ABSTRACT
The plastidic ATP/ADP transporter (AATP) imports adenosine triphosphate (ATP) from the cytosol into plastids, resulting in an increase in the ATP supply to facilitate the anabolic synthesis in heterotrophic plastids of dicotyledonous plants. In this study, a gene encoding the AATP protein, StAATP, was successfully isolated from potato and transformed into Arabidopsis. Constitutive expression of StAATP significantly increased the starch accumulation in the transgenic plants. Real-time quantitative polymerase chain reaction analysis showed that constitutive expression of StAATP up-regulated the expression of key genes involved in the starch biosynthesis pathway in the transgenic Arabidopsis plants: phosphoglucomutase (AtPGM), ADP-glucose pyrophosphorylase (AGPase) small subunit (AtAGPase-S1 and AtAGPase-S2), AGPase large subunit (AtAGPase-L1 and AtAGPase-L2), granule-bound starch synthase (AtGBSS I and AtGBSS II), soluble starch synthases (AtSSS I, AtSSS II, AtSSS III and AtSSS IV) and starch-branching enzyme (AtSBE I and AtSBE II) genes. Furthermore, the major enzymes involved in starch biosynthesis (AGPase, GBSS, SSS and SBE) exhibited higher catalytic activities in the transgenic plants compared to the wild type. These results indicate that StAATP may improve the starch content of Arabidopsis by up-regulating the expression of the related genes and increasing the activities of the major enzymes involved in starch biosynthesis. All these findings suggest that the StAATP gene may be applied for increasing starch accumulation of plants in the future.
Introduction
Biofuel, which can decrease environmental damage by reducing the extraction and use of fossil fuels, is more and more important with the development of society. Breeding of non-food energy crops for economically viable production as environmentally friendly biofuels is on the way [Citation1]. Since the conversion of starch into fermentable sugars is relatively easy, starch has been taken as a major feedstock for first-generation biofuel production [Citation1,Citation2]. Therefore, deeper knowledge of how carbohydrates are metabolized in plants could greatly facilitate the development of crops by means of enhancing starch synthesis and the improvement of biofuel production efficiency [Citation1].
Starch is a main source of nutrition in the human and animal diet and is a major storage form of carbohydrates in plants [Citation3]. It plays a fundamental role in plant survival and adaptation to various environmental conditions [Citation4]. In plants, starch exists as an insoluble glucan comprised of two glucose polymers: amylose and amylopectin. Amylose is mainly comprised of linear chains that are linked by α-1,4-O-glycosidic bonds, whereas amylopectin is highly branched and contains 5%–6% α-1,6-O-glycosidic bonds to generate glucan branches of various lengths [Citation5]. Four major enzymes are involved in starch biosynthesis: adenosine diphosphate (ADP)-glucose pyrophosphorylase (AGPase), starch synthase (SS), starch-branching enzyme (SBE) and starch-debranching enzyme [Citation6].
The best option to enhance starch biosynthesis is by increasing the adenosine triphosphate (ATP) supply to the plastid in below-ground storage organs [Citation2]. ATP, as the basic energy currency in living cells, is needed for almost every step of biochemical reactions. Meanwhile, ATP is also an indispensable participant in the AGPase reaction, which is a rate-limiting step of starch biosynthesis catalysing the formation of ADP-glucose (ADPG) [Citation7]. ATP exchange between organelles and the cytosol is mediated by adenylate carrier proteins (ACPs). There are two types of ACPs. The mitochondrial ADP/ATP carrier (AAC), one type of ACPs, can export ATP, which is produced previously via oxidative phosphorylation in a one-to-one exchange of cytosolic ADP [Citation8]. The mitochondrial AAC is a dimer, and each monomer contains six transmembrane helices [Citation9]. Another type of ACPs is the plastidic ATP/ADP transporter protein (AATP) discovered in spinach chloroplasts. AATP, an important energy transporter, is generally found in the heterotrophic plastids (amyloplasts, chromoplasts and leucoplasts) of higher and lower land plants [Citation10–16]. Further studies found that its main function is involved in providing the plastid stroma with cytosolic ATP, to further participate in anabolic processes such as starch and fatty acid synthesis [Citation13].
