ABSTRACT
In this study, a watermelon (Citrullus lanatus) zeaxanthin epoxidase gene, ClZE, was isolated by reverse transcription-polymerase chain reaction (PCR) together with RNA ligase-mediated rapid amplification of cDNA ends (RLM-RACE). The full cDNA sequence of ClZE is 2535 bp in length containing a 1998 bp open reading frame (ORF) that encodes 665 amino acids. ClZE was shown to share high homology with the putative ZE genes in other plant species. Prediction analysis revealed that ClZE bears two large conservative domains, including Pyr_redox and FHA (forkhead-associated domain). Phylogenetic analysis suggested that ClZE shares high similarity (94.8%) with CsZE from Cucumis sativus, but is far from the ZE genes of other species. Prokaryotic expression indicated that ClZE possessed the apparent molecular mass consistent with its calculated molecular mass of 73.1 KDa. Based on quantitative real-time PCR (qRT-PCR), ClZE was shown to be down-regulated under chilling–low irradiance stress in watermelon leaves. Transgenic Arabidopsis lines harbouring ClZE were generated to test the gene function in mediating plant response to chilling stress. The results indicated that the transgenic lines exhibited decreased non-photochemical quenching (NPQ) and maximum quantum efficiency of photosystem II (PSII) photochemistry (Fv/Fm), increased activities of peroxidase (POD), superoxide dismutase (SOD) and elevated content of malondialdehyde (MDA) relative to the wild type (WT) under chilling–low irradiance stress. In addition, overexpression of ClZE resulted in impaired xanthophyll cycle and aggravated PSII photoinhibition under chilling–low irradiance. All the results together suggested that ClZE plays major roles in regulating the photoinhibition behaviour.
Introduction
In natural conditions, plants absorb light and convert it into chemical energy through photosynthesis. However, excessive absorption of light can be deleterious to plants when its amount exceeds the capacity for conversion into photochemical energy. Such energy imbalance is exacerbated under chilling conditions due to the primarily limited photosynthetic energy utilization, which is restricted by the low temperature-imposed limitations on enzymes involved in carbon metabolism. Thus, much more excessive energy is converted into excited energy and reactive oxygen species (ROS) [Citation1] causing inhibition of the repair mechanism for photosystem II (PSII) [Citation2]. Thus, ROS are an extremely harmful stress factor for photosynthetic organisms [Citation3]. During chilling stress, ROS are mainly formed in chloroplasts, resulting in cellular oxidative damage [Citation4] and inhibiting the repair of PSII by suppressing D1 protein synthesis, which acts as the primary target for photodamage of the PSII complex when plants suffer from photoinhibition [Citation5,Citation6]. In addition, ROS have also been proved to induce degradation of the D1 peptide bonds [Citation7]. A range of protective mechanisms have evolved to reduce or prevent the excess light damage to photosynthetic tissues [Citation8]. Among these, thermal dissipation of excess light energy, which is measured as non-photochemical quenching (NPQ), exerts important photoprotective functions during plant photosynthesis [Citation9]. Research has shown that when the high intensity is the only stress factor, the light energy absorbed by plants is consumed by thermal dissipation (about 75%). It is well known that thermal dissipation mainly depends on the xanthophyll cycle, which involves two enzymes, violaxanthin de-epoxidase (VDE) and ZE. VDE catalyses the conversion of violaxanthin (V) to zeaxanthin (Z) via anthraxanthin (A) under excess light conditions, whereas ZE catalyses the reverse reaction under the low light conditions or in darkness. These two enzymes are both categorized into the lipocalin superfamily. Thus, it has been demonstrated that the lipocalin superfamily is involved in the photoinhibition response.
Several mutants affecting the function of the xanthophyll cycles have been characterized in Arabidopsis. In comparison with wild-type (WT) plants, NPQ is reduced in the npq1 mutant (abolishment of VDE and failure to form Z upon illumination) and increased in the npq2 mutant (affected in ZE and accumulation of high levels of Z under all conditions) [Citation10]. In overwintering evergreen plants, high levels of Z can be permanently retained during winter along with the sustained down-regulation of PSII activity [Citation11]. It has further been shown in many studies that Z is an intermediate in the biosynthetic pathway of abscisic acid (ABA), an important plant hormone associated with stress response and dormancy regulation [Citation12]. Full-length cDNA of the VDE gene has been cloned in Arabidopsis, spinach, wheat, tomato and other plants [Citation3,Citation13]. But the ZE genes have only been cloned in a few plants [Citation14,Citation15] and there are few reports on the purification of the translated proteins. That is why there is still just limited research on the above gene functions in mediating pigment biosynthesis and energy interconversion and further characterization of the photoinhibition response mechanism is necessary.
Watermelon (Citrullus lanatus), a typical chilling-sensitive plant species, is an important economic crop and is often named the king of summer fruits. Low temperature and weak light are the main limiting factors in the production of greenhouse-grown watermelon in early spring or late autumn in North China.
