ABSTRACT
Pregabalin and gabapentin are amino-acid derivatives of gamma-aminobutyric acid. They have a high affinity to the α2δ protein in the central nervous system and both have been shown to be effective for neuropathic pain disorder. The aim of this study was to investigate the efficacy of gabapentin and pregabalin in animal models of neuropathic pain, and to correlate with clinical outcomes in patients with diabetic neuropathy. Gabapentin (60 mg/kg) and pregabalin (30 mg/kg) attenuate mechanical, tactile and heat hypersensitivity in rats with chronic constriction injury of the sciatic nerve and streptozotocin (STZ)-induced diabetes. There is no evidence that one of the drugs is superior to another at the different rat models and tests. In the incisional pain model, there was partial efficacy of gabapentin. Our clinical data suggest that relative to the baseline pain score, the treatment with pregabalin at doses of 300 mg/day or gabapentin at doses of 900 mg/day would be effective and well tolerated in patients diagnosed with moderate diabetic polyneuropathy. The study suggested that pregabalin may provide better analgesic outcomes than gabapentin on the sixth month of treatment. In conclusion, the comparative effects of gabapentinoids in animal models of neuropathic pain and neuropathic patients are suggestive of similar pathophysiological mechanisms being involved, but successful outcome is determined by a patient's individuality and the drug nature as well as the drug tolerability.
Introduction
Diabetic neuropathy is the most common complication of diabetes type 1 and type 2 and as many as 50% of patients develop neuropathy in the course of the disease [Citation1]. According to a definition of the Special Interest Group on Neuropathic Pain (NeuPSIG), neuropathic pain is ‘pain arising as a direct consequence of a lesion or disease affecting the somatosensory system’ [Citation1]. Peripheral neuropathic pain can be caused by nerve injury or disease, including radiculopathy (sciatica), postherpetic neuralgia (PHN) (persistent pain after shingle episode), diabetic neuropathy, human immunodeficiency virus (HIV)-related neuropathy or chronic postsurgical pain [Citation2]. Some major characteristics of neuropathic pain are spontaneous pain, paresthesia, dysesthesia, hyperalgesia and allodynia. Numerous animal models of nerve damage and subsequent neuropathic pain have been utilized to study different pain mechanisms. Neuropathic pain secondary to lesions of traumatic origin (constriction, partial section, spinal nerve ligation, etc.), metabolic (diabetes) or toxic origin (anticancer or anti-retroviral agents) could imitate principal signs of human neuropathic pain [Citation3] and animal models are used as important tools in the studies of the mechanisms of neuropathic pain [Citation4]. Different strategies to alleviate the neuropathic symptoms in these models might be predictive of the efficacy of analgesic drugs in humans. The recommended pharmacotherapy of neuropathic pain includes anticonvulsant drugs such as gabapentinoids (pregabalin and gabapentin), several antidepressants (amitriptyline, venlafaxine, duloxetine), tramadol and opioids [Citation5,Citation6]. Gabapentinoids are first-line treatment for neuropathic pain. The efficacy of gabapentin and pregabalin has been established in painful diabetic neuropathy (PDN) and PHN [Citation7]. Pregabalin, like gabapentin, is an amino-acid derivative of gamma-aminobutyric acid. They exert their actions by binding to the α2δ accessory subunits of voltage-gated Ca2+ channels [Citation8]. The binding at pre- and post-synaptic membranes results in inhibition of the release of excitatory neurotransmitters [Citation9]. This α2δ1 subunit-binding is considered to account for both antinociceptive and probably antiseizure effects as well [Citation10]. Additionally, this mechanism correlates with a growing number of clinical trials indicating that gabapentin and pregabalin could be effective as postoperative analgesics [Citation11].
There are conflicting reports regarding the efficacy of gabapentinoids in inflammatory and neuropathic animal models against mechanical and thermal hypersensitivity [Citation12]. While indirect comparisons show similar efficacy and tolerability profile [Citation13], there are few comparative studies of pregabalin and gabapentin for neuropathic pain including PHN, PDN and spinal cord injury-related neuropathic pain.
The aim of this study was to investigate the efficacy of gabapentin and pregabalin in animal models of neuropathic pain (chronic constriction injury (CCI) and streptozotocin-induced diabetes) and the correlation with the clinical outcomes in patients with diabetic neuropathy.
Subjects and methods
Animals and treatment
Adult male Wistar rats (200–220 g body mass) obtained from the Animal House at the Medical University of Sofia were housed at standard laboratory environment (22 ± 2 °C and a 12:12 h light/dark cycle) with ad libitum access to food and water. Animal care and experimentation were carried out in accordance with the Animal Care Guidelines of the Ethics Committee of the Medical University of Sofia.
Ample evidence suggests that chronic constriction nerve injury could simulate major symptoms of chronic nerve compression at clinical conditions of trauma or tumour growth [Citation5]. CCI of the sciatic nerve was performed under general anesthesia (ketamine, 50 mg/kg, i. p. plus nembutal, 12 mg/kg, i. p.). Being isolated/exposed between the gluteal and biceps femoral muscles, the nerve trunk was ligated with four loose ligatures placed at 3 mm apart [Citation14].