There are a few reports about the function of AATP in starch biosynthesis in plants. Constitutive expression of the Arabidopsis AtAATP1 increased ADPG level up to twofold and starch content by 16%–36% [Citation17]. Down-regulation of the potato StAATP decreased ADPG level by 25%–70% and starch content by 19%–51% in potato tubers [Citation18]. Overexpression of IbAATP increased starch and amylose content in transgenic sweet potato [Citation19]. Ectopic expression of SlAATP from tomato increased the starch accumulation in the leaves of transgenic Arabidopsis plants [Citation20]. These results indicate that the manipulation of the enzymes that modulate the ATP supply in plastids is an effective way to enhance starch biosynthesis in plants.
In this study, we isolated StAATP (Genbank accession No. Y10821) from potato and studied its role in transgenic Arabidopsis. Ectopic expression of StAATP significantly increased the starch accumulation in the leaves of transgenic plants, indicating a great potential of StAATP in the development of high starch-accumulating plants.
Materials and methods
Plant materials
Potato (Solanum tuberosum) cultivar Zhongshu No. 5 (Huaiyin Institute of Technology, Huai'an, China) was employed for StAATP gene cloning in this study. Arabidopsis thaliana [ecotype Columbia-0, wild type (WT); Huaiyin Institute of Technology, Huai'an, China] was used as a model plant to investigate the functions of StAATP.
Cloning of the potato StAATP gene
Total RNA was extracted from 0.5 g of fresh leaves of four-week-old in vitro-grown plants of Zhongshu No. 5 with the RNAprep Pure Kit (Tiangen Biotech, Beijing, China). RNA samples were reverse-transcribed according to the instructions of the Quantscript Reverse Transcriptase Kit (Tiangen Biotech, Beijing, China). Based on the sequence of StAATP (Genbank accession No. XM_006345713), we designed gene-specific primers (GC-F/R) for reverse transcription polymerase chain reaction (RT-PCR) (Table S1 in the Online Supplementary Appendix) to obtain its full-length cDNA sequence. PCR was performed in a TaKaRa PCR Thermal Cycler Dice (TaKaRa, Beijing, China), with an initial denaturation step at 94 °C for 3 min, followed by 35 cycles of 94 °C for 30 s, 55 °C for 30 s, 72 °C for 1 min and final extension at 72 °C for 10 min. The PCR products were separated in a 1.0% (w/v) agarose gel. Target DNA bands were recovered by gel extraction, then cloned into PMD19-T (TaKaRa, Beijing, China) and finally transformed into competent cells of Escherichia coli strain DH5α (Transgen, Beijing, China). White colonies were checked by PCR and the positive colonies were sequenced (Invitrogen, Beijing, China). The genomic sequence of StAATP was amplified with primers GA-F/R (Table S1 in the Online Supplementary Appendix) using genomic DNA extracted from the leaves of Zhongshu No. 5 as a template.
Sequence analysis of the StAATP gene
The open-reading frame (ORF) of the cloned StAATP gene was predicted with ORF Finder (http://www.ncbi.nlm.nih.gov/projects/gorf/). The homology of the StAATP protein was identified using protein BLAST (Basic local alignment search tool) in the National Center for Biotechnology Information (NCBI) database (http://blast.ncbi.nlm.nih.gov/Blast.cgi). The conserved domain of the StAATP protein was scanned by the InterProScan Program (http://www.ebi.ac.uk/Tools/ pfa/iprscan/). The theoretical molecular weight and isoelectric point (pI) were calculated using the ProtParam tool (http://web.expasy.org/protparam/). The genomic structure of StAATP was analysed using the Spidey Program (http://www.ncbi.nlm.nih.gov/spidey/). The transmembrane helices in the StAATP protein were detected using the TMHMM Server (v. 2.0, http://www.cbs.dtu.dk/services/TMHMM/). For multiple sequence alignment analysis, the amino acid sequences of StAATP and other AATP homologs from different plant species retrieved from NCBI were aligned using the DNAMAN software (Lynnon Biosoft, Quebec, Canada). Phylogenetic analysis was conducted with the MEGA4 software (http://www.megasoftware.net/) [Citation21].