The aim of this study was to characterize a new ClZE gene of watermelon and further demonstrate its function under low temperature and weak light conditions. To achieve this goal, we inserted ClZE into the Arabidopsis genome and studied the NPQ and the maximum efficiency of PSII (Fv/Fm), as well as the peroxidase (POD) and superoxide dismutase (SOD) activities and the malondialdehyde (MDA) content in overexpression lines and WT plants.
Materials and methods
Plant material and growth conditions
A wild variety of watermelon with tolerance to chilling–low irradiance was used in this study. Seeds of the watermelon variety (Agricultural University of Hebei, North China Key Laboratory for Crop Germplasm Resources of Education Ministry, Baoding, China) were first soaked in sterile water for 10 min at 55 °C, followed by 6–7 h of treatment at 30 °C. The seeds were then sown in vermiculite and incubated at 25 °C and light intensity of 500 μmol·m−2·s−1 under long-day conditions (14 h light /8 h dark).
Isolation of the cDNA of ClZE and sequence analysis
Total RNA was isolated from fresh leaves of three-leaf-stage watermelon plants, using Trizol reagent (Invitrogen, Shanghai, China). The first-strand cDNA synthesis from total RNA and RT (reverse transcription)-polymerase chain reaction (PCR) for the target gene were performed using a One Step RNA PCR kit (Takara, Dalian, China) with a pair of primers: ZE-F (5ˊ-TGGACTCGGCCCACTTTC-3ˊ) and ZE-R (5ˊ-ACCGTCGTCCTTCGTGAT-3ˊ), which were designed using Primer 5.0 based on the chloroplast ZE genes from tomato, Arabidopsis and muskmelon. The amplified product was cloned into the pMD18-T vector using a TA cloning kit (Takara, Dalian, China) and was subjected to sequencing(Sangon Biotech, Shanghai, China). It was aligned by BLAST (Basic Local Alignment Search Tool) analysis in GenBank. The 5ˊ- and 3ˊ-end regions were obtained using RNA ligase-mediated rapid amplification for 5ˊ and 3ˊ cDNA ends (RLM-RACE) (Invitrogen, Shanghai, China). The products of the amplified fragment were sequenced and assembled to generate a full-length ClZE cDNA. The molecular mass and isoelectric point were analysed by Protparam software (http://expasy.org/tool/protparam.html). The hydrophobic distribution curve of ClZE amino acids was assessed by proScal (http://web.expasy.org/protscale/). The transmembrane domain of the deduced ClZE protein was detected by using TMHMM (http://www.cbs.dtu.dk/services/TMHMM-2.0). The deduced ClZE protein was subjected to analyses of homology and phylogenesis by CLUSTAL X and MEGA 3.1.
Prokaryotic expression analysis
The ClZE gene was cloned into the vector pET-32a, transferred and expressed in Escherichia coli BL21(DE3)pLysS (Tiangen Biotech, Beijing, China) according to the conventional approach, in which the empty vector was used as the control. They were grown on LB medium at 28 °C and isopropyl β-D-1-thiogalactopyranoside (IPTG; 1 mmol L−1) was acted as the inductor. The expressed products were separated by electrophoresis in a 10% sodium dodecyl sulfate-polyacrylamide gel (SDS-PAGE) [Citation16].
Expression analysis of the ClZE gene
Roots, stems and leaves were harvested from chilling–low-irradiance-treated and control plants at different time points (0, 1, 3, 6, 9 and 12 h), then frozen in liquid nitrogen and stored at −80 °C until the isolation of RNA. Total RNA in the tissues was extracted using RNAprep pure Plant Kit (Tiangen Biotech, Beijing, China), according to the manufacturer's protocol. Then, 1 μg of total RNA was used to synthsize the first-strand cDNA using FastQuant RT Kit (with gDNase, Tiangen Biotech, Beijing, China) in a 20 μL reaction mixture volume. The gene-specific primers for PCR amplification of the ClZE transcript were RT-F: 5ˊ-GAGAAAGTGACCCAAGTGAAAGCC-3ˊ and RT-R: 5'-CCAGCCACAAGTATCCGAACAT-3', which were designed using Primer 5.0. The quantitative real-time PCR (qRT-PCR) analysis for ClZE was performed by using SuperReal PreMix Plus (SYBR Green; Tiangen Biotech, Beijing, China) in a 20-μL reaction volume containing 10 μL of SuperReal PreMix Plus (2×), 0.6 μL of PCR forward primer (10 μmol L−1), 0.6 μL of PCR reverse primer (10 μmol L−1), 1 μL of template cDNA and 7.8 μL of double distilled water (ddH2O). Reactions were carried out under the following conditions: 95 °C/15 s; 57 °C/20 s, 72 °C/20 s (40 cycles) in a Mastercycler® ep realplex (Eppendorf, Hamburg, Germany). 18s rRNA was used as a reference gene for the normalization of ClZE expression in watermelon tissues, using a forward primer 18s rRNA-F: 5'-AGTCGGGGGCATTCGTATTT-3', and a reverse primer 18s rRNA-R: 5'-CCCTGGTCGGCATCGTTTAT-3' [Citation17]. Because the expression level was relatively higher in leaves, this tissue was used as material in the following qRT-PCR analysis.
