ABSTRACT
Interest in nanoparticles has increased rapidly over the last few years, becoming one of the most compelling scopes of research fields. The microbial methods utilized for biomediated nanoparticle synthesis are the most favourable and well-established substitute for the classic chemical and physical methods. In this study, silver nanoparticles (AgNPs) were biosynthesized by reducing Ag+ ions using a cell-free supernatant derived from Bacillus aerius culture and silver nitrate (AgNO3) solution as a precursor. The reaction mixture exhibited a colour change from yellow to brown, and ultraviolet-visible spectroscopy showed a surface plasmon resonance peak at 420 nm. The nanoparticles were monodispersed and spherical with an average particle size of 20.12–29.48 nm as determined by transmission electron microscopy. The Fourier transform infrared spectrum revealed the capping of AgNPs with biomolecular compounds that were responsible for the reduction of AgNO3 to AgNPs. The biosynthesized AgNPs had powerful and potent antibacterial activity against many multidrug-resistant bacterial pathogens. Moreover, the in vitro dose-dependent antioxidant activity of the aqueous extract of AgNP components showed good antioxidant activity as compared to the standard antioxidant ascorbic acid. These outcomes support the advantages of green techniques for synthesizing AgNPs that can be utilized effectively in the production of potential antioxidant and antibacterial AgNPs for commercial application.
Introduction
Nanosilver is one of the most important molecules in recent science and has drawn the attention of scientists from different disciplines [Citation1]. Several methods are used in nanoparticle production, such as physical, chemical and hybrid methods [Citation2–4] such as the reduction methods [Citation5], sonochemical [Citation6], microemulsion [Citation7] and microwave techniques [Citation8]. Unfortunately, despite many of these methods being rapid-production, they require the use of unsafe chemicals, are labour-intensive, produce undesirable byproducts and have difficult and wasteful purification processes [Citation9]. Moreover, these methods lead to the attachment of toxic materials onto the produced nano-sized materials, or nanoparticles, which may have an undesirable effect on their medical implementation [Citation10].
Nanoparticles have unique and improved properties because of their larger surface area-to-volume ratio and are considered the building blocks of nanotechnology for designing materials with interesting properties. In the last decade, noble metal nanoparticles have attracted much attention from scientific researchers due to their applications in medicine, biology, optoelectronics and material science [Citation11]. Among the noble metals, silver is the most extensively used and studied because of its unique properties and its use in the biomedical field in particular [Citation12].
Accordingly, there is an urgent need to discover harmless, effective and safe methods for nanoparticle production. In the last few years, many scientists have made potential efforts to synthesize eco-friendly, economical and safe nanoparticles from different biological sources. A large number of organisms have been employed in nanoparticle biosynthesis, including prokaryotes (bacteria and actinomycetes); several authors [Citation13–15] have reported the extracellular synthesis of silver nanoparticles (AgNPs) using several Gram-positive and Gram-negative bacteria, and eukaryotes (yeasts, fungi and plants) [Citation16].
Among the various noble metals, silver, gold and platinum nanoparticles have been widely applied as new therapeutic agents against viral and microbial diseases, and cancer, and as anti-inflammatory agents [Citation17,Citation18]. The antimicrobial characteristics of AgNPs promote their use in a large number of medical and ecological applications as well as in various consumer products [Citation19].
The present work concentrated on the discovery of new AgNP-reducing strains and the evaluation of their antioxidant and bactericidal activity against various human pathogenic bacteria. The study also included energy dispersive X-ray spectroscopy (EDX), transmission electron microscopy (TEM), Fourier transform infrared (FTIR) and ultraviolet-visible (UV-vis) spectroscopy of AgNP characterization.
Materials and methods
Biomass production
The bacterial strain Bacillus aerius was isolated from native soil and characterized performing biochemical tests. The strain was maintained at 4 °C on nutrient agar slants as well as sub-cultured from regularly to regulate its viability. Pure culture of B. aerius was cultured on nutrient broth medium of the following composition: peptone (5.0 g/L), sodium chloride (5.0 g/L), beef extract (1.5 g/L), at pH 7 ± 0.1 to synthesize the AgNPs [Citation20]. The bacterial culture was incubated for 24 h and agitated on an orbital shaker at 150 rpm and 35 °C. The supernatant of the overnight bacterial culture was collected by centrifugation at 5000 rpm for 10 min. The pellet was discarded and the supernatant suspension was retained for AgNP biosynthesis.