Vector construction and transgenic Arabidopsis generation
The coding region of StAATP was cut out from the PMD19-T vector with the restriction enzymes BamHI and SacI, and then inserted into the same enzyme sites in pCAMBIA1301 to create the plant expression vector pCAMBIA1301-StAATP, under the control of the cauliflower mosaic virus (CaMV) 35S promoter and the nopaline synthase (NOS) terminator. This vector also contains β-glucuronidase (gusA) and hygromycin resistance (hptII) genes driven by the CaMV 35S promoter. Both pCAMBIA1301-StAATP and the control vector (VC) pCAMBIA1301 were transformed into Agrobacterium tumefaciens strain LBA4404 cells (Transgen, Beijing, China) by the electroporation method for Arabidopsis transformation [Citation22]. Transgenic plants were produced according to methods described previously [Citation23]. Transformants were selected based on their resistance to hygromycin (Hyg). Putative transformant seeds were germinated on agar-solidified Murashige and Skoog (MS) [Citation24] medium containing 25 mg/L Hyg. Positive transgenic seedlings were grown in pots containing a mixture of soil, vermiculite and humus (1:1:1, v/v/v) for T2 and T3 seed selection. The incubation and growth conditions of Arabidopsis were the same as described previously [Citation23].
Molecular confirmation of transgenic plants
The presence of StAATP in hygromycin-resistant plants was assessed by PCR analysis using specific primers (Table S1 in the Online Supplementary Appendix) to amplify fragments of the hptII coding sequence. DNA was first extracted from Arabidopsis leaves according to the instructions of the EasyPure Plant Genomic DNA Kit (Transgen, Beijing, China). PCR amplifications were performed with an initial denaturation step at 94 °C for 3 min, followed by 35 cycles of 94 °C for 30 s, 55 °C for 30 s, 72 °C for 1 min and final extension at 72 °C for 10 min. The PCR products were separated by electrophoresis in a 1.0% (w/v) agarose gel.
Starch content assays
Starch extraction and quantification were performed as described previously [Citation25]. Seeds were grown on MS medium for two weeks and transferred to pots containing a mixture of soil, vermiculite and humus (1:1:1, v/v/v). Plants were grown in a growth chamber for four weeks at 22 °C under standard long day conditions (14 h light and 10 h dark). Leaves of four-week-old plants were harvested to determine the starch content in light at 10–11 am. All treatments were performed in triplicate.
Gene expression analysis
The expression of StAATP and starch biosynthesis-related genes was analysed by real-time quantitative PCR (qRT-PCR). Transgenic, VC and WT plants were grown in pots for four weeks under normal conditions. Total RNA was extracted from the leaves of these plants, respectively, using the RNAprep Pure Plant Kit (Tiangen Biotech, Beijing, China). RNA samples were reverse-transcribed using a Quantscript Reverse Transcriptase Kit (Tiangen Biotech, Beijing, China). The cDNA solution was used as templates for PCR amplification with gene-specific primers (Table S1 in the Online Supplementary Appendix). The Arabidopsis Atactin gene (Genbank accession No. NM112764) was used as an internal control (Table S1) [Citation26].
PCR amplifications were conducted by ABI PRISM 7500 (Software for 7500 and 7500 Fast Real-Time PCR Systems, V2.0.1, USA) using SYBR Green PCR Master Mix (Tiangen Biotech, Beijing, China). The amplifications were performed with initial denaturation at 95 °C for 10 min, followed by 40 cycles of 95 °C for 15 s and 60 °C for 1 min. Quantification of gene expression was done with the comparative CT method [Citation27]. All experiments were repeated three times and each data point represents the average from three experiments.
AGPase, GBSS, SSS and SBE activity analysis
The activity of four starch biosynthetic enzymes (AGPase, GBSS, SSS and SBE) in the leaves of four-week-old transgenic, VC and WT plants was performed according to the method described by Nakamura et al. [Citation28]. One unit of enzyme activity (AGPase, GBSS and SSS) was defined as the formation of 1 nmol ADP per minute at 30 °C and 1 unit of SBE activity was defined as the amount of enzyme required to increase the spectrophotometric absorbance by 1 unit in 1 min.