Construction of transformation vector and transformation of Arabidopsis
The ClZE gene was cloned into the binary vector pBI121 under the control of the cauliflower mosaic virus CaMV35S promoter to generate pBI121-ClZE. The recombinant plasmid was transferred into Agrobacterium GV3101 cells by using the freeze-thawing method.
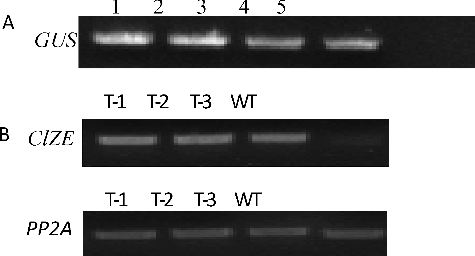
After surface-sterilization and cold treatment at 4 °C for 3 days, Arabidopsis thaliana seeds (Agricultural University of Hebei, North China Key Laboratory for Crop Germplasm Resources of Education Ministry, Baoding, China) were grown on vermiculite under a 16 h light/8 h dark cycle at 21 °C in a plant growth chamber at 500 μmol·m−2·s−1. ClZE gene was transferred into WT Arabidopsis as described by Zhong and Gallie [Citation18]. The seeds were collected and screened on 0.5 MS (Murashige and Skoog) medium containing 50 mg mL−1 kanamycin. Genomic DNA from the transgenic Arabidopsis plants was extracted by the CTAB (cetyl trimethylammonium bromide) method. The integration of ClZE into the genome of transgenic plants was detected by amplifying the β-glucanase gene (GUS) in pBi121 in a 20-μL reaction volume containing 2.0 μL of 10×buffer, 1.6 μL of deoxynucleoside triphosphates (dNTP) mixture (2.5 mmol L−1), 0.2 μL of Taq DNA polymerase (5 U μL−1), 1.0 μL F (10 μmol L−1), R (10 μmol L−1) 1.0 μL, DNA 1.0 μL and ddH2O 13.2 μL. Reactions were carried out in a T Professional Thermal Cycler (Biometra, Jena, Germany) using the following conditions: 94 °C/5 min (1 cycle); 94 °C /30 s, 54 °C/35 s, 72 °C/1 min (30 cycles); and a final extension step at 72 °C/5 min (1 cycle). The primer pairs used were GUS-F: 5'-TCCTGTAGAAACCCCAACCC-3', and GUS-R: 5'-TCATAGAGATAACCTTCACCCG-3', which were designed using Primer 5.0. T0 plants (original transformants) were selfed up to the third generation (T3) under strict screening. The T3 homozygous plants were then used for further study.
Total RNA from the transgenic and WT plants was extracted from young leaves with RNAprep pure Plant Kit (Tiangen Biotech, Beijing, China) according to the manufacturer's protocol. Protein phosphatase PP2A (At1g13320) was used as a reference for the semi-quantitative RT-PCR with the following primers: forward primer PP2A-F: 5'-AGTATCGCTTCTCGCTCCAG-3', and reverse primer PP2A-R: 5'-GTTCTCCACAACCGCTTGGT-3' [Citation18].
Chilling–low irradiance treatment and fluorescence measurement
The Arabidopsis seedlings of transgenic lines and the WT were grown in the conditions described above. Four-week-old transgenic lines and WT were incubated at 4 °C and 100 μmol·m−2·s−1 for 12 h. The plants were then used to determine the photosynthesis parameters. Among them, chlorophyll fluorescence was measured with a portable fluorometer (FMS2, Hansatech, Briningham, England) according to the protocol described by van Kooten and Snel [Citation19]. To measure Fv/Fm, plants were adapted in darkness for 2 h before chilling stress, and for 15 min during chilling stress. The maximum efficiency of PSII was expressed as follows: Fv/Fm = (Fm−Fo)/Fm, where Fo is the initial fluorescence measured after dark adaptation for more than 2 h at room temperature prior to treatments, Fm is the maximum yield of fluorescence measured after dark adaptation for more than 15 min prior to treatments and Fv = Fm−Fo, is variable fluorescence. The NPQ was calculated as described by Wang et al. [Citation20]: NPQ = (Fm−Fm')/Fm', where Fm' is the maximum intensity of fluorescence in light-acclimated leaves.
Measurement of antioxidant enzyme activities and MDA content
An amount of 0.5 g of leaves was ground in a cold phosphate buffer (pH 7.8) The SOD activity was measured by the nitro-blue tetrazolium (NBT) photoreduction method [Citation21], and the POD activity, by the guaiacol method [Citation22] by measuring the absorption at 470 nm and 560 nm, respectively. The total enzyme activities of SOD and POD (in U/mg fresh weight) were both determined using a DU800 Spectrophotometer (Beckman Coulter, Southfield, Michigan, USA) with three replications.
