 °C for 24 h. At the end of incubation, any inhibition zones which formed around the disc were measured. Disks soaked in sterilized water were used as controls. The diameter of any control zones was subtracted from the test zones and the resulting zone diameter and the result obtained was tabulated. Finally, discs loaded with streptomycin (15 μg /disc) were used as positive control. The experiment was performed throughout in triplicate.
Determination of the MIC
The MIC was determined using the 96-well plate method; three 96-well plates being used, one for each Ag-NPs (A, B and C). The first nine columns were assigned for each bacterial strain and were inoculated with 125 µL of bacterial culture. Then, 125 µL of pre-weighted Ag-NPs of each type were inoculated in the first row (A) and then a two-fold serial dilution was performed. Only the row H was left as negative control (without Ag-NPs). The last three columns, 10, 11 and 12, were left as blank. The plates were incubated at 37 °C for 24 h. Plates were read by Microplate reader (Mithras LB 940, Berthold Technologies, Bad Wildbad, Germany) at 690 nm wavelength and MICs were recorded as the lowest concentration of Ag-nanoparticles showing no visible growth in the broth. Samples from wells used in broth microdilution assay that did not exhibit any visible growth after the 24 h incubation were subcultured onto MHA plates.
Scanning electron microscopy
SEM analysis was used to analyse the bactericidal mechanism of these nanoparticles. Ag-NPs was inoculated into a growing culture of S. aureus and incubated overnight. Non-treated culture was used as control. Samples from both cultures were then processed and examined by SEM (JSM-6380 LA, Tokyo, Japan).
Results and discussion
Green synthesis and characterization of Ag-NPs
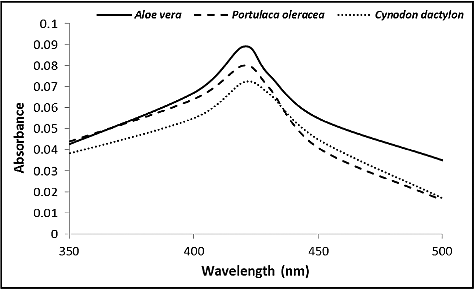
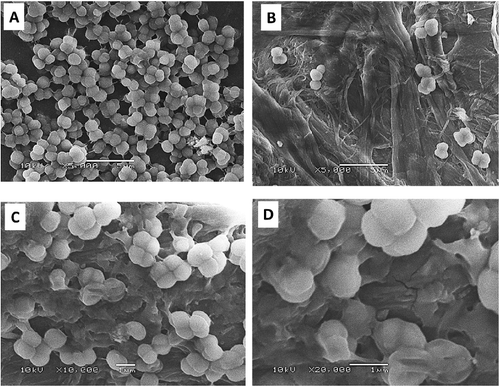
Ag-NPs were formed immediately by mixing silver nitrate solution and leaf-broth as indicated by an observed colour change, with solution colour becoming completely dark after 2 h. The identity of Ag-NPs was confirmed from absorption spectra recorded by UV–visible spectrophotometer (). The various types of Ag-NPs, A. vera (A), P. oleracea (B) and C. dactylon (C), had different peak values of 405, 415 and 420 nm, respectively. Stable and monodispersed Ag-NPs were recovered and monitored by SEM (). The resultant SEM images showed that the nanoparticles were spherical shaped and from 10 to 30 nm for type A, 15–40 nm for type B and 25–60 nm for type C.
Figure 2. SEM images showing well-dispersed silver nanoparticles formed by extracts of plants: A. vera (A), P. oleracea (B) and C. dactylon (C).
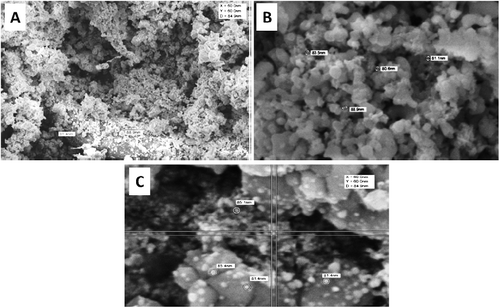
Ag-NPs have recently received much attention due to their bactericidal activities against a broad spectrum of pathogenic bacteria. A number of physical, chemical and biological methods have been described in order to synthesize nanoparticles of silver. Of these, biological, or green, synthesis is the most environmentally benign and cost effective approach [Citation33–36]. Numerous biological materials from different sources can be used to synthesize Ag-NPs, and bacterial, fungal and plant extracts have been employed for Ag-NPs biosynthesis [Citation37,Citation38]. Several papers report the use of different plant extracts such as Emblica officinalis [Citation35], Parthenium [Citation39], Aloe vera [Citation40], Pisonia grandis [Citation41], Jatropha curcas [Citation8] and Justicia genderussa [Citation42].