Statistical analysis
All experiments were repeated three times and the data are presented as mean values with standard error of the means (±SEM). Where applicable, data were analysed by Student's t-test in a two-tailed analysis. Values of P < 0.05 or P < 0.01 were considered to indicate statistically significant differences.
Results and discussion
Cloning and sequence analysis of StAATP
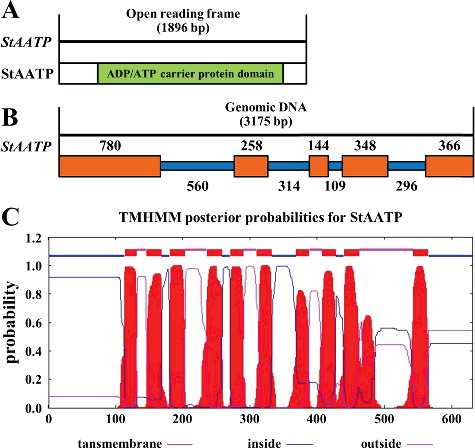
The StAATP (Genbank accession No. XP_004235723) gene was cloned by RT-PCR. StAATP contained an 1896 bp ORF, encoding a polypeptide of 631 amino acids, with a molecular weight of 68.89 kDa and a theoretical isoelectric point (pI) of 9.52. Sequence analysis via the InterProScan program (http://www.ebi.ac.uk/Tools/pfa/iprscan/) showed that the StAATP protein contained an AAC protein domain ((A)). The genomic sequence of StAATP was 3175 bp long and contained 5 exons and 4 introns ((B)). Similar to sweet potato IbAATP [Citation19] and tomato SlAATP [Citation20], StAATP was predicted to contain 10 transmembrane (TM) helices ((C)), according to the online software TMHMM (v. 2.0), which is a typical structural characteristic of the AATP family [Citation9,Citation16].
Figure 1. (Color online). Gene structure of plastidic ATP/ADP transporter protein from Solanum tuberosum (StAATP). Structure of StAATP and StAATP (A): the StAATP protein contained an ADP/ATP carrier protein domain. Exon/intron organization of the StAATP gene (B) with location of exons (brown boxes) and introns (blue lines): the genomic DNA of StAATP gene was 3175 bp. Prediction of transmembrane helices (C) in the StAATP protein.
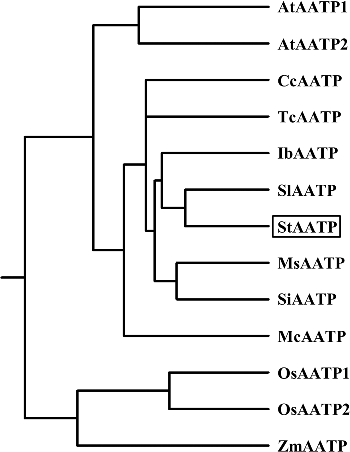
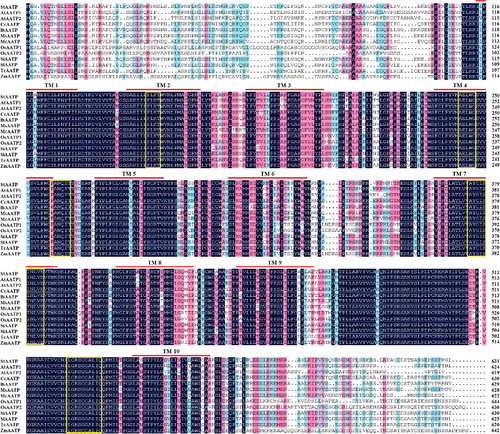
The alignment of the deduced amino acid sequence showed that StAATP was highly conserved and homologous to the AATPs from Arabidopsis thaliana (AtAATP1, 2), Coffea canephora (CcAATP), Ipomoea batatas (IbAATP), Mentha spicata (MsAATP), Mesembryanthemum crystallinum (McAATP), Oryza sativa (OsAATP1, 2), Sesamum indicum (SiAATP), Solanum lycopersicum (SlAATP), Theobroma cacao (TcAATP) and Zea mays (ZmAATP), especially in the TM regions (). Further homology analyses using DNAMAN showed that StAATP had 66.10% to 87.50% amino acid similarity to OsAATP1, OsAATP2, ZmAATP, AtAATP2, AtAATP1, McAATP, TcAATP, CcAATP, IbAATP, MsAATP, SiAATP and SlAATP (). In agreement with our previous studies [Citation19,Citation20], five highly conserved motifs [FLKT, AELWG, FANQIT, AYG(I/V)S(I/V)NLVE and (L/I)GKSGGA(L/I)IQ] present in the plant and bacterial AATPs were also identified (), providing further support to the hypothesis that these could be considered key motifs for AATP function [Citation13,Citation15]. Phylogenetic analyses revealed that StAATP had a close relationship with SlAATP ().