The content of MDA in the leaves was measured by thiobarbituric acid as described by Zang et al. [Citation22]. The absorbance (optical density, OD) of the supernatant was read at 532 nm and 450 nm. The MDA content was calculated using the following formula: MDA (μmol L−1) = 6.45×OD532−0.56×OD450.
Data/statistical analysis
Statistical analysis of the differences between the measured parameters in WT and transgenic plants was done by one-tailed paired t-test using SPSS v.19.0. Values are means from five experiments with standard deviation (±SD).
Results and discussion
Isolation and sequence analysis of the watermelon ClZE gene
A 482-bp cDNA fragment was obtained by RT-PCR from the watermelon leaves. GenBank BLAST search revealed that this fragment appeared to be a cDNA fragment of ZE. The full-length sequence of ClZE (accession no. HM107775) was obtained using RLM-RACE, which is 2535 bp in length, with a 1998-bp open reading frame encoding 665 amino acids with an approximate molecular mass of 73.1 kDa and isoelectric point of 7.25.
Sequence analysis indicated that the protein instability index of ClZE was 36.94, indicating a stable protein. The hydrophobic distribution curve of ClZE amino acids assessed showed a minimal sore of -2.978 and a maximal score of 2.433, which represent the hydrophilicity and hydrophobicity, respectively. A transmembrane domain was detected in deduced ClZE protein. The subcellular localization prediction results for ClZE showed that it is most likely located at the chloroplast thylakoid membrane. This prediction is consistent with the tomato LeZE which is functional as lipocalins located in the thylakoid membrane [Citation15].
The deduced amino acid sequence of ClZE is homologous to its counterparts in other plant species. ClZE was shown to share a homology of 72.4% with the ZE in Ricinus communis (XP-002523587.1), 72.1% with that in Vitis vinifera (NP-001268202.1), 69.6% in Eutrema halophilum (AAV85824.1), 73.4% in Prunus armeniaca (O81360.1), 71% in Ipomoea nil (ADX99209.1) and 94.8% in Cucumis sativus (KGN61963.1) (). These results suggested that ClZE shared high sequence identity with ZEs in other plants and that ClZE is functional as a member of the lipocalin family. The amino acid sequence alignment analysis revealed that ClZE harbours four conserved motifs termed blocks I-IV that were previously defined in various ZEs from different species [Citation23,Citation24]. It also bears two large conservative domains referred to as Pyr_redox in block I-II, which is the characteristic region of the lipocalin protein family, and a flavoprotein momooxygenase domain in block III. The forkhead-associated domain (FHA) motif in block IV has been noted to play an important role in protein–protein interaction [Citation25,Citation26]. The amino acid sequence alignments of ClZE with the ZEs in other species were performed and a phylogenetic tree was constituted (). The phylogenetic relation results showed that ClZE is closer to CsZE from Cucumis sativus, but far from the ZEs in other plant species.
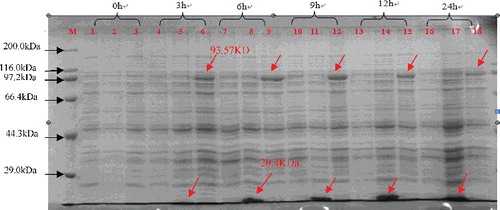
When the recombinant ClZE was expressed in E. coli with IPTG induction, along with induction progression, a large amount of the target protein was induced in a 12 h regime. As expected, an approximately 93-kDa recombinant protein (with the His*Tag at the N-terminus encoding 20.4 kDa) was detected through SDS-PAGE (), suggesting that ClZE was expressed in the prokaryotic system.
Figure 3. SDS-PAGE analysis of the expressed product of pET-ClZE 0 to 24 h after induction with IPTG. M: protein marker ladder (Premixed Protein Marker (Broad), Takara, Dalian, China); Lanes 1, 4, 7, 10, 13, 16: BL21 (DE3) pLysS; Lanes 2, 5, 8, 11, 14, 17: pET-32a (+); Lanes 3, 6, 9, 12, 15 and 18: pET-ClZE.
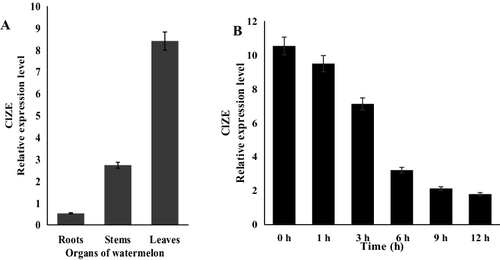
Expression of the ClZE gene under cold–low irradiance condition
A constitutive expression pattern of ClZE was observed in all tissues of watermelon by qRT-PCR analysis. However, more transcripts were detected in leaves than in roots and stems ((A)). These results indicated that ClZE is abundantly expressed in green tissue, being consistent with the result of ClZE's subcellular localization in chloroplasts. Under chilling–low irradiance stress, the expression levels of ClZE gradually decreased after 3 h of treatment, then were reduced rapidly and reached the minimal levels after 12 h of treatment ((B)). These expression levels of ClZE were consistent with those previously reported in alfalfa homologue [Citation27]. However, some studies indicate that the expression of ZE genes is not induced by light and low temperature, but by diurnal rhythm, as those characterized in tomato [Citation20], Nicotiana [Citation28] and Arabidopsis [Citation29]. In addition, Schwarz et al. [Citation30] revealed a tissue- and stress-specific expression pattern of the ZE genes, which is associated with the physiological processes of ABA biosynthesis and the xanthophyll cycle. It is therefore necessary to further study the ClZE expression pattern under other growth conditions.