AgNP biosynthesis
Fifty millilitre of cell-free supernatant was added to individual reaction vessels each containing 50 mL of AgNO3 solution (1 mmol/L final concentration). Control (without silver nitrate) was also run along with the experimental condition. The reaction mixture was incubated in the dark for 24 h. The ability of the bacteria to AgNP biosynthesis was determined visually based on change in colour of the reaction mixture.
Characterization of AgNPs
The preliminary detection of AgNO3 reduction was achieved by visual observation of the colour change of the reaction mixture. The nanoparticle reduction dynamics were assessed by plotting the maximum absorbance reached at each time of measurement (12, 24, 48 and 72 h). These samples were later measured optically using a UV-vis spectrophotometer (Labomed, Korea) and scanning the spectra between 200 and 1000 nm.
Fourier transform infrared
The interaction between protein in the supernatant (bacterial cell-free) and the AgNPs was detected using FTIR analysis. The FTIR spectrum of the dried sample was recorded on a Perkin Elmer instrument in the 450–4000 cm−1 range at 4 cm−1 resolution.
Energy dispersive X-ray spectroscopy
Elemental analysis was performed for EDX determination operating at 20 KeV, where a fine film of the sample was prepared on aluminium foil (1 cm × 1 cm) by dropping 100 μL supernatant on the foil sheet and allowing it to dry for 30 min before further use.
Transmission electron microscopy
The TEM investigation samples were prepared by removing AgNPs from the extract by centrifugation (5000 rpm for 15 min) and washing them twice in sterile distilled water. One drop of sample was applied onto a carbon-coated copper grid. After about 1 min, the excess solution was removed using absorbent paper and the grid air-dried before analysis.
Characterization of biosynthesized AgNPs
The effects of the various phys-chemical factors of the AgNPs were determined by varying the temperature, pH and AgNO3 concentration.
The temperature was maintained at 30, 40, 50, 60, 70 and 80 °C. The effect of pH was studied at pH 5–11, which was adjusted using 0.1 N NaOH and 0.1 N HCl. To detect AgNP concentrations, 0.5–5 mmol/L AgNO3 was used. The parameter absorbance values were measured using a UV-vis spectrophotometer.
In vitro analysis of antimicrobial activity of biosynthesized AgNPs by agar well diffusion method
The agar well diffusion method was used to test the antimicrobial sensitivity of the biosynthesized AgNPs against the following drug-resistant pathogenic bacterial strains: Acinetobacter baumannii (A. baumannii, ATCC BAA-1605), Escherichia coli (E. coli, ATCC BAA-2523), Klebsiella pneumoniae (K. pneumoniae, ATCC BAA-2524), Pseudomonas aeruginosa (P. aeruginosa, ATCC BAA-2108), Micrococcus luteus (ATCC 10240); methicillin-resistant Staphylococcus aureus (MRSA, ATCC 43330); extended-spectrum beta-lactamase strains such as E. coli (ATCC BAA-2326), K. oxytoca (ATCC 51983), vancomycin-resistant enterococci: Enterococcus faecalis (E. faecalis, ATCC 51299) and Moraxella catarrhalis (M. catarrhalis, ATCC 25238), Salmonella typhimurium (ATCC 14028), Shigella sonnei (Sh. sonnei, ATCC 25931), Staphylococcus epidermidis (S. epidermidis, ATCC 1228) and Streptococcus bovis (ATCC 49147).
The pure cultures of microorganisms were sub-cultured in nutrient broth at 37 °C in a shaker incubator. Then, the bacterial suspension was prepared and spectrophotometrically adjusted to match the turbidity of 1.5 × 10−8 cell forming unit (CFU) (in OD600). Subsequently, 100 µL of fresh culture of each test organism was spread on Mueller–Hinton agar plates. To evaluate the antibacterial activity of nanoparticles, 5 mm wells were punched into the nutrient agar plates. Subsequently, 100 μL of AgNPs suspension was loaded into each well. Ampicillin (10 μg/mL) was used as positive control and the cell-free medium as negative control. The plates were incubated for 18 h incubation at 35 °C; and then, diameters of the zones of inhibition were measured.
Antioxidant activity
To evaluate the in vitro free radical scavenging activity of the biosynthesized AgNPs, the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay was used. Reduced AgNPs were dissolved in 10, 20, 30, 40, 50, 60, 70, 80, 90 and 100 μg/mL methanol; an equal amount of methanol was added to the control. DPPH solution (2 mL) in ethanol (100 μmol/L) was separately mixed with 2 mL of each concentration of methanol [Citation21]. The reaction mixture was incubated in the dark for 15 min; thereafter, the optical density was recorded at 517 nm against the blank. The scavenging percentages of DPPH with the AgNPs were calculated using the following formula: Sfr (%) = (Ac − As/Ac) × 100%, where Sfr is free radical scavenging, Ac is the absorbance of the control and As is the absorbance obtained from the sample.