Figure 2. (Color online). Multiple sequence alignment of AATP proteins from Solanum tuberosum (StAATP), Arabidopsis thaliana (AtAATP1, 2), Coffea canephora (CcAATP), Ipomoea batatas (IbAATP), Mentha spicata (MsAATP), Mesembryanthemum crystallinum (McAATP), Oryza sativa (OsAATP1, 2), Sesamum indicum (SiAATP), Solanum lycopersicum (SlAATP), Theobroma cacao (TcAATP) and Zea mays (ZmAATP).
Increased starch content in Arabidopsis expressing StAATP
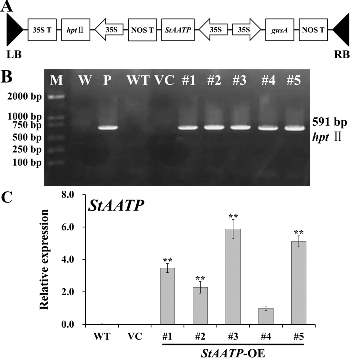
The ORF of StAATP was ectopically expressed in Arabidopsis (Col-0, WT) using the binary vector pCAMBIA1301-StAATP ((A)). Five independent transgenic lines constitutively expressing StAATP (T1 generation) were obtained by Hyg-resistance selection, named #1–#5, respectively, and their progenies (T3 generation) were generated. PCR analyses of genomic DNA confirmed the successful integration of the transgene ((B)). qRT-PCR analyses showed that the highest expression levels of StAATP were observed in transgenic lines #3 and #5, while no transgene expression was observed in VC and WT ((C)). Therefore, transgenic lines #3 and #5 were selected for further analyses.
Figure 4. Molecular confirmation of transgenic plants. Schematic diagram (A) of the T-DNA region of binary plasmid pCAMBIA1301-StAATP: LB, left border; RB, right border; hptII, hygromycin phosphotransferase II gene; StAATP, potato plastidic ATP/ADP transporter protein gene; gusA, β-glucuronidase gene; 35S, cauliflower mosaic virus (CaMV) 35S promoter; 35S T, CaMV 35S terminator; NOS T, nopaline synthase terminator. PCR analysis (B) of StAATP-expressing Arabidopsis plants: Lane M, DL2000 DNA marker (Transgen, Beijing, China); Lane W, water as a negative control; Lane P, plasmid pCAMBIA1301-StAATP as a positive control; Lane WT, wild type; VC, control vector; Lanes #1–#5, different transgenic lines. Expression levels (C) of StAATP in different transgenic lines. The Arabidopsis actin gene was used as an internal control.
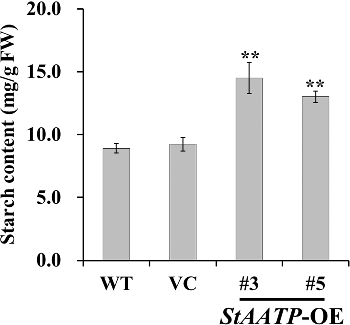
Starch is the major dietary source of carbohydrates and the most abundant storage of polysaccharides in higher plants. The AATP gene has been shown to be involved in starch accumulation in plants [Citation17–20]. In our work, two-week-old WT, VC and transgenic plants (lines #3 and #5) were grown in pots under normal condition for four weeks. Expression of StAATP did not change the growth of transgenic plants, since no significant differences in growth were observed between WT, VC and the transgenic plants under normal conditions. However, the starch content in the leaves of these plants was different. The starch content in StAATP expressing plants increased 66%–87% compared to that in WT (), whereas no significant difference was observed between VC and WT plants ().