Chilling–low irradiance resistance analysis of transgenic Arabidopsis overexpressing ClZE
To determine the physiological function of ClZE, transgenic Arabidopsis plants constitutively overexpressing ClZE were generated. The putative transgenic plants were first screened with kanamycin (50 mg/mL) (data not shown), then the insertion of GUS gene in transgenic plants was detected by PCR ((A)). The semi-quantitative RT-PCR results showed that all of the kanamycin-resistant plants had significantly higher target mRNA expression than that of WT plants ((B)). These results indicated that ClZE had been introduced into the Arabidopsis genome and expressed driven by the CaMV35S promoter.
Effects of chilling–low light treatment on NPQ and Fv/Fm of transgenic plants
To understand the function of ClZE, we investigated the tolerance capacity to chilling–low irradiance stress in transgenic Arabidopsis plants. The dynamics of Fv/Fm and NPQ in transgenic and WT plants after chilling–low irradiance treatment are shown in . Thermal dissipation acts as a main way to alleviate photoinhibition [Citation11,Citation31]. NPQ is a light-inducible process that competes and diverts absorbed energy from photochemistry to balance light harvesting and photosynthetic performance [Citation16,Citation32]. NPQ has been shown to be quickly responsive to high light stress and functional in preventing the over-excitation of photochemical reactions via dissipating excessive excitation energy into heat [Citation11,Citation33]. In transgenic lines and WT, NPQ showed no significant difference at 25 °C but increased when the plants were exposed to chilling–low irradiance stress. Research has indicated that the overall NPQ includes three components with different relaxation times after acclimation under a period of high light illumination, i.e. high-energy-state-quenching (qE), quenching due to state transitions (qT) and photoinhibitory quenching (qI) [Citation34]. Among them, qE responds the fastest to high light stress and accounts for the main component of NPQ [Citation35]. It has been shown that qE is activated and deactivated quickly (1–2 min) in response to rapidly changing light intensities under lab conditions and in the field [Citation36,Citation37] through the modulation of Z [Citation38]. In this study, at the first two hours of chilling–low irradiance treatment, NPQ increased rapidly in WT plants mainly due to the promoted dissipation of qE resulting from the high concentration of Z (NPQ in WT increased by about 22.7% compared to the initial level). In contrast, the NPQ in transgenic lines (T-1, T-2 and T-3) was lower than that of WT (whose NPQ increased by 13%, 12% and 14.6%, respectively, compared to the initial level). Some reports indicate that once plants are transferred to 25 °C, the ratio of NPQ is recovered in the first 2 h because of loss of the light-induced pH gradient across the thylakoid membrane [Citation20]. In this study, the NPQ ratio still remained a relatively high value after a 2-h recovery, suggesting that a portion of NPQ could be xanthophyll-cycle-independent in watermelon.
Figure 6. Effect of chilling–low irradiance stress on NPQ (A) and Fv/Fm (B) of transgenic and WT Arabidopsis plants.
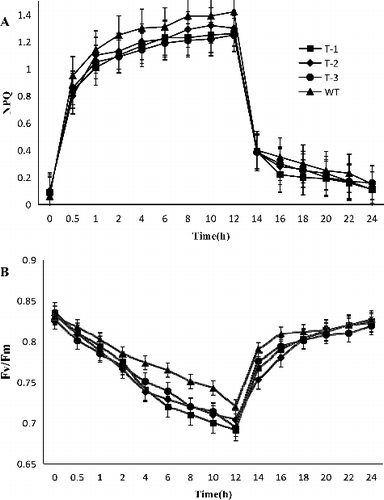
Low temperature can prevent the excitation energy from being effectively utilized for CO2 assimilation and thus induce photoinhibition even under low irradiance. Reduction of thermal dissipation in transgenic lines can lead to increase of the excitation pressure, and ultimately cause damage of the photosynthetic apparatus [Citation20]. Since Fv/Fm represents the maximum potential of PSII quantum efficiency and is used as a parameter for measurement of the function of PSII reaction centres, the decrease in Fv/Fm mainly reflects the characteristics of photoinhibition [Citation27].
