 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
In this study, the phytochemical profile of extracts from the flowers of Bromelia laciniosa was characterized. The total phenolic and flavonoids contents were determined and the antioxidant and photoprotective activities were evaluated using in vitro assays. Phytochemical analysis demonstrated that the extracts were found to be positive for the presence of anthracene derivatives, anthraquinones, coumarins, flavonoids and tannins, lignans, mono and diterpenes. The ethanol extract (Bl-EtOH) presented the most significant total phenolic content (53.75 ± 1.25 mg GAE/g). The dichloromethane extract (Bl-CH2Cl2) showed the highest flavonoid content (46.06 ± 1.18 mg CE/g). The methanol extract (Bl-MeOH) was the most effective extract in the DPPH (2,2-diphenyl-1-picrylhydrazil) assay (EC50 141.90 ± 3.01 μg/mL). Bl-EtOH and Bl-MeOH showed higher sun protection factor at a concentration of 100 mg/L (3.38 ± 0.04 and 3.78 ± 0.11, respectively). Therefore, the extracts proved to be interesting for the development of new studies aiming their incorporation into photoprotective cosmetic formulations.
Introduction
The incidence of skin cancer and photoaging induced by solar radiation grows around the world. This is due to the prolonged exposure to ultraviolet (UV) radiation, which is normally divided into UVA (from 320 to 400 nm), UVB (from 290 to 320 nm) and UVC (from 100 to 290 nm) [Citation1]. UVA and UVB radiations are associated with cumulative damage to the skin, especially the triggering of the inflammatory process characterized by the development of erythema or burns in variable levels, oedema, heat and elevated levels of substances such as prostaglandins and leukotrienes [Citation1,Citation2].
An alternative to combat the damage caused by UV radiation is the search for molecules with antioxidant and photoprotective potential [Citation3,Citation4]. In this context, plants have provided a wide variety of phenolic compounds with antioxidant and photoprotective activity, especially flavonoids. These compounds have been used by the pharmaceutical industry for incorporation into cosmetic formulations for topical use [Citation5].
The Bromeliaceae family is distributed predominantly in the neotropical region and comprises approximately 3172 species belonging to 58 genera [Citation6]. Plants from this family have been studied as sources of a range of phytochemical compounds, such as flavonoids, triterpenoids, steroids, diterpenes, cinnamic acid derivatives, lignans and nitrogen compounds, among others [Citation7]. Our research group has demonstrated that extracts from Bromeliaceae species are source of chemically defined molecules with antinociceptive [Citation8], antioxidant [Citation9,Citation10], antimicrobial [Citation9,Citation10], photoprotective [Citation5,Citation11] and gastroprotective [Citation12,Citation13] activities.
Bromelia laciniosa is a Bromeliaceae species known under the common names ‘macambira’ and ‘macambira-de-porco.’ This plant is used in popular medicine against intestinal disorders and as a natural diuretic [Citation7], but to the best of our knowledge, there are no chemical and pharmacological studies of this species until now. Thus, this study presents the phytochemical profile, photoprotective and antioxidant potential of flowers extracts of B. laciniosa.
Materials and methods
Plant material
The flowers of Bromelia laciniosa Mart. ex Schult. f. were collected in the city of Petrolina (coordinates: S 08°59′16.90″; W 40°35′20.60″), State of Pernambuco, Brazil, in January 2013. The samples were identified by André Paviotti Fontana, a botanist from Centro de Recuperação de Áreas Degradadas da Caatinga (CRAD). A voucher specimen (6442) was deposited at the Herbário Vale do São Francisco of the Universidade Federal do Vale do São Francisco.