Enhanced starch biosynthetic gene transcription and enzyme activity in Arabidopsis expressing StAATP
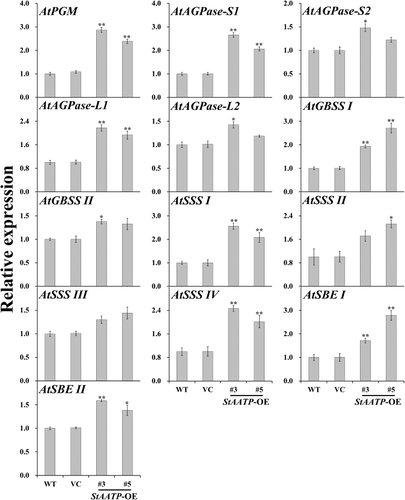
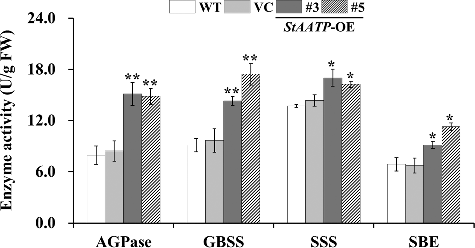
To dissect how the expression of StAATP increased the starch content in transgenic plants, the transcript levels of 13 starch biosynthetic genes in WT, VC and transgenic plants (lines #3 and #5) were examined by qRT-PCR (). The activity of major enzymes (AGPase, GBSS, SSS and SBE) involved in starch biosynthesis was also investigated in the leaves of WT, VC and transgenic plants (lines #3 and #5) ().
Figure 7. AGPase, GBSS, SSS and SBE enzyme activity assays in the leaves of WT and transgenic plants.
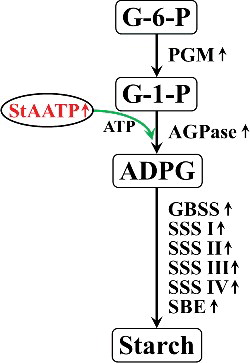
The carbon precursors and ATP are mainly imported from the cytoplasm to be involved in anabolic processes in heterotrophic organs [Citation9,Citation12]. Increasing the levels of ATP or ADPG are important targets in genetic manipulation, in order to attempt to enhance starch biosynthesis in amyloplasts. ATP uptake by amyloplasts is mediated by a plastidic AATP [Citation11]. Ectopic expression of AtAATP1 led to higher levels of ATP imported from the cytosol into the stroma, which accelerated the increase in ADPG, resulting in an increase in starch accumulation in tubers [Citation17]. In the present study, the transgenic Arabidopsis plants expressing StAATP exhibited increased starch content (). It is thought that constitutive expression of StAATP could also increase the ATP import levels into amyloplasts, further activating the key AGPase reaction in starch biosynthesis, leading to the increase in the starch content in the transgenic plants.
Furthermore, we found that the expression of AtAGPase-S1 and AtAGPase-S2, encoding the AGPase small subunit, and AtAGPase-L1 and AtAGPase-L2, encoding the AGPase large subunit, was up-regulated in transgenic plants (). Consistently, AGPase activity was also significantly increased in the StAATP-expressing Arabidopsis plants (). These results showed that ADPG, as a large amount of the ultimate precursor for starch synthesis, could be accumulated to a greater extent (). Meanwhile, the consumption of glucose-1-phosphate (G-1-P) in the AGPase reaction required the accelerated conversion of glucose-6-phosphate (G-6-P) to G-1-P, which was catalysed by phosphoglucomutase (PGM) [Citation29], and consequently, the transcription level of AtPGM was increased in the transgenic plants (). These results support our previous suggestion that expression of IbAATP/SlAATP increased starch accumulation by the up-regulation of starch biosynthetic-related genes [Citation19,Citation20], by showing that expression of StAATP enhances the starch biosynthesis due to the increased ATP supply into amyloplasts, which further increase the production of precursors (ADPG and G-1-P) and the expression of starch biosynthesis-related genes ().
