ABSTRACT
The main flavonoid compound rutin is highly expressed in the crop plant buckwheat (Fagopyrum tataricum (L.) Gaertn.) and plays important roles in the human diet. In this study, phenylpropanoid production in buckwheat hairy roots was evaluated following ethephon treatment. Using quantitative real-time polymerase chain reaction and high-performance liquid chromatography, we analysed the relationship between flavonoid and anthocyanin biosynthetic pathway gene regulation and the relative accumulation of the secondary compounds in ethephon-treated buckwheat hairy roots. Generally, the transcription of the biosynthetic pathway genes varied between the treated samples and controls. Most of the flavonoid biosynthetic genes were upregulated by ethephon, typically after four days of treatment. The application of 0.5 mg/L ethephon markedly induced anthocyanin production in hairy roots compared to that induced by the other concentrations tested (0, 1 and 2 mg/L). These data indicate that anthocyanin biosynthesis may play an important role in the response of buckwheat to ethephon-induced stress.
Introduction
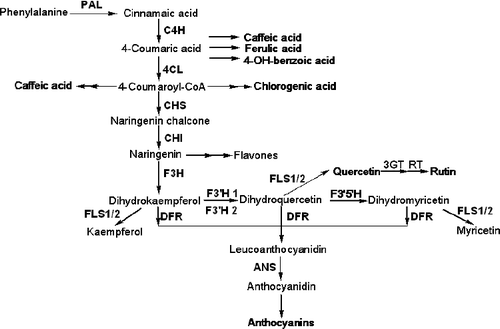
Phenylpropanoids comprise the largest group of secondary metabolites produced by higher plants; they have powerful antioxidant properties and protect plants against biotic and abiotic stresses [Citation1]. Among the various phenylpropanoid compounds, flavonoids and anthocyanins mainly contribute to the colour or pigmentation of flowers and fruits [Citation2,Citation3]. The flavonoids and anthocyanins are biosynthesized via the branched flavonoid biosynthetic pathways [Citation2,Citation3]. Phenylalanine ammonium lyase (PAL), cinnamic acid 4-hydroxylase (C4H), 4-coumarate-CoA ligase (4CL), chalcone synthase (CHS), chalcone isomerase (CHI), flavanone-3-hydroxylase (F3H), flavonoid-3′-hydroxylase (F3′H), flavonol synthase (FLS), dihydroflavonol reductase (DFR), anthocyanin synthase (ANS), flavonoid 3-O-glucosyltransferase (3GT) and 3-O-rhamnosyltransferase (RT) are the main genes involved in flavonoid and anthocyanin biosynthetic pathways ().
Physical, chemical and biological methods have been applied to regulate secondary metabolite biosynthetic pathways in plants. It has been reported that precursor feeding with shikimic acid and phenylalanine increases anthocyanin synthesis in grape cell cultures [Citation4]. Feeding with the inhibitors and precursors of the phenylpropanoid pathway has a differential stimulatory effect on the accumulation of p-hydroxybenzoic acid, lignin and flavonoids in Daucus carota hairy roots [Citation5]. Anthocyanin biosynthesis was also enhanced 2.3-fold in grape cell cultures treated with ethephon [Citation6]. Treatment with abscisic acid, jasmonate, ethylene or salicylic acid has been shown to induce higher production of secondary metabolites in plants [Citation7–10]. These elicitor molecules exert their effects by taking part, either directly or indirectly, in multiple signalling pathways or by activating plant transcription factors [Citation11–13].