Extraction
The dried and pulverized flowers of B. laciniosa (40.12 g) were subjected to maceration initially with dichloromethane (500 mL) for 72 h. Then, the solution was removed, filtered and concentrated under reduced pressure on a rotatory evaporator at 50 °C, producing 1.33 g of dichloromethane extract (Bl-CH2Cl2, 3.31%). The plant material was subjected to a second extraction by maceration, using ethanol (500 mL) as solvent also for 72 h. Then, the solution was removed, filtered and concentrated, resulting in 1.78 g of ethanol extract (Bl-EtOH, 4.44%). Finally, the plant material was submitted to a final maceration (72 h) using methanol (500 mL) as solvent. The extraction solution was removed and processed as the other, resulting in 4.10 g of methanol extract (Bl-MeOH, 10.22%). All solvents used were purchased from Synth® (Diadema, Brazil).
Phytochemical analysis
Extract solutions (1 mg/mL, in CHCl3) were evaluated on thin-layer chromatography (TLC) plates of silica gel 60 F254 in aluminium supports (Merck®, São Paulo, Brazil), applied with a micropipette and eluted in different solvent systems as described by Wagner and Bladt [Citation14], seeking to highlight the main groups of secondary metabolism (). After elution, the plates were visualized under a UV camera at 254 and 365 nm wavelengths. Specific revelators were used for each secondary metabolite class to ensure a better assessment. The presence of the phytochemicals in the extracts was evaluated based on the spots profile, comparing with standards (quercetin, coumaric acid, lupeol, harmane, sitosterol), whenever possible. A photographic register of the most interesting plates was made.
Table 1. Elution systems and revelators used to characterize the main secondary metabolites from the extracts of flowers of B. laciniosa by TLC.
Determination of total phenolic content
Total phenolic content was assayed using the Folin--Ciocalteu reagent, based on the method reported by Slinkard and Singleton [Citation15] and only the volumes have been adjusted [Citation16]. An aliquot (40 μL) of suitably diluted Bl-CH2Cl2, Bl-EtOH and Bl-MeOH extracts was added to 3.16 mL of distilled water and 200 μL of the Folin–Ciocalteu reagent and was mixed well. The mixture was shaken and allowed to stand for 6 min before adding 600 μL of sodium carbonate solution, and was shaken to mix. The solutions were left at 25 °C for 2 h and the absorbance of each solution was determined at 765 nm against the blank and plot absorbance vs. concentration. Total phenolic contents of the extracts (three replicates per treatment) were expressed as milligrams of gallic acid equivalents per gram (mg GAE/g) through the gallic acid calibration curve. The calibration curve range was 50–1000 mg/L (R2 = 0.9998). All measurements were performed in triplicates.
Determination of total flavonoid content
Total flavonoid content was determined by using a colorimetric method described previously [Citation17]. Briefly, 0.30 mL of the Bl-CH2Cl2, Bl-EtOH and Bl-MeOH extracts, or (+)−catechin standard solution was mixed with 1.50 mL of distilled water in a test tube followed by the addition of 90 μL of a 5% NaNO2 solution. After 6 min, 180 μL of a 10% AlCl3·6H2O solution was added and allowed to stand for another 5 min before 0.6 mL of 1 mol/L NaOH was added. The mixture was brought to 330 μL with distilled water and mixed well. The absorbance was measured immediately against the blank at 510 nm using a spectrophotometer (Quimis®, Diadema, Brazil) in comparison with the standards prepared similarly with known (+)−catechin concentrations. The results were expressed as milligrams of catechin mg CE/g of extracts through the catechin calibration curve (R2 = 0.9948). The calibration curve range was 50–1000 mg/L. All measurements were performed in triplicates.
In vitro antioxidant activity
DPPH radical assay
The free radical scavenging activity was measured using the 2,2-diphenyl-1-picrylhydrazil (DPPH) assay [Citation18]. Sample stock solutions (1.0 mg/mL) of extracts were diluted to final concentrations of 243, 81, 27, 9, 3 and 1 μg/mL, in ethanol. One millilitre of a 50 μg/mL DPPH ethanol solution was added to 2.5 mL of sample solutions of different concentrations, and allowed to react at room temperature. After 30 min, the absorbance values were read at 518 nm and converted into percentage antioxidant activity (AA) using the following formula: AA% = ((absorbance of the control − absorbance of the sample)/absorbance of the control) x 100. Ethanol (1.0 mL) plus plant extracts solutions (2.5 mL) were used as a blank. Subsequently, the percentage of antioxidant activity was converted to EC50 (half maximal effective concentration). DPPH solution (1.0 mL) and ethanol (2.5 mL) were used as a negative control. The positive controls were ascorbic acid, butyl-hydroxyanisole (BHA) and butyl-hydroxytoluene (BHT) standard solutions. Assays were carried out in triplicate.