The higher level of starch content is related to the increased expression of starch biosynthesis genes [Citation5,Citation30]. SS can be grouped into five types, granule-bound starch synthase (GBSS) and four types of soluble starch synthases (SSS): SSS I, SSS II, SSS III and SSS IV [Citation5,Citation6,Citation31]. A large body of evidence has illustrated that up-regulation of these genes can increase starch accumulation in plants [Citation30–36]. In our study, systematic up-regulation of these genes (AtGBSS I, AtGBSS II, AtSSS I, AtSSS II, AtSSS III, AtSSS IV, AtSBE I and AtSBE II) involved in the starch biosynthesis pathway was observed in transgenic plants (). Consistent with this phenomenon, the activities of the major enzymes (GBSS, SSS and SBE) involved in starch biosynthesis were also increased in transgenic plants (). Therefore, it is thought that constitutive expression of StAATP increased the expression of the genes and the activity of the major enzymes involved in starch biosynthesis, leading to the increased starch accumulation in transgenic plants (). These foundings indicate that the StAATP gene may be applied for increasing starch accumulation in plants in the future.
Conclusions
The StAATP gene was successfully isolated from potato. Constitutive expression of StAATP significantly increased the starch accumulation in transgenic Arabidopsis plants. Our results suggest that StAATP has potential in the engineering of alternative energy crop plants with increased starch accumulation.
Supplementary_Data.pdf
Download PDF (148.8 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Sanz-Barrio R, Corral-Martinez P, Ancin M, et al. Overexpression of plastidial thioredoxin f leads to enhanced starch accumulation in tobacco leaves. Plant Biotechnol J. 2013;11:618–627.
- Smith AM. Prospects for increasing starch and sucrose yields for bioethanol production. Plant J. 2008;54:546–558.
- Blennow A, Jensen SL, Shaik SS, et al. Future cereal starch bioengineering cereal ancestors encounter gene technology and designer enzymes. Cereal Chem. 2013;90:274–287.
- Skryhan K, Cuesta-Seijo JA, Nielsen MM, et al. The role of cysteine residues in redox regulation and protein stability of Arabidopsis thaliana starch synthase 1. PLoS One [ Internet]. 2015[ cited 2016 Aug 8];10:e0136997. Available from: http://journals.plos.org/plosone/article?id=10.1371/journal.pone.0136997.
- Delvallé D, Dumez S, Wattebled F, et al. Soluble starch synthase I: a major determinant for the synthesis of amylopectin in Arabidopsis thaliana leaves. Plant J. 2005;43:398–412.
- Fujita N, Yoshida M, Asakura N, et al. Function and characterization of starch synthase I using mutants in rice. Plant Physiol. 2006;140:1070–1084.
- Jeon JS, Ryoo N, Hahn TR, et al. Starch biosynthesis in cereal endosperm. Plant Physiol Bioch. 2010;48:383–392.
- Fiore C, Trézéguet V, Saux AL, et al. The mitochondrial ADP/ATP carrier: structural, physiological and pathological aspects. Biochimie. 1998;80:137–150.
- Winkler HH, Neuhaus HE. Non-mitochondrial ATP transport. Trends Biochem Sci. 1999;24:64–68.
- Heldt HW. Adenine nucleotide translocation in spinach chloroplasts. FEBS Lett. 1969;5:11–14.
- Schünemann D, Borchert S, Flügge UI, et al. ADP/ATP translocator from pea root plastids-comparison with translocators from spinach chloroplasts and pea leaf mitochondria. Plant Physiol. 1993;103:131–137.
- Emes MJ, Neuhaus HE. Metabolism and transport in non-photosynthetic plastids. J Exp Bot. 1997;48:1995–2005.
- Möhlmann T, Tjaden J, Schwöppe C, et al. Occurrence of two plastidic ATP/ADP transporters in Arabidopsis thaliana L-Molecular characterization and comparative structural analysis of similar ATP/ADP translocators from plastids and Rickettsia prowazekii. Eur J Biochem. 1998;252:353–359.