Statistical analysis
The acquired data were analysed using SPSS 9.0, and the results are presented as the mean ± SD of three replicates. The mean comparison between the various assessed groups was performed using one-way analysis of variance. Statistical significance was defined when p < 0.05.
Results and discussion
The preliminary AgNO3 reduction efficiency of B. aerius was observed visually through colour change of the mixture solution (), where it changed from yellow to dark brown. The colour change is based on the surface plasmon resonance (SPR) property of the AgNPs; no similar colour changes took place in both the positive and negative controls. The brown colour confirmed that the reduction had occurred (Ag+ to Ag0) in the aqueous solution [Citation22].
Figure 1. The changing in colour of supernatant free bacterial cell after addition of silver nitrate for synthesis silver nanoparticles.

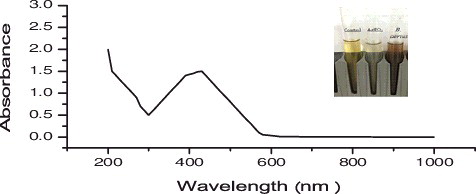
Further confirmation of AgNP synthesis was monitored using UV-vis spectrophotometry. This scanning has been proven as a useful technique for detecting the AgNP SPR [Citation23,Citation24]. Strong SPR bands were detected at 430 nm, which is similar to the absorbance spectra seen in the B. aerius cell-free filtrate. The data in show the UV-vis spectra values for B. aerius. The bands at 430 nm are the distinctive AgNP peaks as reported by Petit et al. [Citation5].
Figure 2. UV-visible spectra for silver nanoparticles (1 mM aqueous solution of AgNO3) synthesized by Bacillus aerius. The inset of the figure shows a test tube of the silver nanoparticle solution formed at the end of the reaction.
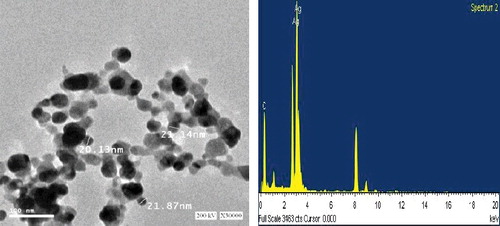
The presence of the elemental silver particles can be seen in the EDX graph (). EDX analysis proved the chemical purity and the existence signal of the elemental silver particles. In the present study, elemental composition analysis utilizing EDX detected a strong and sharp signal of silver atoms produced by B. aerius, where the silver nanocrystals arose at an optical absorption band peak at about 3 KeV, which is typical for the absorption of metallic AgNPs and that confirmed the presence of AgNPs in the bacterial supernatant [Citation19].
Figure 3. TEM micrograph at 30.000 time magnification and energy dispersive spectroscopy (EDX) spectrum of silver nanoparticles that were synthesized by Bacillus aerius. The scale bar corresponds to 100 ηm.
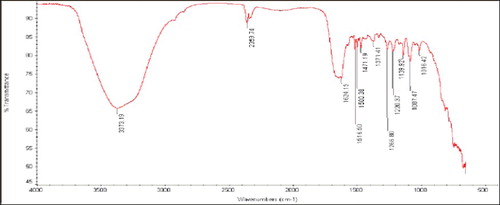
FTIR measurement was carried out to determine the capping agent, functional groups and possible mechanism behind AgNP formation (). A broad existence band was observed at 3500–3000 cm−1 (O–H bending and stretching); more bands observed at about 1743 cm−1 were assigned to C = O. The bands observed in the FTIR spectra at 1650 cm−1 were a definite indicator of amide I and amide II linkages [Citation25]. The following vibrations may be attributed to the oxidation of the hydroxyl groups in bacterial hydrolysates coupled to the reduction of silver ions [Citation24]. The FTIR spectrogram, which can be specific to the frequency of extending vibration of primary amines, could be the reason for the O–H stretching and N–H, O–H, = C–H, C = C and C = O functional groups, which may be present between the amino acid residues and protein during microbial AgNP synthesis. The FTIR spectrogram indicates the existence of protein as a capping agent enclosing the AgNPs [Citation13].