Buckwheat (Fagopyrum esculentum and Fagopyrum tataricum) is an important annual crop. It is considered as one of the most important alternative crops and functional foods because it is a rich dietary source of starch, proteins and trace elements [Citation14]. In addition, buckwheat contains high amounts of active compounds, including rutin, quercetin, myricetin, isoquercitrin, catechin and some anthocyanins [Citation14,Citation15]. To date, only a few studies have investigated methods to increase the production of secondary metabolites of nutritional value in buckwheat. The nutritional quality, levels of phenolic and carotenoid compounds and antioxidant activity have been reported to increase significantly in common buckwheat sprouts in the presence of 100 mmol/L sodium chloride (NaCl) [Citation16]. It has also been reported that the flavonoid, anthocyanin and proanthocyanidin contents were markedly changed following methyl jasmonate treatment in common buckwheat [Citation17,Citation18]. We previously reported the effect of light/dark environments on the biosynthesis of flavonoids and anthocyanins in buckwheat sprouts [Citation19]. Different Agrobacterium rhizogenes strains had different effects on the phenylpropanoid biosynthesis in buckwheat hairy roots [Citation20]. Rutin content was also increased by overexpressing the Arabidopsis transcription factor AtMYB12 in buckwheat hairy root culture [Citation21]. In the present study, buckwheat hairy roots were exposed to exogenous ethephon treatment, and we subsequently examined the effect of ethephon on the regulation of flavonoid and anthocyanin biosynthetic pathway genes and the relative accumulation of secondary metabolites in buckwheat hairy roots.
Materials and methods
Plant materials and hairy root culture
The tartary buckwheat (F. tataricum (L.) Gaertn.) cultivar ‘Hokkai T10’ was obtained from the Hokkaido Agricultural Research Center (Hokkaido, Japan). We used seven-day-old buckwheat sprouts of ‘Hokkai T10’ for hairy root induction. A. rhizogenes strain R1000-induced hairy roots were transformed and cultured as previously described [Citation21]. Briefly, the collected hairy roots were cultivated in a half strength Murashige and Skoog liquid medium in 100 mL flasks and maintained at 25 °C on a shaker (100 r/min) in a growth chamber under standard, cool, white fluorescent tubes with a flux rate of 35 μmol/(s/m2) and a 16-h photoperiod.
Ethephon treatment on buckwheat hairy root
A. rhizogenes-induced hairy roots were sub-cultured as described earlier. After 21 days of multi-culture, hairy roots were ready for treatment. The ethephon stock was prepared and added to hairy root culture liquid medium, resulting in final concentrations of 0.5, 1 and 2 mg/L. Three flasks were treated in duplicate. Samples were collected at different time points after ethephon treatment (0, 6, 12, 24, 48, 72 and 96 h). The collected samples were frozen in liquid nitrogen and finally stored at −80 °C until further use in RNA isolation.
Total RNA extraction and qRT-PCR
For quantitative real-time polymerase chain reaction (qRT-PCR), RNeasy Plant Mini Kit (Qiagen, Valencia, CA, USA) was used for the isolation of total RNA from the F. tataricum hairy root samples. The purity of the RNA was checked by gel electrophoresis and the RNA samples were then used for synthesis of cDNA using the GeneRacer Kit (Invitrogen, Carlsbad, CA, USA). Superscript II First Strand Synthesis Kit procured from Invitrogen was used for the transcription study using qRT-PCR. Different gene-specific primers were designed and used for the quantification of qRT-PCR () and the histone 3 (H3) gene was used as the house-keeping gene. The qRT-PCR experiment was carried out in triplicates using a MiniOpticon system (Bio-Rad Laboratories, Hercules, CA, USA) together with the Quantitect SYBR Green PCR Kit (Qiagen, Valencia, CA, USA).
Table 1. Primers used in this study.
Extraction of phenylpropanoid from buckwheat
For extraction of total phenylpropanoid components from buckwheat, the samples were collected and washed thoroughly with sterile distilled water and packed in aluminium foil for freeze-drying at −30 °C. After that, the frozen samples were powdered for the extraction study. Briefly, 100 mg of the powdered samples were transferred into sterile tubes containing 5 mL of 80% methanol (MeOH) together with 10% formic acid (0.1% (v/v)). The samples were mixed thoroughly, vortexed for 5 min and sonicated at 60 Hz in a sonicator (KODO, JAC 4020, Korea) for 1 h at 37 °C for complete extraction, with 1 min of vortexing after every 20 min of sonication. Later, the slurry was centrifuged at 1000 × g for 5 min for removal of the debris. After centrifugation, the debris-free supernatant was carefully collected and filtered through a 0.45-μm polytetrafluoroethylene syringe for high-performance liquid chromatography (HPLC) analysis. Separately, 100 mg powder was sonicated with MeOH containing 5% formic acid (v/v) for 20 min for total anthocyanin extraction.