β-Carotene bleaching assay
The β-carotene bleaching method is based on the loss of the yellow colour of β-carotene due to its reaction with radicals formed by linoleic acid oxidation in an emulsion [Citation19]. The rate of β-carotene bleaching can be slowed down in the presence of antioxidants. β-carotene (2 mg) was dissolved in 10 mL chloroform and to 2 mL of this solution, linoleic acid (40 µL) and Tween 40 (400 µL) were added. Chloroform was evaporated under vacuum at 40 °C and 100 mL of distilled water was added, then the emulsion was vigorously shaken for 2 min. Reference compounds (ascorbic acid, BHA and BHT) and sample extracts were prepared in ethanol. The emulsion (3.0 mL) was added to a tube containing 0.12 mL of 1 mg/mL solutions of reference compounds and sample extracts. The absorbance was immediately measured at 470 nm and the test emulsion was incubated in a water bath at 50 °C for 120 min, when the absorbance was measured again. Ascorbic acid, BHA and BHT were used as positive control. In the negative control, the extracts were substituted with an equal volume of ethanol. The antioxidant activity (%) was evaluated in terms of the bleaching of β-carotene, using the following formula: % antioxidant activity = (1 − (A0– At)/(A00 – At0)) x 100, where A0 is the initial absorbance and At is the final absorbance measured for the test sample, A00 is the initial absorbance and At0 is the final absorbance measured for the negative control (blank). The results are expressed as percentage of antioxidant activity (% AA). Tests were carried out in triplicate.
In vitro photoprotective activity
Maximum absorption wavelength and sun protection factor (SPF) determination
In this assay, the extracts were diluted in absolute ethanol, obtaining final concentrations of 100 mg/L. Subsequently, spectrophotometric scanning was performed at wavelengths between 260 and 400 nm, with intervals of 5 nm. The readings were carried out using a 1-cm quartz cell, and ethanol was used as blank [Citation20]. Calculation of sun protection factor (SPF) was obtained according to the equation developed by Mansur et al. [Citation21]:where EE(λ) is the erythemal effect spectrum; I(λ) is the solar intensity spectrum; Abs(λ) is the absorbance of sunscreen product and CF is the correction factor (CF = 10). The values of EE x I are constants. They were determined by Sayre et al. [Citation22]. Benzophenone-3 (10 mg/mL) and quercetin (10 mg/L) were used as positive control.
Statistical analysis
The data obtained were analysed using the GraphPad Prism® version 6.0 and expressed as mean values with standard deviation (±SD). The EC50 values were calculated by nonlinear regression. Statistically significant differences were calculated using one-way analysis of variance (ANOVA) followed by Tukey's test. Values were considered significantly different at p < 0.05.
Results and discussion
Preliminary phytochemical analysis demonstrated that all extracts were positive for the presence of anthraquinones, coumarins, flavonoids and tannins and lignans (). Flavonoids and tannins were considered the main class of secondary metabolites identified in the extracts. After staining with NEU's reagent, the chromatographic plate showed strong absorption at a wavelength of 365 nm (). Bl-MeOH and Bl-CH2Cl2 also showed positive reaction for the presence of anthracene derivatives and mono- and diterpenes, respectively. These results are in agreement with the major classes of secondary metabolites found in the Bromeliaceae family [Citation7].
Table 2. Phytochemical characterization of extracts (1 mg/mL) from the flowers of B. laciniosa by TLC analysis.
Figure 1. Characterization of the presence of flavonoids and tannins in extracts of flowers of B. laciniosa after staining with NEU's reagent and visualization in a UV camera (365 nm): (1) Bl-EtOH; (2) Bl-CH2Cl2; (3) Bl-MeOH; (4) quercetin standard.