Figure 4. The Fourier transform infrared (FTIR) spectrums of silver nanoparticles synthesized by Bacillus aerius.
The morphology and size details of the biosynthesized AgNPs were analysed using TEM (). TEM images show that the biosynthesis-mediated AgNPs were triangular, hexagonal and spherical and had some undefined morphology with traces of agglomeration due to the binding of biological molecules with the nanoparticles present in the bacteria [Citation26]. The TEM study showed that the AgNPs ranged 20.13–21.87 nm in size.
Different phys-chemical parameters (temperature, reaction mixture pH, AgNO3 concentration), which had been identified as factors affecting AgNP yields [Citation27], were optimized.
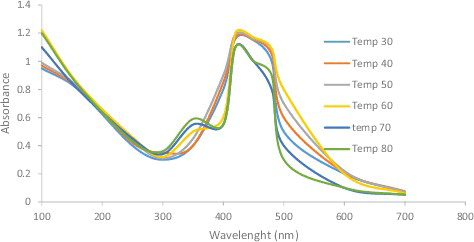
As illustrated in , the absorption peak was reached as the temperature increased, so the rate of AgNP synthesis was also increased, which was indicated by the rapid change in the colour of the reaction mixture. However, absorption was not increased at >70 °C. These results correlate completely with the work reported by Veerasamy et al. [Citation27], where increasing the temperature beyond 75 °C led to decreased absorption.
Figure 5. UV-visible spectra of silver nanoparticles obtained at different temperature of 30, 40, 50, 60, 70 and 80 °C.
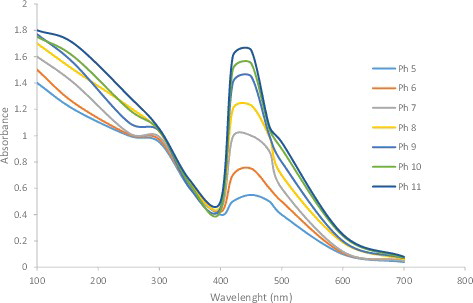
Verma and Mehata [Citation28] reported that altering the reaction mixture pH affects the shape and size of the nanoparticles, as pH can alter the charge of biomolecules, which might affect their capping as well as stabilizing abilities. shows that an acidic pH suppresses AgNP formation, while a basic pH increases it. In addition to the spectral shift, the absorption intensity increases with the pH. Nanoparticles were formed at a lower pH (pH 4), whereas small and highly dispersed nanoparticles were formed at a high pH (pH 8) [Citation27]. It has been stated [Citation28] that AgNP absorbance increases as the pH of the solution increases from 2 to 8; the absorbance decreases upon further increase of the solution pH.
Figure 6. UV-visible spectra of silver nanoparticles at different pH values (pH = 5–11) of the reaction mixture.
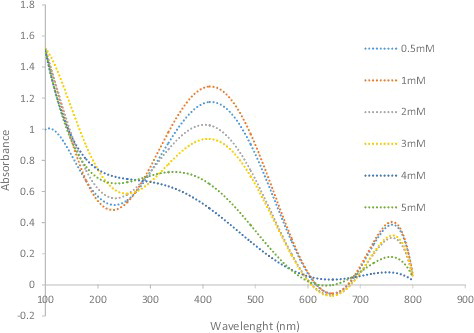
The reaction mixture AgNO3 concentration is the third factor affecting nanoparticle synthesis (). More AgNPs are formed as the AgNO3 concentration in the reaction mixture increases, until a maximum yield is achieved with 1 mmol/L AgNO3, where the absorption maximum shifts from 380 to 425 nm. No increase in absorption was observed beyond 1 mmol/L AgNO3.
Figure 7. UV-visible spectra of silver nanoparticles obtained at different silver nitrate concentrations of 0.5–5 mM.
The in vitro antimicrobial activity of the biosynthesized AgNPs against three Gram-positive and five Gram-negative bacteria are presented in .
Table 1. Antimicrobial activity of silver nanoparticles (AgNPs) synthesized by two bacterial isolates on some different pathogenic strains.
The highest antibacterial activity was observed against S. typhimurium and Sh. sonnei, followed by P. aeruginosa, S. epidermidis, S. aureus and K. oxytoca. These findings are in agreement with previous studies that examined the antibacterial activity of AgNPs against many human pathogens [Citation29]. Therefore, AgNPs could be considered excellent broad-spectrum antibacterial agents.