In this study, extracts from three plants, A. vera, P. oleracea and C. dactylon were used to reduce silver ions into Ag-NPs (). The morphology and resonance properties of Ag-NPs were examined by SEM and UV–visible spectrophotometer, respectively. The size, distribution and stability of Ag-NPs were seen to vary with the plant extract, or the organism used for the biosynthesis process. Surface plasmon resonance of Ag-NPs reported in previous studies displayed different peak values: E. coli (400 nm) [Citation43], Aspergillus niger (420 nm) [Citation44], Pisonia grandis (420 nm) [Citation41], Merrimia tridendata (440 nm) [Citation45], Kappaphycus alvarezii (420 nm) [Citation46], Citrullus colocynthis (440 nm) [Citation47] and Allium cepa (412 nm) [Citation48]. The Ag-NPs obtained in this study were uniform spherical and varied according to the plant extract. Other investigators have synthesized different sizes of Ag-NPs: 30–40 nm, for example, by Boswellia ovalifoliolata [Citation49]; 30–50 nm by Merremia tridendata [Citation45], Carcia papaya [Citation50] and Emblica officinalis [Citation35].
Antimicrobial activities of Ag-NPs
In order to evaluate the bactericidal activities of Ag-NPs, the disc diffusion method was used here. The inhibition zones around Ag-NPs-loaded disks were measured and recorded. Biosynthesized Ag-NPs (A, B and C) were found to have potent bactericidal activities against both Gram positive and Gram negative bacteria. However, the bactericidal potential was more effective against Gram positive bacteria than against Gram negative ones (). Ag-NPs type A formed by the extract from A. vera was more effective than extracts B and C. For the three types of Ag-NPs, the highest effect was observed at a concentration of 50 µg/mL. The diameter of the inhibition zone was smaller at a concentration of 5 µg/mL and considerably smaller at 0.5 µg/mL.
Table 1. Antibacterial activities of Ag-NPs biosynthesized by extracts from A. vera (A), P. oleracea (B) and C. dactylon (C).
Ag-NPs exhibited antibacterial activity against B. subtilis, B. cereus, S. aureus, Enterococcus faecalis, S. typhi, Shigella sp., E. coli, P. aeruginosa and A. baumannii. Similar antibacterial activity of Ag-NPs has been reported against E. coli and P. aeruginosa; B. cereus and P. aeruginosa [Citation51]; P. aeruginosa, E. coli, Streptococcus pyrogens and Samonella enteritis [Citation48]; Proteus vulgaris, Vibrio [Citation52]; S. aureus, B. subtilis, E. coli and Klebsiella pneumoniae [Citation53]; Samonella typhi and E. coli [Citation54]; Proteus morgani and S. aureus [Citation55].
MIC
shows the MIC values of different Ag-NPs as measured by Microplate reader at 690 nm wavelength. Higher concentrations of Ag-nanoparticles (A, B and C) were found to completely inhibit bacterial growth. The effect gradually decreased with decreasing concentrations. The average MIC value of Ag-NPs from A. vera against Gram positive bacteria was 2.1 mg/L and 3.2 mg/L against Gram negative bacteria. The average MIC values for Ag-NPs of P. oleracea were 3 mg/L for Gram-positive bacteria and 4.27 mg/L for Gram negative ones. Finally, for Ag-NPs of C. dactylon, the average MIC values were 4.57 mg/L and 5.82 mg/L for Gram positive and Gram negative bacteria, respectively.
Table 2. MIC values of different types of Ag-NPs biosynthesized by extracts from A. vera (A), P. oleracea (B) and C. dactylon (C).
Although the bactericidal activities of Ag-NPs were seen in this study to vary among bacterial species, the effect was greater against Gram positive than against Gram negative bacteria (). This is due to the fact that the cell walls of Gram positive bacteria bind larger quantities of metals than do Gram negative bacteria [Citation56]. Another factor that plays a major bactericidal role is the size of Ag-NPs, where small size Ag-NPs are more effective than larger ones (). To investigate the relation between the size of Ag-NPs and their bactericidal activities, the MIC values for each type (A, B and C) were measured using the micro-dilution method. Small Ag-NPs (type A) showed more pronounced bactericidal activities than types B and C. These results are similar to those reported by other investigators [Citation57–59].