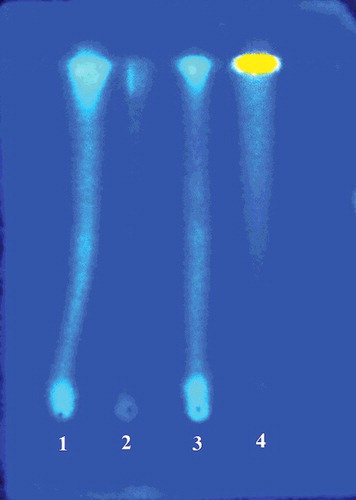
The phenolic content varied between 10.83 ± 1.91 and 53.75 ± 1.25 mg GAE/g for Bl-CH2Cl2 and Bl-EtOH extracts, respectively. The level of flavonoids varied from 11.59 ± 3.11 to 46.06 ± 1.18 mg CE/g for Bl-MeOH and Bl-CH2Cl2, respectively. In relation to the antioxidant activity in vitro, Bl-MeOH exhibited the most significant activity among the extracts, presenting EC50 of 141.90 ± 3.01 mg/mL. Ascorbic acid was the most effective antioxidant in this test (EC50 8.81 ± 0.27 mg/mL). The antioxidant activity of the extracts was also evaluated by the β-carotene/linoleic acid bleaching method. In this model, the extracts showed moderate to weak antioxidant activity (10.19%–17.88%), and the most active extract was Bl-EtOH ().
Table 3. Total phenolics (TP), total flavonoids (TF)a and antioxidant activity in vitro of extracts from the flowers of B. laciniosa.
Historically, the chemistry of natural products has contributed to the search for antioxidant molecules to combat several diseases. Most of these molecules were obtained from plants. As noted previously, B. laciniosa extracts showed relevant antioxidant activity in the DPPH radical assay. This result could probably be explained by the phenolic content determined, as reported in other previous studies [Citation16,Citation23,Citation24]. In fact, there are other Bromeliaceae species besides B. laciniosa that present satisfactory antioxidant activity. Studies performed with extracts from the leaves and flowers of Neoglaziovia variegata showed significant antioxidant activity in the DPPH and β-carotene assays [Citation5,Citation10]. Similar results were observed for Encholirium spectabile extracts, which have demonstrated relevant antioxidant activity, possibly related to the total phenolic and flavonoid contents [Citation9].
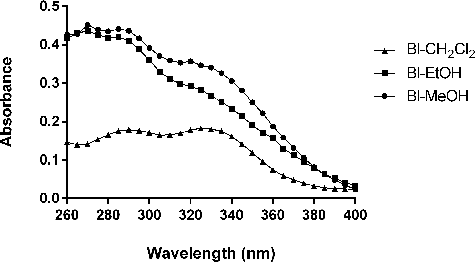
shows the spectrophotometric absorption profile of B. laciniosa extracts. It was observed that Bl-CH2Cl2, Bl-EtOH and Bl-MeOH showed characteristic absorption bands in the UVB and UVA regions, suggesting a possible photoprotective potential. The maximum absorption wavelength (λmax) for Bl-CH2Cl2, Bl-EtOH and Bl-MeOH was 325, 270 and 270 nm, respectively.
Figure 2. Spectrophotometric absorption spectra (260–400 nm) of Bl-CH2Cl2, Bl-EtOH and Bl-MeOH (100 mg/mL).
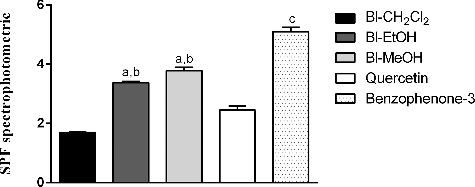
SPF in vitro was determined by the spectrophotometric method developed by Mansur et al. [Citation21] using the UVB region, considered to be the region of greatest incidence during the day, i.e. the region to which people are exposed for longer [Citation1]. In , it is possible to observe that the Bl-EtOH and Bl-MeOH extracts showed higher SPF (3.38 ± 0.04 and 3.78 ± 0.11, respectively, at a concentration of 100 mg/mL) than quercetin (2.45 ± 0.13, at a concentration of 10 mg/mL), a flavonoid with known photoprotective activity. Bl-CH2Cl2 showed a low value of SPF (1.69 ± 0.02) and benzophenone-3 was considered the most effective photoprotective agent (5.09 ± 0.14).