Quantitative HPLC analysis for phenylpropanoid
The concentrations of flavanoids in the freeze-dried samples were determined by HPLC using refractive index (RI) and photodiode array (PDA) detectors (Agilent Technologies, Palo Alto, CA, USA) equipped with a Capcell PAK ODS column (250 mm × 4.6 mm, 5 μm; Shiseido, Tokyo, Japan). The operating conditions (mixture of (1) MeOH:water:acetic acid (5:92.5:2.5, v/v/v) and (2) MeOH:water:acetic acid (95:2.5:2.5, v/v/v)) in gradient conditions, column temperature 30 °C, flow rate of 1.0 mL/min, injection volume 20 μL, detector wave length 280 nm) were studied to optimize peak separation. On the basis of the retention time and the peak area, individual flavanoids were quantified with respect to standard reference compounds. Data were expressed in milligrams per gram dry weight. All the experiments were conducted in triplicate in HPLC analysis.
The anthocyanin filtrate was analysed by HPLC using RI and PDA detectors (Agilent Technologies, Palo Alto, CA, USA) equipped with a Synergi 4μ POLAR-RP 80A column (250 × 4.6 mm, i.d., particle size 4 μm; Phenomenex, Torrance, CA, USA) [Citation22]. The operating conditions (mixture of (1) water:formic acid (95:5, v/v) and (2) acetonitrile:formic acid (95:5, v/v)) in gradient conditions, column temperature of 30 °C, flow rate of 1.0 mL/min, injection volume of 20 μL and detector wave length 520 nm) were studied to optimize the peak separation [Citation22]. On the basis of the retention time and the peak area, individual anthocyanins were quantified with respect to cyanidin-3-O-glucoside as a standard. The values were expressed in milligrams per gram dry weight. All the experiments were conducted in triplicate in HPLC analysis.
Data analysis
The results shown in the figures are mean values with standard deviations (±SD) from three measurements. Statistical analysis was performed using statistical analysis software 8.2 (SAS Institute, Cary, NC, USA). The significant difference between treatments was assessed by Duncan's multiple-range test and statistical significance for all tests was set at a level of p < 0.05.
Results and discussion
Transcript level of phenylpropanoid biosynthetic genes in tartary buckwheat hairy roots
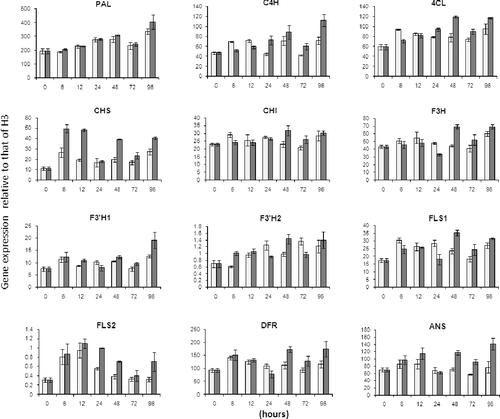
qRT-PCR using specific primers showed that the expression patterns of the main biosynthetic pathway genes varied in a time-dependent manner (). We also observed differential transcriptional levels between controls and ethephon-treated hairy roots. The transcript levels of FtC4H, Ft4CL, FtCHI and FtFLS1 were depressed after a 6-h stress treatment; conversely, the levels of FtCHS and FtF3H2 were increased. Ethephon induced higher transcription of most of the studied genes after 48 h, including Ft4CL, FtCHS, FtCHI, FtF3H, FtF3´H1, FtF3´H2, FtFLS1, FtFLS2, FtDFR and FtANS. The relative higher expression levels of these genes lasted up to 96 h after ethephon treatment compared to control (). Overall, most genes in the flavonoid biosynthetic pathway were upregulated and remained highly active for 48–96 h after treatment. One exception was FtPAL, which remained slightly upregulated after 96 h. Our results indicate that Ft4CL, FtCHS, FtCHI, FtF3H, FtF3´H1, FtF3´H2, FtFLS1, FtFLS2, FtDFR and FtANS may play important roles in the processes that occur during ethephon treatment of buckwheat.