Mechanism of Ag-NPs action
The mechanism of Ag-NPs action was also investigated in the present study. Ag-NPs-treated S. aureus brought about cell-shrinkage, indicating permeability and a loss of viability (). These findings support the observation that Ag-NPs adhere to, and then form pits on bacterial cell walls. The exact mechanisms by which Ag-NPs exert their bactericidal action are not known, although a number of theories have been suggested. Gogoi et al. [Citation60], for example, proposed that Ag-NPs have no direct effect on cellular DNA and proteins [Citation60]; adherence of Ag-NPs to bacterial cell wall results in the formation of pits which, in turn, lead to permeability loss and cell death [Citation59]. Free radicals generation and the release of silver ions by Ag-NPs are also possible mechanisms that may lead to cell death [Citation61,Citation62].
Conclusions
Green synthesis of Ag-NPs was attempted using extracts from A. vera, P. oleracea and C. dactylon. Characterization of Ag-NPs by SEM and UV–visible spectrophotometer revealed spherical nanoparticles exhibiting a range of different sizes. Biosynthesized Ag-NPs exhibited bactericidal activities against both Gram positive and Gram negative bacteria used in this study. The relation between Ag-NPs size and bactericidal activity was elucidated by measuring MIC values of different Ag-NPs. Small-sized Ag-NPs synthesized by A. vera were more effective than those synthesized by P. oleracea and C. dactylon. We also investigated the mechanism of Ag-NPs and observed that treated bacteria undergo cell-shrinkage, thereby indicating loss of permeability and viability. Finally, the results of this study suggest an eco-friendly, cost-effective and rapid method for the generation of bactericidal Ag-NPs.
Acknowledgements
The authors extend their appreciation to the Deanship of Scientific Research at the King Saud University for funding the work through the research group project No. RGP-VPP-332.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Okeke IN, Abudu AB, Lamikanra A. Microbiological investigation of an outbreak of acute gastroenteritis in Niger State, Nigeria. Clin Microbiol Infect. 2001;7:514–516.
- Webb GF, D'Agata EM, Magal P, et al. A model of 499 antibiotic-resistant bacterial epidemics in hospitals. Proc Nat Acad Sci. 2005;102:13343–13348.
- Lok CN, Ho CM, Chen R, et al. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res. 2006;5:916–924.
- Cho KH, Park JE, Osaka T, et al. The study of antimicrobial activity and preservative effects of nanosilver ingredient. Electrochim Acta. 2005;51:956–960.
- Silver S. Bacterial silver resistance: molecular biology and uses and misuses of silver compounds. FEMS Microbiol Rev. 2003;27:341–353.
- Goia DV, Matijević E. Preparation of monodispersed metal particles. New J Chem. 1998;22:1203–1215.
- Taleb A, Petit C, Pileni MP. Synthesis of highly monodisperse silver nanoparticles from AOT reverse micelles: a way to 2D and 3D self-organization. Chem Mater. 1997;9:950–959.
- Bar H, Bhui DK, Sahoo, P, et al. Green synthesis of silver nanoparticles using latex of Jatropha curcas. Colloids Surf A Physicochem Eng Aspects. 2009;339:134–139.
- Rodriguez-Sanchez L, Blanco MC, Lopez-Quintela MA. Electrochemical synthesis of silver nanoparticles. J Phys Chem B. 2000;104:9683–9688.
- Gericke M, Pinches A. Biological synthesis of metal nanoparticles. Hydrometallurgy. 2006;83:132–140.
- Harris AT, Bali R. On the formation and extent of uptake of silver nanoparticles by live plants. J Nanopart Res. 2008;10:691–695.
- Parashar UK, Saxena SP, Srivastava A. Bioinspired synthesis of silver nanoparticles. Digest J Nanomater Biostruct. 2009;4(1):159–166.
- Saifuddin N, Wong CW, Yasimura AN. Rapid biosynthesis of silver nanoparticles using culture supernatant of bacterial with microwave irradiation. E J Chem. 2009;6(1):61–70.
- Bhainsa KC, D'Souza SF. Extracellular biosynthesis of silver nanoparticles using the fungusAspergillus fumigatus. Colloids Surf B Biointerfaces. 2006;47:160–164.
- Willner B, Basnar B, Willner B. Nanoparticle-enzyme hybrid systems for nanobiotechnology. FEBS J. 2007;274:302–309.
- Ghaedi M, Yousefinejad M, Safarpoor M, et al. Rosmarinus officinalis leaf extract mediated green synthesis of silver nanoparticles and investigation of its antimicrobial properties. J Industr Eng Chem. 2015;31:167–172.