Figure 3. Spectrophotometric determination of SPF of B. laciniosa extracts (Bl-CH2Cl2, Bl-EtOH and Bl-MeOH, 100 mg/mL) and standards (quercetin and benzophenone-3, 10 mg/mL).
The presence of flavonoids in the extracts can also justify the observed photoprotective activity. In general, the UV–Vis absorption spectrum of flavonoids shows two peaks of maximum absorption (240–280 nm and 300–550 nm), similar to what was presented by B. laciniosa extracts [Citation25]. Therefore, the flavonoids content produced by plants is considered an important protection factor against UV radiation [Citation26]. Furthermore, although the test is carried out in vitro, this method correlates well with in vivo tests, since it relates the absorbance of the substance of interest with the erythematogenic effect of radiation and the intensity of UVB light at specific wavelengths (between 290 and 320 nm) [Citation20].
The discovery of natural compounds as photoprotective agents has increasingly contributed to obtaining new pharmaceuticals [Citation27,Citation28]. Recent studies have demonstrated that N. variegata and E. spectabile extracts also have relevant photoprotective effect and are considered promising for incorporation into cosmetic formulations [Citation5,Citation11]. This shows the evident presence of flavonoids in Bromeliaceae species as antioxidant and photoprotective molecules. In addition, these compounds have been considered chemotaxonomic markers of this family, justifying their biological properties. In this context, the present study highlights the importance of Bromeliaceae species, especially B. laciniosa, as a promising source of new photoprotective and antioxidant molecules.
Conclusions
This study reported the phytochemical screening of B. laciniosa as well as the determination of the phenolic and flavonoids content, which may be linked to the antioxidant and photoprotective activities observed for the tested extracts. Compared to the literature data, the extracts also proved to be interesting for designing new studies aiming incorporation of the extracts into photoprotective cosmetic formulations.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Dutra EA, Oliveira DAGC, Kedor-Hackmann ERM, et al. Determination of sun protection factor (SPF) of sunscreens by ultraviolet spectrophotometry. Rev Bras Cienc Farm. 2004;40:381–385.
- Vilela FMP, Fonseca YM, Vicentini FTMC, et al. Determination of three ultraviolet filters in sunscreen formulations and from skin penetration studies by high-performance liquid chromatography. Quim Nova. 2011;34:879–883.
- Oliveira-Junior RG, Almeida JRGS. Prospecção tecnológica de fotoprotetores derivados de produtos naturais. Geintec. 2013;3:32–40.
- Lima-Saraiva SRG, Guimarães AL, Oliveira AP, et al. Antioxidant activity and acute toxicity of Neoglaziovia variegata (Bromeliaceae). Afr J Biotechnol. 2012;11:13998–13906.
- Oliveira-Junior RG, Araújo CS, Souza GR, et al. In vitro antioxidant and photoprotective activities of dried extracts from Neoglaziovia variegata (Bromeliaceae). J Appl Pharm Sci. 2013;3:122–127.
- Luther HE. An alphabetical list of bromeliad binomials. Sarasota: The Bromeliad Society International; 2008.
- Manetti LM, Delaporte RH, Laverde-Júnior A. Secondary metabolites of the family Bromeliaceae. Quim Nova. 2009;32:1885–1897.
- Lima-Saraiva SRG, Saraiva HCC, Silva JC, et al. Antinociceptive effect of the ethanolic extract of Neoglaziovia variegata (Bromeliaceae) in mice. J Med Plant Res. 2012;6:5330–5336.