The mechanisms of the antimicrobial activity of the AgNPs remain to be identified. The literature states that AgNPs not only interact with the membrane surface, but may also penetrate a bacterial cell, causing cell death [Citation30]. We surmise that the observed effect of the AgNPs could be due to action along any of these reported pathways. The main drawback of using AgNPs is the accompanying nontoxicity, which can be overcome by using surface coatings. In the present work, biomolecules present in the bacterial supernatant acted as the surface coating material.
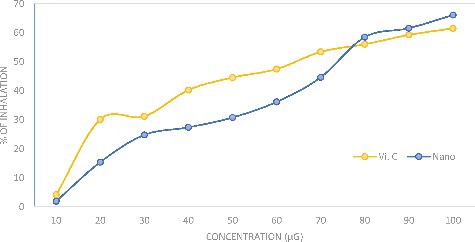
The antioxidant activity of the AgNPs is shown in as the DPPH inhibition percentage. The AgNPs were a potent free radical scavenger when compared with the ascorbic acid standard. The antioxidant activity of the AgNPs was 66.23% inhibition at 100 μg/mL and that for ascorbic acid was 61.14% inhibition. The principle of the DPPH assay is based on the reduction of DPPH in the presence of a hydrogen-donating antioxidant due to the formation of diphenylpicrylhydrazine, where sample compounds alter the DPPH colour due to their hydrogen-donating ability. The colour change of the violet DPPH to yellow clearly demonstrated the effect of AgNPs as an antioxidant. In a previous study [Citation11], silver phyto nanoparticles exhibited high antioxidant properties compared with the plant extract alone; in order to neutralize the free radicals, the antioxidant itself undergoes oxidation. The activity and stability of the AgNPs are affected during the antioxidation process and also they are oxidized in the presence of air; therefore, metal nanoparticles could be used for treating many diseases caused by oxidative stress.
Conclusions
Bionanotechnology renders nanoparticles safe, economical and more compatible for making a significant contribution to many medical applications such as anti-cancer, antibacterial and antiviral activities, and disease detection. Moreover, nanoparticle biosynthesis eliminates many problems originating from chemical synthesis methods. In the present study, about 20 microbial isolates were screened to investigate their ability to produce AgNPs. The AgNPs formed were characterized by UV-vis spectra, FTIR, TEM and EDX studies. The size of the AgNPs ranged 20.12–29.48 nm. UV-vis spectroscopy and FTIR analysis revealed the possibility of using proteins as a stabilizing agent in the AgNPs. In addition, AgNPs have potent and wider antimicrobial activities against S. typhimurium, Sh. sonnei, K. pneumonia, S. bovis, P. aeruginosa, S. aureus and E. coli. Accordingly, a minimum amount of these particles can be used to inhibit the growth of these pathogens without any harmful side effects, replacing conventional antibiotics. The nanoparticle production process described here is eco-friendly, as it is free from solvent or toxic chemicals and it is easily modified for large-scale production. The present study indicates that AgNPs can be applied as an effective antibacterial material against various micro-organisms that can cause disease in humans.
Acknowledgments
The authors acknowledge with thanks Deanship of Scientific Research (DSR) for technical and financial support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Galdiero S, Falanga A, Vitiello M, et al. Silver nanoparticles as potential antiviral agents. Molecules. 2011;16:8894–8918.
- Tiwari DK, Behari J, Sen P. Time and dose dependent antimicrobial potential of Ag nanoparticles synthesized by top-down approach. Curr Sci. 2008;95:647–655.
- Grass LRN, Athanassiou EK, Stark WJ. Bottom up fabrication of metal/metal nanocomposites from nanoparticles of immiscible metals. Chem Mater. 2010;22:155–160.
- Liu J, Qiao SZ, Hu QH, et al. Magnetic nanocomposites with mesoporous structures: synthesis and applications. Small. 2011;7:425–443.
- Petit C, Lixon P, Pileni MP. In situ synthesis of silver nanocluster in AOT reverse micelles. J Phys Chem. 1993;97:12974–12983.
- Pol VG, Srivastava DN, Palchik O, et al. Sonochemical deposition of silver nanoparticles on silica spheres. Langmuir. 2002;18:3352–335337.
- Solanki JN, Murthy ZVP. Highly monodisperse and sub-nano silver particles synthesis via microemulsion technique. Colloids Surf A. 2010;359:31–38.
- Li X, Wang L, Lu X. Preparation of silver-modified TiO 2 via microwave-assisted method and its photocatalytic activity for toluene degradation. J Hazard Mater. 2010;177:639–647.