- Mohammadi S, Pourseyedi S, Amini A. Green synthesis of silver nanoparticles with a long lasting stability using colloidal solution of cowpea seeds (Vigna sp. L). J Environ Chem Engin. 2016;4:2023–2032.
- Hanumanta Rao N, Lakshmidevi N, Pammi SN, et al. Green synthesis of silver nanoparticles using methanolic root extracts of Diospyros paniculata and their antimicrobial activities. Mater Sci Eng C. 2016;62:553–557.
- Chandra SE, Krishna Rao KV, Madhusudana Rao K. Bio-synthesis and characterization of silver nanoparticles using Terminalia chebula leaf extract and evaluation of its antimicrobial potential. Mater Let. 2016;174:129–133.
- Sayed SRM, Bahkali AH, Marwa MB, et al. Antibacterial activity of biogenic silver nanoparticles produced by Aspergillus terreus. Int J Pharmacol. 2015;11:858–863.
- Colin P, Zheng D, Muhi MZ, et al. Silver nanoparticle synthesis using monosaccharides and their growth inhibitory activity against Gram-negative and positive bacteria. ISRN Nanotechnol. 2014; 2014:480284.
- Jha AK, Prasad K, Prasad K, et al. Plant system: nature's nanofactory. Colloids Surf B Biointerfaces. 2009;73:219–223.
- Baker C, Pradhan A, Pakstis L, et al. Synthesis and antibacterial properties of silver nanoparticles. J Nanosci Nanotechnol. 2005;5:244–249.
- Gemmell CG, Edwards DI, Frainse AP. Guidelines for the prophylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the UK. J Antimicrob Chemother. 2006;57:589–608.
- Morones JR, Elechiguerra JL, Camacho A, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346–2353.
- Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2012;27:76–83.
- Birla SS, Tiwari VV, Gade AK, et al. Fabrication of silver nanoparticles by Phoma glomerata and its combined effect against Escherichia coli, Pseudomonas aeruginosa and Staphylococcus aureus. Lett Appl Microbiol. 2009;48:173–179.
- Gade A, Gaikwad S, Tiwari V, et al. Biofabrication of silver nanoparticles by Opuntia ficus-indica: in vitro antibacterial activity and study of the mechanism involved in the synthesis. Curr Nanosci. 2010;6:370–375.
- Jia J, Duan YY, Wang S H, et al. Preparation and characterization of antibacterial silver-containing nanofibers for wound dressing applications. J US China Med Sci. 2007;4:52–54.
- Kirsner R, Orsted H, Wright B. Matrix metalloproteinases in normal and impaired wound healing: a potential role of nanocrystalline silver. Wounds. 2001;13:5–10.
- Tian J, Wong KK, Ho CM, et al. Tropical delivery of silver nanoparticles promotes wound healing. Chem Med Chem. 2007;2:129–136.
- Shin SH, Ye MK, Kim HS, et al. The effects of nano-silver on the proliferation and cytokine expression by peripheral blood mononuclear cells. Int Immunopharmacol. 2007;7:1813–1818.
- Raja SK, Ganesh S. Evaluation of anti-bacterial activity of silver nanoparticles synthesized from Candida glabrata and Fusarium oxysporum. Inter J Medicobiol Res. 2011;1(3):130–136.
- Shankar SS, Rai A, Ankamwar B, et al. Biological synthesis of triangular gold nanoprisms. Nature Mater. 2004;3:482–488.
- Ankamwar B, Chaudhary M, Sastry M. Gold nanotriangles biologically synthesized using tamarind leaf extract and potential application in vapor sensing. Synth Reaction Inorg Metal Org Nanotech. 2005;35:19–26.
- Huang J, Li Q, Sun D, et al. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology. 2007;18:105104–105115.
- Mohanpuria P, Rana KN, Yadav SK. Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart. Res. 2008;10:507–517.
- Anil Kumar S, Majid KA, Gosavi SW, et al. Nitrate reductase mediated synthesis of silver nanoparticles from AgNO3. Biotechnol Lett. 2007;29:439–445.
- Vyom P, Rashmi P, Bechan S, et al. Parthenium leaf extract mediated synthesis of silver nanoparticles a novel approach towards weed utilization. Digest J Nanomater Nanostruct. 2009;4:45–53.
- Chandran SP, Chaudhary M, Pasricha R, et al. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog. 2006;22(2):577–583.