- Santana CRR, Oliveira-Junior RG, Araújo CS, et al. Phytochemical screening, antioxidant and antibacterial activity of Encholirium spectabile (Bromeliaceae). Int J Sci. 2012;1:1–19.
- Oliveira-Junior RG, Araujo CS, Santana CRR, et al. Phytochemical screening, antioxidant and antibacterial activity of extracts from the flowers of Neoglaziovia variegata (Bromeliaceae). J Chem Pharm Res. 2012;4:4489–4494.
- Oliveira-Junior RG, Souza GR, Guimarães AL, et al. Dried extracts of Encholirium spectabile (Bromeliaceae) present antioxidant and photoprotective activities in vitro. J Young Pharm (Print). 2013;5:102–105.
- Carvalho KIM, Fernandes HB, Machado FDF, et al. Antiulcer activity of ethanolic extract of Encholirium spectabile Mart. ex Schult and Schult f. (Bromeliaceae) in rodents. Biol Res. 2010;43:459–465.
- Machado FDF, Silva FV, Fernandes HB, et al. Gastroprotective effect of ethanolic extract from Neoglaziovia variegata (Arruda) Mez. (Bromeliaceae) in rats and mice. J Biosci. 2013;68:97–107.
- Wagner H, Bladt S. Plant drug analysis: a thin layer chromatography atlas. Berlin Heidelberg: Springer Verlag; 1996. p. 384.
- Slinkard K, Singleton VL. Total phenol analysis: Automation and comparison with manual methods. Am J Enol Viticult. 1977;28:49–55.
- Almeida JRGS, Oliveira MR, Guimarães AL, et al. Phenolic quantification and antioxidant activity of Anaxagorea dolichocarpa and Duguetia chrysocarpa (Annonaceae). Int J Pharm Bio Sci. 2011;2:367–374.
- Dewanto V, Wu X, Adom K, et al. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50:3010–3014.
- Falcão DQ, Costa ER, Alviano DS, et al. Atividade antioxidante e antimicrobiana de Calceolaria chelidonioides Humb.Bonpl. & Kunth. Braz J Pharmacogn. 2006;16:73–76.
- Wannes WA, Mhamdi B, Sriti J, et al. Antioxidant activities of the essential oils and methanol extracts from myrtle (Myrtus communis var. italica L.) leaf, stem and flower. Food Chem Toxicol. 2010;48:1362–1370.
- Violante IMP, Souza IM, Venturini CL, et al. Avaliação in vitro da atividade fotoprotetora de extratos vegetais do cerrado de Mato Grosso. Braz J Pharmacogn. 2009;19:452–457.
- Mansur JS, Breder MVR, Mansur MCA, et al. Determinação do fator de proteção solar por espectrofotometria. An Bras Dermatol. 1986;61:121–124.
- Sayre RM, Agin PP, Levee GJ, et al. Comparison of in vivo and in vitro testing of sunscreening formulas. Photochem Photobiol. 1979;29:559–566.
- Silva MEGC, Guimarães AL, Oliveira AP, et al. HPLC-DAD analysis and antioxidant activity of Hymenaea martiana Hayne (Fabaceae). J Chem Pharm Res. 2012;4:1160–1166.
- Adefuye AO, Ndip RN. Phytochemical analysis and antibacterial evaluation of the ethyl acetate extract of the stem bark of Bridelia micranta. Pharmacogn Mag. 2013;9(33):45–50.
- Souza TM, Santos LE, Moreira RRD, Rangel VLBI. Avaliação da atividade fotoprotetora de Achillea millefolium L. (Asteraceae). Braz J Pharmacogn. 2005;15:36–38.
- Bobin MF, Raymond M, Martini MC. Propriedades de absorção UVA/UVB de produtos naturais. Cosmet Toil. 1995;7:44–50.
- Saewan N, Jimtaisong, A. Natural products as photoprotection. J Cosmet Dermatol. 2015;14(1):47–63.
- Fernandes AS, Alencar AS, Evangelista H, et al. Photoprotective and toxicological activities of extracts from the Antarctic moss Sanionia uncinata. Pharmacogn Mag. 2015;11(41):38–43.