- Linka N, Hurka H, Lang BF, et al. Phylogenetic relationships of non-mitochondrial nucleotide transport proteins in bacteria and eukaryotes. Gene. 2003;306:27–35.
- Meng K, Chang TJ, Liu X, et al. Cloning and expression pattern of a gene encoding a putative plastidic ATP/ADP transporter from Helianthus tuberosus L.. J Integr Plant Biol. 2005;47:1123–1132.
- Yuen CYL, Leelapon O, Chanvivattana Y, et al. Molecular characterization of two genes encoding plastidic ATP/ADP transport proteins in cassava. Biol Plantarum. 2009;53:37–44.
- Tjaden J, Möhlmann T, Kampfenkel K, et al. Altered plastidic ATP/ADP-transporter activity influences potato (Solanum tuberosum L.) tuber morphology, yield and composition of tuber starch. Plant J. 1998;16:531–540.
- Geigenberger P, Stamme C, Tjaden J, et al. Tuber physiology and properties of starch from tubers of transgenic potato plants with altered plastidic adenylate transporter activity. Plant Physiol. 2001;125:1667–1678.
- Wang YN, Li Y, Zhang H, et al. A plastidic ATP/ADP transporter gene, IbAATP, increases starch and amylose content and alters starch structure in transgenic sweetpotato. J Integr Agr. 2016;15:1968–1982.
- Wang FB, Ye YX, Niu Y, et al. A tomato plastidic ATP/ADP transporter gene SlAATP increases starch content in transgenic Arabidopsis. Physiol Mol Biol Plants. 2016;22:497–506.
- Tamura K, Dudley J, Nei M, et al. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599.
- Lou XM, Yao QH, Zhang Z, et al. Expression of human hepatitis B virus large surface antigen gene in transgenic tomato. Clin Vaccine Immunol. 2007;14:464–469.
- Zhang X, Henriques R, Lin SS. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat Protoc. 2006;1:641–646.
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497.
- Smith AM, Zeeman SC. Quantification of starch in plant tissues. Nat Protoc. 2006;1:1342–1345.
- Li X, Ma H, Huang H, et al. Natural anthocyanins from phytoresources and their chemical researches. Nat Prod Res. 2013;27:456–469.
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative CT method. Nat Protoc. 2008;3:1101–1108.
- Nakamura Y, Yuki K, Park SY, et al. Carbohydrate metabolism in the developing endosperm of rice grains. Plant Cell Physiol. 1989;30:833–839.
- Harrison CJ, Mould RM, Leech MJ, et al. The rug3 locus of pea encodes plastidial phosphoglucomutase. Plant Physiol. 2000;122:1187–1192.
- Jiang T, Zhai H, Wang FB, et al. Cloning and characterization of a carbohydrate metabolism-associated gene IbSnRK1 from sweetpotato. Sci Hortic. 2013;158:22–32.
- Szydlowski N, Ragel P, Raynaud S, et al. Starch granule initiation in Arabidopsis requires the presence of either class IV or class III starch synthase. Plant Cell. 2009;21:2443–2457.
- Burton RA, Jenner H, Carrangis L, et al. Starch granule initiation and growth are altered in barley mutants that lack isoamylase activity. Plant J. 2002;31:97–112.
- Bustos R, Fahy B, Hylton CM, et al. Starch granule initiation is controlled by a heteromultimeric isoamylase in potato tubers. PNAS. 2004;101:2215–2220.
- Roldan I, Wattebled F, Lucas MM, et al. The phenotype of soluble starch synthase IV defective mutants of Arabidopsis thaliana suggests a novel function of elongation enzymes in the control of starch granule formation. Plant J. 2007;49:492–504.
- Wang FB, Guo XT, Qiao XQ, et al. The maize plastidic thioredoxin F-type gene ZmTrxF increases starch accumulation in transgenic Arabidopsis. Sci Hortic. 2016;210:205–212.
- Wang FB, Kong WL, Fu YR, et al. Constitutive expression of SlTrxF increases starch content in transgenic Arabidopsis. Biol Plantarum. 2016. DOI:10.1007/s10535-016-0675-6