- Kowshik M, Ashtaputre S, Kharrazi S, et al. Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology. 2003;14:95
- Jain D, Kachhwaha S, Jain R, et al. Novel microbial route to synthesize sliver nanoparticles using spore crystal mixture of Bacillus thuringiensis. Indian J Exp Biol. 2010;48:1152–1156.
- Bunghez IR, Barbinta patrascu ME, Badea N, et al. Antioxidant silver nanoparticles green synthesized using ornamental plants. J Optoelectron Adv Mater. 2012;14(11):1016–1022.
- El-Rafie HM, Hamed MA. Antioxidant and anti-inflammatory activities of silver nanoparticles biosynthesized from aqueous leaves extracts of four Terminalia species. Adv Nat Sci: Nanosci Nanotechnol. 2014;5:1–11.
- Vigneshwaran N, Ashtaputre NM, Varadarajan PV, et al. Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater Lett. 2007;61:1413–1418.
- Sadowski Z, Maliszewska I, Polowczyk I, et al. Biosynthesis of colloidal-silver particles using microorganisms. Polish J Chem. 2008;82:377–382.
- Sintubin L, De Windt W, Dick J, et al. Lactic acid bacteria as reducing and capping agent for the fast and efficient production of silver nanoparticles. Appl Microbiol Biotechnol. 2009;84:741–749.
- Jha AK, Prasad K, Kumar V, et al. Biosynthesis of silver nanoparticles using Eclipta leaf. Biotechnol Prog. 2009;25:1476–1479.
- Hu M, Chen J, Li ZY, et al. Gold nanostructures: engineering their plasmonic properties for biomedical applications. Chem Soc Rev. 2006;35:1084–1094.
- Jain PK, Huang X, El-Sayed IH, et al. Noble metals on the nanoscale: optical and photothermal properties and some applications in imaging, sensing, biology, and medicine. Acc Chem Res. 2008;41:1578–1586.
- Kumar A, Vemula PK, Ajayan PM, et al. Silver-nanoparticle-embedded antimicrobial paints based on vegetable oil. Nat Mater. 2008;7:236–241.
- Elbeshehy EKF, Elazzazy AM, Aggelis G. Silver nanoparticles synthesis mediated by new isolates of Bacillus spp., nanoparticle characterization and their activity against Bean yellow mosaic virus and human pathogens. Front Microbiol. 2015;6:453.
- Chen JC, Yeh JY, Chen PC, et al. Phenolic content and PPH radical scavenging activity of yam-containing surimi gels influenced by salt and heating. Asian J Health Inform Sci. 2007;2:1–11.
- Banu AN, Balasubramanian C. Extracellular synthesis of silver nanoparticles using Bacillus megaterium against malarial and dengue vector (Diptera: Culicidae). Parasitol Res. 2015;11:4069–4079.
- Shahverdi AR, Fakhimi A, Shahverdi SR, et al. Synthesis and effect of silver nanoparticles on the antibacterial activity of different antibiotics against Staphylococcus aureus and Escherichia coli. Nanomed. 2007;3:168–171.
- Ponarulselvam S, Panneerselvam C, Murugan K, et al. Synthesis of silver nanoparticles using leaves of Catharan thusroseus Linn. G. Don and their antiplasmodial activities. Asian Pac J Trop Biomed. 2013;574–580.
- Magudapathy P, Gangopadhyay P, Panigrahi BK, et al. Electrical transport studies of Ag nanocrystallites embedded in glass matrix. Phys. B: Condens Matter. 2001;299:142–146.
- Malarkodi C, Annadurai G. A novel biological approach on extracellular synthesis and characterization of semiconductor zinc sulfide nanoparticles. Appl Nanosci. 2013;3:389–395.
- Veerasamy R, Xin TZ, Gunasagaran S, et al. Biosynthesis of silver nanoparticles using mangosteen leaf extract and evaluation of their antimicrobial activities. J Saudi Chem Soc. 2011;15(2):113–120.
- Verma A, Mehata SM. Controllable synthesis of silver nanoparticles using Neem leaves and their antimicrobial activity. J Radiat Res Appl Sci. 2016;9:109–115.
- Sulaiman MH, Hiba TH, Maysoon MS. Biosynthesis of silver nanoparticles synthesized by Aspergillus flavus and their antioxidant, antimicrobial and cytotoxicity properties. Bull Mater Sci. 2015;38:639–644.
- Chen CW, Hsu CY, Lai SM, et al. Metal nano bullets for multidrug resistant bacteria and biofilms. Adv Drug Deliv Rev. 2014.