In this study, the dynamics of Fv/Fm were analysed under the same treatment conditions as NPQ. Along with the chilling–low irradiance, the decrease values of Fv/Fm in both WT and transgenic lines indicated that chilling largely affected the carbon assimilation efficiency. At the end of the chilling–low irradiance stress treatment, the Fv/Fm values in transgenic lines varied from 0.691 to 0.704 (being 0.691, 0.704, and 0.694 for T1, T2 and T3, respectively). These values were smaller than that in WT plants (0.720). Additionally, the Fv/Fm values decreased more significantly in transformed lines and recovered faster than in WT plants after 12 hours of recovery treatment (). Takahashi and Murata [Citation2] proposed that the photoinhibition degree is determined by the balance between the rate of PSII photodamage and the rate of its repair. Environmental stresses do not affect photodamage, but inhibit the PSII repair cycle through suppression of the synthesis of PSII proteins, especially D1, which is a key protein in the process of the PSII repair cycle [Citation39]. Thus, environmental stress conditions are unfavourable for repairing the photodamaged PSII and, then, intensify the extent of photoinhibition. In this study, the overexpression of ClZE decreased the relative content of Z and the thermal dissipation under the chilling–low irradiance conditions, which thus aggravated the degree of photoinhibition. These results indicated that the PSII in plants overexpressing ClZE was more vulnerable than that in WT plants under chilling–low irradiance stress.
Physiological changes involved in chilling–low light stress
To further understand the function of ClZE, the activities of POD and SOD in both the transgenic and WT Arabidopsis plants were also analysed ((A,B)). When plants are under adverse conditions, ROS are greatly induced, which ultimately causes membrane lipid peroxidation. SOD and POD are considered to be the most important protective enzymes for removal of ROS. The degree of cell injury upon exposure to ROS initiated under chilling–low irradiance has been studied in various plants based on the peroxidase activity as an indicator [Citation40]. In this study, under normal growth conditions, there was no significant difference in the activities of SOD and POD between the transgenic lines and WT plants. However, after 12 h of treatment at 4 °C and light intensity of 100 μmol·m−2·s−1, the SOD and POD activities in transgenic lines and WT plants both increased significantly, with higher activities of SOD and POD in transgenic lines than those in WT plants ((A, B)). The POD activities increased by 21.8%, 35.5%, 37.8% and 42.8% in WT, T-1, T-2 and T-3, respectively; whereas the SOD activities increased by 224.7%, 262.3%, 241.7% and 258.9% in WT, T1, T2 and T3, respectively. These results indicated that the SOD activities were much more induced in plants than the POD activities, which is in line with previous studies that have shown that SOD activity in plants is more sensitive to chilling stress than POD activity [Citation40]. Our quantitative analysis of the POD and SOD activities confirmed this and revealed that transgenic lines had more accumulation of ROS irrespective of the higher activities of SOD and POD.
Figure 7. Activities of POD (A) and SOD (B) and MDA content (C) in transgenic and WT Arabidopsis plants under chilling–low irradiance stress.
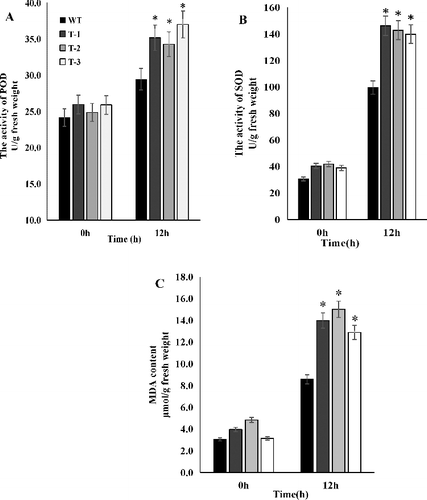
Tocopherol and Z also function as antioxidant substances involved in photoprotection [Citation41,Citation42]. More ROS accumulation in the transgenic lines is possibly associated with the biosynthesis of Z and tocopherol, which are needed to be further characterized. The MDA content was used as an indicator of the degree of membrane lipid peroxidation. In this study, the levels of MDA were increased under chilling stress by 180.2%, 253.7%, 210.9% and 311.5% in WT, T-1, T-2 and T-3, respectively, after 12 h of stress treatment ((C). These results demonstrated that membrane damage was more serious in the transgenic plants than in WT plants under the chilling–low irradiance conditions. The translation of psbA mRNA encoding the D1 protein is inhibited by ROS [Citation2,Citation43], suggesting the role of ROS in directly degrading D1 peptide bonds [Citation44]. Our results showed that plants overexpressing ClZE were more prone to lipid peroxidation under the chilling–low light stress. Overall, the findings suggest that ClZE can be of potential interest in genetic engineering for improvement of cultivars with tolerance to chilling and low light intensity in watermelon via mediation of photoinhibition and cellular ROS homeostasis.