Identification of phenylpropanoids and anthocyanins in tartary buckwheat hairy roots
HPLC detected the polyphenol compounds chlorogenic acid, 4-hydroxybenzoic acid, caffeic acid, ferulic acid, rutin and quercetin and two anthocyanin compounds, cyanidin 3-O-glucoside and cyanidin 3-O-rutinoside, in buckwheat hairy root treated with 0, 0.5, 1 and 2 mg/L ethephon for four days (). We found that almost all the above compounds were affected by ethephon treatment at the concentrations 0.5 and 1 mg/L. We observed the effects of ethephon treatment on chlorogenic acid, 4-hydroxybenzoic acid and caffeic acid synthesis (). In particular, the main flavonoid compound rutin was increased by 20% (15.56 and 19.06 mg/g in the control and 0.5-mg/L ethephon-treated groups, respectively). The quercetin content was 0.45 mg/g in the 1-mg/L ethephon-treated group but only 0.33 mg/g in the control group.
Figure 3. Accumulation of the main compounds in F. tataricum hairy roots following ethephon treatment.
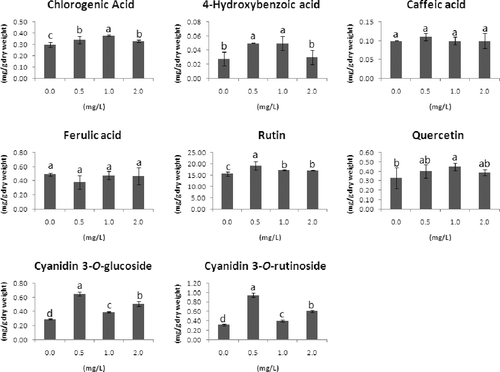
When the concentration of ethephon was increased to 2 mg/L, it only contributed to further accumulation of two anthocyanin compounds, cyanidin 3-O-glucoside and cyanidin 3-O-rutinoside, but not to that of the other polyphenols. However, the accumulation of cyanidin 3-O-glucoside and cyanidin 3-O-rutinoside was predominantly increased () twofold and threefold higher in the 0.5 mg/L ethephon treatment group compared to that in the control. In addition, the accumulated amount of ferulic acid was slightly decreased by 0.5 mg/L ethephon treatment, while no effect was induced by the other two concentrations of ethephon tested. Our observation of enhanced anthocyanin synthesis induced by ethephon is consistent with that reported in previous studies [Citation7,Citation23].
Phenylpropanoid compounds play important roles in protecting plants against biotic and abiotic stresses such as ultraviolet (UV) irradiation, water, infections, wounding and consumption by herbivores [Citation1,Citation24]. Environmental factors, including light, water, temperature, nutrition, phytohormones and salinity, could directly affect the biosynthesis of secondary metabolites in plants [Citation4,Citation18,Citation19,Citation23,Citation25–27]. With increasing knowledge of the complicated metabolic networks involved in the biosynthesis of secondary metabolites, it is possible to understand the influences of environmental factors on biosynthetic pathways.