- Firdhouse MJ, Lalitha P, Sripathi SK. Novel synthesis of silver nanoparticles using leaf ethanol extract of Pisonia grandis (R. Br). Der Pharma Chemica. 2012;4(6):2320–2326.
- Chinna M, Hema P. Green synthesis of highly stable silver nanoparticles using Justicia gendenussa. Int J Nanotech Appl. 2012;1;39–57.
- Natarajan K, Selvaraj S, Murty RV. Microbial production of silver nanoparticles. Digest J Nanomater Biostruct. 2010;1:135–140.
- Jaidev LR, Narasimha G. Fungal mediated biosynthesis of silver nanoparticles, characterization and antimicrobial activity. Colloids Surface B Biointerfaces. 2010;81:430–433.
- Ganesan V, Astalakshmi A, Nima P, et al. Synthesis and characterization of silver nanoparticles using Merremia tridentata (L.) Hall.f. Int J Curre Sci. 2013;6:87–93.
- Ganesan V, Aruna Devi J, Astalakshmi A, et al. Eco-friendly synthesis of silver nanoparticles using a sea weed, Kappaphycus alavarezii. Int J Engin Advanced Res. 2013;2:559–563.
- Satyavani K, Gurudeeban S, Ramanathan T, et al. Biomedical potential of silver nanoparticles synthesized from calli cells of Citrullus colocynthis (L.) Schrad. J Nanobiotechnol [ Internet]. 2011 [ cited 2016 Nov 24];9:43. Available from: https://jnanobiotechnology.biomedcentral.com/articles/10.1186/1477-3155-9-43
- Antariksh S, Tripathi RM, Singh RP. Biological synthesis of silver nanoparticles using onion (Allium cepa) extract and their antibacterial activity. Digest J Nanomater Biostruct. 2010;5:427–432.
- Savithramma N, Rao ML, Suvarnalatha Devi P. Evaluation of antibacterial efficacy of biologically synthesized silver nanoparticles using stem barks of Boswellia ovalifoliolata Bal. and Henry and Shorea tumbuggaia Ruxb. J Biol Sci. 2011;11:39–45.
- Jain D, Daima HK, Kachnwaha S, et al. Synthesis of plant mediated silver nanoparticles using papaya fruit extract and evaluation of their antimicrobial activities. Digest J Nanomater Biostr. 2009;4:557–563.
- Elumalai EK, Prasad TN, Hemachandran J, et al. Extracellular synthesis of silver nanoparticles using leaves of Euphorbia hirta and their antibacterial activities. J Pharm Sci Res. 2010;2:549–554.
- Prabhu N, Divya TR, Yamuna G. Synthesis of silver nanoparticles and their antibacterial efficacy. Digest J Nanomater Biostruct. 2010;5:185–189.
- Pasupuleti VR, Prasad TN, Shiekh RA, et al. Biogenic silver nanoparticles using Rhinacanthus nasutus leaf extract: synthesis, spectral analysis and antimicrobial studies. Int J Nanomedicine. 2013;8:3355–3364.
- Lalitha A, Subbaiya R, Ponmurugan P. Green synthesis of silver nanoparticles from leaf extract of Azadiractaindica and to study its antibacterial and antioxidant property. Int J Cur Microbiol Appl Sci. 2013;2:228–235.
- Nalwade AR, Badhe MN, Pawale CB, et al. Rapid biosynthesis of silver nanoparticles using fern leaflet extract and evaluation of their antibacterial activity. Int J Biol Technol. 2013;4(2):12–18.
- Beveridge TJ, Fyfe WS. Metal fixation by bacterial cell walls. Can J Earth Sci. 1985; 22:1893–1898.
- Khaydarov RA, Khaydarov RR, Gapurova O, et al. Electrochemical method for the synthesis of silver nanoparticles. J Nanopart Res. 2009;11:1193–1200.
- Sarkar S, Jana AD, Samanta SK, et al. Facile synthesis of silver nano particles with highly efficient anti-microbial property. Polyhedron. 2007;26:4419–4426.
- Sondi I, Salopek-Sondi B. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 2004;275:177–182.
- Gogoi SK, Gopinath P, Paul A, et al. Green fluorescent protein-expressing Escherichia coli as a model system for investigating the antimicrobial activities of silver nanoparticles. Langmuir. 2006;22:9322–9328.
- Danilcauk M, Lund A, Saldo J, et al. Conduction electron spin resonance of small silver particles. Spectrochimaca Acta Part A. 2006;63:189–191.
- Kim JS, Kuk E, Yu K, et al. Antimicrobial effects of silver nanoparticles. Nanomedicine. 2007;3:95–101.