Conclusions
In this study, the ClZE gene from watermelon was cloned. ClZE was predicted to contain two conservative domains, Pyr_redox and FHA. According to the qRT-PCR results, ClZE transcripts were more abundant in leaves and were shown to be sensitive to chilling–low irradiance stress. Overexpression of ClZE decreased the thermal dissipation capacity in Arabidopsis. The decrease in NPQ impaired the xanthophyll cycle-mediated photosynthesis apparatus protection through consumption of excess light energy. The transgenic Arabidopsis lines expressing ClZE exhibited more sensitivity to chilling–low irradiance stress than WT plants. These results suggest that ClZE probably plays important roles in alleviating photoinhibition of the photosynthetic apparatus and in associating with regulation of the cellular ROS homeostasis under chilling–low irradiance conditions.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Xu CC, Li LB, Kuang TY. The inhibited xanthophyll cycle is responsible for the increase in sensitivity to low temperature photoinhibition in rice leaves fed with glutathione. Photosynthesis Res. 2000;65:107–114.
- Takahashi S, Murata N. How do environmental stresses accelerate photoinhibition? Trends Plant Sci. 2008;13:178–182.
- Han H, Gao S, Dong XC, et al. Overexpression of violaxanthin de-epoxidase gene alleviates photoinhibition of PSII and PSI in tomato during high light and chilling stress. J Plant Physiol. 2010;167:176–183.
- Zhou B, Deng YS, Kong FY, et al. Overexpression of a tomato carotenoid 3-hydroxylase gene alleviates sensitivity to chilling stress in transgenic tobacco. Plant Physiol Biochem. 2013;70:235–245.
- Sun XL, Yang S, Wang LY, et al. The unsaturation of phosphatidylglycerol in thylakoid membrane alleviates PSII photoinhibition under chilling stress. Plant Cell Rep. 2011;30:1939–1947.
- Pokorska B, Zienkiewicz M, Powikrowska M, et al. Differential turnover of the photosystem II reaction centre D1 protein in mesophyll and bundle sheath chloroplasts of maize. Biochim Biophys Acta. 2009;1787:1161–1169.
- Nishiyama Y, Allakhverdiev SI, Murata N. A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim Biophys Acta. 2006;757:742–749.
- Johnson MP, Goral TK, Duffy CDP, et al. Photoprotective energy dissipation involves the reorganization of photosystem II light harvesting complexes in the grana membrane of high plant chloroplasts. Plant Cell. 2011;23:1468–1479.
- Belgio E, Kapitonova E, Chmeliov J. Economic photoprotection in photosystem II that retains a complete light-harvesting system with slow energy traps. Nature Commun [Internet]. 2014 [ cited 2016 Jul 24];5:4433. Available from: http://www.nature.com/articles/ncomms5433
- Kalituho L, Beran KC, Jahns P. The transiently generated nonphotochemical quenching of excitation energy in Arabidopsis leaves is modulated by zeaxanthin. Plant Physiol. 2007;143:1861–1870.
- Reinhold C, Niczyporuk S, Christian K, et al. Short-term down-regulation of zeaxanthin epoxidation in Arabidopsis thaliana in response to photo-oxidative stress conditions. Biochim Biophys Acta. 2008;1777:462–469.
- Nowicka B, Strzalka W, Strzalka K. New transgenic line of Arabidopsis thaliana with partly disabled zeaxanthin epoxidase activity displays changed carotenoid composition, xanthophyll cycle activity and non-photochemical quenching kinetics. J Plant Physiol. 2009;166:1045–1056.
- Bugos RC, Hieber AD, Yamamoto HY. Xanthophyll cycle enzymes are members of the lipocalin family, the first identified from plants. J Biol Chem. 1998;273:15321–15324.
- Lin RC, Xu CC, Li LB, et al. Xanthophyll cycle and its molecular mechanism in photoprotection. Acta Botanica Sinica. 2002;44:379–383.
- Wang N, Li B, Feng HL, et al. Antisense-mediated suppression of tomato zeaxanthin epoxidase alleviates photoinhibition of PSII and PSI during chilling stress under low irradiance. Photosynthetica. 2010;48:409–416.
- Xing X, Liu YK, Kong XP, et al. Overexpression of a maize dehydrin gene, ZmDHN2b, in tobacco enhances tolerance to low temperature. Plant Growth Regul. 2011;65:109–118.
- Yang BY, Liu YT, Hu WJ, et al. Depend on cDNA-AFLP and MSAP technical analysis of homologous diploid and tetraploid watermelon under cold stress. J Plant Genet Res. 2015;16(6):1298–1306.
- Zhong C, Gallie DR. Violaxanthin de-epoxidase is rate-limiting for non-photochemical quenching under subsaturating light or during chilling in Arabidopsis. Plant Physiol Biochem. 2012;58:66–82.
- Van Kooten O, Snel JFH. The use of chlorophyll fluorescence nomenclature in plant stress physiology. Photosynthesis Res. 1990;25:147–150.
- Wang N, Fang W, Han H, et al. Overexpression of zeaxanthin epoxidase gene enhances the sensitivity of tomato PSII photoinhibition to high light and chilling stress. Physiol Plant. 2008;132:384–396.