Plants have complicated mechanisms that respond to different environmental factors. It is widely accepted that transcription factors play an important role in regulating secondary metabolite biosynthetic pathways in plants under diverse environmental and developmental stimuli, including different abiotic stresses such as darkness, drought, temperatures and exposure to UV-B light, as well as wounding, salinity and salicylic acid [Citation28–32]. Several R2R3-type MYB genes cloned from buckwheat have been shown to exhibit different transcription levels in different buckwheat organs and seedlings; these genes were found to be involved in proanthocyanidin biosynthesis [Citation33,Citation34]. The floral transcriptome information of buckwheat has been sequenced with 454 pyrosequencing technology, which has greatly contributed towards understanding the secondary metabolite biosynthesis and metabolism in buckwheat [Citation35]. A total of 509 transcript fragments (TDFs) have been generated by differential transcript profiling through complementary DNA-amplified fragment length polymorphism (cDNA-AFLP) in the seed-maturing stages of buckwheat [Citation36]. We speculate that certain TDFs represented genes that were involved in regulating the response to ethephon in buckwheat hairy root cultures.
Previous reports have revealed the signal transduction steps underlying the activation of plant secondary metabolism [Citation11,Citation37]. First, elicitor signal perception initiates a signal transduction network that leads to activation or de novo biosynthesis of transcription factors, which subsequently regulate the expression of biosynthetic genes involved in plant secondary metabolism [Citation11]. In addition, interactions among the elicitors signal transduction network (such as methyl jasmonate, wounding and fungal elicitation) have been found to exist [Citation13,Citation37,Citation38].
The genetic mechanisms underlying ethephon's effects on biosynthesis of secondary metabolites are not well documented. The present study investigated the genes involved in the flavonoid biosynthetic pathway of buckwheat. The expressions of most genes in the flavonoid biosynthetic pathway were significantly induced by ethephon treatment. HPLC results demonstrated that the contents of rutin and quercetin slightly increased in response to ethephon treatment, which may result from FtFLS, which also plays an important role (especially in quercetin synthesis) in the response of buckwheat to salinity stress [Citation39]. In the secondary metabolite pathway, DFR and ANS mainly contribute to anthocyanin biosynthesis (). In buckwheat, ANS was highly expressed in different organs of the whole buckwheat plant during the blooming stage and in maturing seeds; it was also found to have a direct relationship with anthocyanin accumulation in two buckwheat cultivars [Citation40]. Interestingly, levels of the two anthocyanin compounds were sharply increased by ethephon treatment (), which corresponded to the upregulated expressions of FtDFR and FtANS. Based on our findings, we speculate that ethephon induces or stimulates the expression of certain transcription factors, ultimately resulting in the regulation of secondary metabolite biosynthesis in buckwheat hairy root. Further studies are needed for a deeper understanding of the regulatory system governing ethephon-induced secondary metabolism in buckwheat.
Conclusions
To date, there is little evidence regarding the genetic mechanism underlying the response of buckwheat to ethephon stress condition. In addition to investigating the effect of ethephon effect on buckwheat nutritional properties, we assessed the effect of ethephon on the biosynthesis of the main secondary metabolites in the buckwheat hairy root system by analysing the gene-to-metabolite pathway. Our results indicated that ethephon largely activated most of the phenylpropanoid pathway genes and markedly increased the accumulation of several phenylpropanoid compounds, including flavonoids and anthocyanins, which play an important role in the response of buckwheat to ethephon-induced stress.
Disclosure statement
The authors have declared no conflict of interest.
Additional information
Funding
References
- Korkina L. Phenylpropanoids as naturally occurring antioxidants: from plant defense to human health. Cell Mol Biol. 2007;53:15–25.
- Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longevity. 2009;2:270–278.
- Winkel-Shirley B. Flavonoid biosynthesis. A colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol. 2001;126:485–493.
- Saw N, Riedel H, Kütük O, et al. Effect of elicitors and precursors on the synthesis of anthocyanin in grape (Vitis vinifera) cell cultures. Energy Rec J. 2010;1:189–192.
- Sircar D, Mitra A. Accumulation of p-hydroxybenzoic acid in hairy roots of Daucus carota 2: confirming biosynthetic steps through feeding of inhibitors and precursors. J Plant Physiol. 2009;166:1370–1380.