- Chongchatuporn U, Ketsa S, van Doorn WG. Chilling injury in mango (Mangifera indica) fruit peel: relationship with ascorbic acid concentrations and antioxidant enzyme activities. Biol Technol. 2013;86:409–417.
- Zang DD, Wang C, Ji XY. Tamarix hispida zinc finger protein ThZFP1 participates in salt and osmotic stress tolerance by increasing proline content and SOD and POD activities. Plant Sci. 2015;235:111–121.
- Baroli I, Do AD, Yamane T, et al. Zeaxanthin accumulation in the absence of a functional xanthophyll cycle protects Chlamydomonas reinhardtii from photooxidative stress. Plant Cell. 2003;15:992–1008.
- Durocher D, Jackson SP. The FHA domain. FEBS Lett. 2002;513:58–66.
- Hieber AD, Bugos RC, Yamamoto HY. Plant lipocalins: violaxanthin de-epoxidaseand zeaxanthin epoxidase. Biochim Biophys Acta, 2000;482:84–91.
- Grzyb J, Latowski D, Strzalka K. Lipocalins - a family portrait. Plant Physiol. 2006;163:895–915.
- Zhang Z, Wang Y, Chang L, et al. MsZEP, a novel zeaxanthin epoxidase gene from alfalfa (Medicago sativa), confers drought and salt tolerance in transgenic tobacco. Plant Cell Rep. 2016;35:439–453.
- Audran C, Borel C, Frey A, et al. Expression studies of zeanxanthin epoxidase gene in Nicotiana plumbaginifolia. Plant Physiol. 1998;118:1021–1028.
- North HM, Fry A, Boutin JP, et al. Analysis of xanthophyll cycle gene expression during the adaption of Atabidopsis to excess lihgt dan drought stress: changes in RNA steady-state levels do no contribute to short-term response. Plant Sci. 2005;169:115–124.
- Schwarz N, Armbruster U, Lven T, et al. Tissue-specific accumulation and regulation of zeaxanthin epoxidase in Arabidopsis reflect the multiple function of the enzyme in plastids. Plant Cell Physiol. 2015;56:346–257.
- Quaas T, Berteotti S, Ballottari M, et al. Non-photochemical quenching and xanthophyll cycle activities in six green algal species suggest mechanistic differences in the process of excess energy dissipation. J Plant Physiol. 2015;172:92–103.
- Demmig-Adams B, Adams III WW. Carotenoid composition in sun and shade leaves of plants with different life forms. Plant Cell Environ. 1992;15:411–419.
- Baker NR. Chlorophyll fluorescence: a probe of photosynthesis in vivo. Annu Rev Plant Biol. 2008;59:89–113.
- Müller P, Li XP, Niyogi KK. A response to excess light energy non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001;125:1558–1566.
- Pinnola A, Dall'Osto L, Gerotto C, et al. Zeaxanthin binds to light-harvesting complex stress-related protein to enhance nonphotochemical quenching in Physcomitrella patens. Plant Cell. 2013;25:3519–3534.
- Johnson MP, Davison PA, Ruban AV, et al. The xanthophyll cycle pool size controls the kinetics of non-photochemical quenching in Arabidopsis thaliana. FEBS Lett. 2008;582:259–263.
- Johnson MP, Zia A, Horton P, et al. Effect of xanthophyll composition on the chlorophyll excited state lifetime in plant leaves and isolated LHCII. Chem Phys. 2010;373:23–32.
- Eilers U, Dietzel L, Breitenbach J, et al. Identification of genes coding for functional zeaxanthin epoxidases in the diatom Phaeodactylum tricornutum. J Plant Physiol. 2016;192:64–70.
- Takahashi S, Murata N. Glycerate-3-phosphate, produced by CO 2 fixation in the Calvin cycle, is critical for the synthesis of the D1 protein of photosystem II. Biochim Biophys Acta. 2006;1757:198–205.
- Lin KH, Kuo WS, Chiang CM, et al. Study of sponge gourd ascorbate peroxidase and winter squash superoxide dismutase under respective flooding and chilling stresses. Scientia Horticulturae. 2013;162:333–340.
- Havaux M, Dall'Osto L, Bassi R. Zeaxanthin has enhanced antioxidant capacity with respect to all other xanthophylls in Arabidopsis leaves and functions independent of binding to PSII antennae. Plant Physiol, 2007;1451:1506–1520.
- Havaux M, Eymery F, Porfirova S, et al. Vitamin E protects against photoinhibition and photooxidative stress in Arabidopsis thaliana. Plant Cell. 2005;17:3451–3469.
- Nishiyama Y, Allakhverdiev SI, Murata N. Inhibition of the repair of photosystem II by oxidative stress in cyanobacteria. Photosynth Res. 2005;84:1–7.
- Lupinkova L, Komenda J. Oxidative modifications of the photosystem II D1 protein by reactive oxygen species: from isolated protein to cyanobacterial cells. Photochem Photobiol. 2004;79:152–162.