- Lan X, Chang K, Zeng L, et al. Engineering salidroside biosynthetic pathway in hairy root cultures of Rhodiola crenulata based on metabolic characterization of tyrosine decarboxylase. PloS One [Internet]. 2013 [ cited 2016 Aug 11];8:e75459. Available from: http://dx.doi.org/10.1371/journal.pone.0075459
- Saw NMMT, Riedel H, Cai Z, et al. Stimulation of anthocyanin synthesis in grape (Vitis vinifera) cell cultures by pulsed electric fields and ethephon. Plant Cell Tiss Organ Cult. 2012;108:47–54.
- Ghasemzadeh A, Talei D, Jaafar HZ, et al. Plant-growth regulators alter phytochemical constituents and pharmaceutical quality in sweet potato (Ipomoea batatas L.). BMC Complement Altern Med [Internet]. 2016 [ cited 2016 Aug 11];16:152. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4884425/
- Yan J, Lipka AE, Schmelz EA, et al. Accumulation of 5-hydroxynorvaline in maize (Zea mays) leaves is induced by insect feeding and abiotic stress. J Exp Bot. 2015;66:593–602.
- Vaddadi S, Parvatam G. Impacts of biotic and abiotic stress on major quality attributing metabolites of coffee beans. J Environ Biol. 2015;36:377–382.
- Zhao J, Davis LC, Verpoorte R. Elicitor signal transduction leading to production of plant secondary metabolites. Biotechnol Adv. 2005;23:283–333.
- Coqueiro DS, de Souza AA, Takita MA, et al. Transcriptional profile of sweet orange in response to chitosan and salicylic acid. BMC Genomics [Internet]. 2015 [ cited 2016 Aug 11];16:288. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4415254/
- Yang YX, Ahammed GJ, Wu C, et al. Crosstalk among jasmonate, salicylate and ethylene signaling pathways in plant disease and immune responses. Curr Prot Peptide Sci. 2015;16:450–461.
- Krkosková B, Mrázová Z. Prophylactic components of buckwheat. Food Res Int. 2005;38(5):561–568.
- Kim S-J, Zaidul I, Suzuki T, et al. Comparison of phenolic compositions between common and tartary buckwheat (Fagopyrum) sprouts. Food Chem. 2008;110:814–820.
- Lim J-H, Park K-J, Kim B-K, et al. Effect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food Chem. 2012;135:1065–1070.
- Horbowicz M, Wiczkowski W, Koczkodaj D, et al. Effects of methyl jasmonate on accumulation of flavonoids in seedlings of common buckwheat (Fagopyrum esculentum Moench). Acta Biol Hung. 2011;62:265–278.
- Kim HJ, Park KJ, Lim JH. Metabolomic analysis of phenolic compounds in buckwheat (Fagopyrum esculentum M.) sprouts treated with methyl jasmonate. J Agric Food Chem. 2011;59(10):5707–5713.
- Li X, Thwe AA, Park NI, et al. Accumulation of phenylpropanoids and correlated gene expression during the development of tartary buckwheat sprouts. J Agric Food Chem. 2012;60:5629–5635.
- Thwe A, Arasu MV, Li X, et al. Effect of different Agrobacterium rhizogenes strains on hairy root induction and phenylpropanoid biosynthesis in tartary buckwheat (Fagopyrum tataricum Gaertn). Front Microbiol [ Internet]. 2016 [ cited 2017 11 Jan];7:318. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4789558/
- Park NI, Li X, Thwe AA, et al. Enhancement of rutin in Fagopyrum esculentum hairy root cultures by the Arabidopsis transcription factor AtMYB12. Biotechnol Lett. 2012;34:577–583.
- Kim YB, Park SY, Thwe AA, et al. Metabolomic analysis and differential expression of anthocyanin biosynthetic genes in white- and red-flowered buckwheat cultivars (Fagopyrum esculentum). J Agric Food Chem. 2013;61:10525–10533.
- Li Y, Huang M, Wu L. The effect of ethephon on the content of anthocyanin and flavonoids in Anthurium andraeanum Spathe. Chin Agric Sci Bull. 2012;28(28):198–202.
- Korkina L, Kostyuk V, De Luca C, et al. Plant phenylpropanoids as emerging anti-inflammatory agents. Mini Rev Med Chem. 2011;11:823–835.
- de Ascensao AR, Dubery IA. Soluble and wall-bound phenolics and phenolic polymers in Musa acuminata roots exposed to elicitors from Fusarium oxysporum f. sp. cubense. Phytochemistry. 2003;63:679–686.
- Hu H, You J, Fang Y, et al. Characterization of transcription factor gene SNAC2 conferring cold and salt tolerance in rice. Plant Mol Biol. 2008;67:169–181.
- Nemie-Feyissa D, Olafsdottir SM, Heidari B, et al. Nitrogen depletion and small R3-MYB transcription factors affecting anthocyanin accumulation in Arabidopsis leaves. Phytochemistry. 2014;98:34–40.
- Nakashima K, Takasaki H, Mizoi J, et al. NAC transcription factors in plant abiotic stress responses. Biochim Biophys Acta. 2012;1819:97–103.
- Kaplan-Levy RN, Brewer PB, Quon T, et al. The trihelix family of transcription factors–light, stress and development. Trends Plant Sci. 2012;17:163–171.
- Payyavula RS, Singh RK, Navarre DA. Transcription factors, sucrose, and sucrose metabolic genes interact to regulate potato phenylpropanoid metabolism. J Exp Bot. 2013;64:5115–5131.
- Chen N, Yang Q, Pan L, et al. Identification of 30 MYB transcription factor genes and analysis of their expression during abiotic stress in peanut (Arachis hypogaea L.). Gene. 2014;533:332–345.
- Cui MH, Yoo KS, Hyoung S, et al. An Arabidopsis R2R3-MYB transcription factor, AtMYB20, negatively regulates type 2C serine/threonine protein phosphatases to enhance salt tolerance. FEBS Lett. 2013;587:1773–1778.
- Hai-Xia Z, Xiao-Feng W, Yue-Chen B, et al. Gene expression analysis of key enzymes and MYB transcription factors in flavonoid biosynthesis pathway during germination of Fagopyrum tataricum. J Agric Biotechnol. 2012;20:121–128.
- Bai YC, Li CL, Zhang JW, et al. Characterization of two tartary buckwheat R2R3-MYB transcription factors and their regulation of proanthocyanidin biosynthesis. Physiol Plant. 2014;152(3):431–140.
- Logacheva MD, Kasianov AS, Vinogradov DV, et al. De novo sequencing and characterization of floral transcriptome in two species of buckwheat (Fagopyrum). BMC Genomics [Internet]. 2011 [ cited 2016 Aug 11];12:30. Available from: https://bmcgenomics.biomedcentral.com/articles/10.1186/1471-2164-12-30
- Gupta N, Naik PK, Chauhan RS. Differential transcript profiling through cDNA-AFLP showed complexity of rutin biosynthesis and accumulation in seeds of a nutraceutical food crop (Fagopyrum spp.). BMC Genomics [Internet]. 2012 [ cited 2016 Aug 11];13:231. Available from: https://bmcgenomics.biomedcentral.com/articles/10.1186/1471-2164-13-231
- Singh G, Gavrieli J, Oakey J, et al. Interaction of methyl jasmonate, wounding and fungal elicitation during sesquiterpene induction in Hyoscyamus muticus in root cultures. Plant Cell Rep. 1998;17:391–395.
- Shoji T, Nakajima K, Hashimoto T. Ethylene suppresses jasmonate-induced gene expression in nicotine biosynthesis. Plant Cell Physiol. 2000;41:1072–1076.
- Li X, Kim YB, Kim Y, et al. Differential stress-response expression of two flavonol synthase genes and accumulation of flavonols in tartary buckwheat. J Plant Physiol. 2013;170:1630–1636.
- Park NI, Li X, Suzuki T, et al. Differential expression of anthocyanin biosynthetic genes and anthocyanin accumulation in tartary buckwheat cultivars ‘Hokkai T8’ and ‘Hokkai T10’. J Agric Food Chem. 2011;59:2356–2361.