Diabetes was induced in rats by a single application of streptozotocin (STZ, 75 mg/kg, i. p.) confirmed by blood glucose monitoring three days afterwards. Rats with blood glucose level as high as 15 mmol/L or above were considered diabetics and were used in further experiments. To verify the development of hyperalgesia or allodynia, pain hypersensitivity was evaluated several times prior to CCI (baseline sensitivity), on day 14 after surgery and on day 25 of STZ-induced diabetes. Gabapentin (60 mg/kg) or pregabalin (30 mg/kg) dissolved in 2 mL saline was administered by oral gavage.
The analgesic effect of gabapentin (60 mg/kg) was evaluated in diabetic rats in a model of postsurgical pain. Under general anaesthesia, the plantar surface of the right hind paw was sterilely prepared and 1 cm longitudinal incision was made through the skin and fascia of the plantar aspect of the paw, starting 0.5 cm from the proximal edge of the heel and extending towards the toes. The plantaris muscle was elevated and incised longitudinally. After hemostasis with gentle pressure, the skin was apposed with two mattress sutures. The wound site was covered with povidone–iodine and the animals were allowed to recover in their home cages. Assessment of the effect of gabapentin was performed 24 h after surgery.
Nociception behavioural tests
Mechanical allodynia was measured in all CCI-operated rats by von Frey filament withdrawal threshold using dynamic plantar aesthesiometer (Ugo Basile, Italy). The filament was applied on the hind paw plantar surface with force increasing by 2.5 g/s. The threshold was calculated as the average of three consecutive effective applications. Mechanical hyperalgesia was estimated in CCI operated rats by paw pressure test using analgesimeter (Ugo Basil, Milan, Italy). The withdrawal threshold to increasing pressure applied on the dorsal surface of the hind paw was determined in grams.
Thermal hyperalgesia was assessed by plantar heat test using Hargreaves Apparatus (Ugo Basile, Italy). The withdrawal latency to calibrated radiant heat applied on the plantar surface of CCI operated or intact hind paw was measured in seconds and estimated as the average of three consecutive measurements. Pain locomotion impairment referred to as pain-induced incapacitance was assessed by incapacitance test using incapacitance meter (Ugo Basile, Italy). Spontaneous incapacitance test estimates the pressure that each left and right hind paw of an upright standing animal exerts on two separate platforms coupled to computerized pressure transducers. Rats with intact hind paws would apply even pressure on the platforms, which is an indication of posture equilibrium. Unilateral painful damage of hind paw would result in an asymmetric body weight distribution, i.e. unequal pressure on the platforms. The need of pain relief in upright standing rats necessitates distribution of lesser body weight on the damaged paw. An innovative approach to quantify the posture equilibrium was the calculation of the ratio pressure of CCI paw/pressure of intact paw. A value of 1 unit is a measure of postural equilibrium, while values of less than 1 unit show asymmetric posture and could quantify the degree of pain intensity in a damaged paw. Before experimentation, the rats were habituated to stand calm upright in an unfloored posture restrainer placed over pressure transducer platforms.
Clinical research design and methods
The research was a retrospective cohort study of adult patients (aged 28–84 years) of both sexes (F = M) diagnosed with PDN treated with gabapentin or pregabalin. The inclusion criteria were: (i) patients with type 1 diabetes receiving gabapentin at doses up to 900 mg (n = 30) or pregabalin at doses up to 300 mg (n = 32) daily for at least 6 months, (ii) patients presenting a pain score of 40 mm or more on a 100 mm Visual Analogue Scale (VAS), (iii) patients having haemoglobin A1c (HbA1c) levels below 10 mg percentage and presence of sensory polyneuropathy for at least three months. The exclusion criteria were pregnancy, severe pain with other etiology, painful comorbidities, central nervous system lesions, depression, epileptic seizures, mood or sleeping disorders all of which could change the pain perception and affect the study objectivity. The primary endpoint was pain intensity measured by VAS. The patients had to describe the pain intensity as none, mild, moderate or severe, with recommended VAS cut points set at 0–4 mm, 5–44 mm, 45–74 mm and 75–100 mm, respectively [Citation15].
The secondary endpoint was the assessment of adverse drug reactions (ADRs). Most frequent ADRs are dizziness and lurch at the beginning of treatment which necessitates gradual dose titration or return to lower dose levels to mitigate the complaints. Complying with good clinical practice, the dosing of gabapentin or pregabalin was titrated gradually in all patients. Pregabalin treatment begun with a daily dose of 75 mg, which increased stepwise by 75 mg every 3–7 days until optimal pain control was reached. Gabapentin treatment begun with a daily dose of 300 mg, which increased stepwise by 100 mg every 4–7 days until optimal pain control was reached. All patients participating in this study signed an informed consent.
Statistical analysis
The results are shown as mean values with standard error of the means (± SEM). Data were analysed by Student's t-test or analysis of variance (ANOVA) followed by post-hoc analysis, using GraphPad Instat 3.06. A P-value of less than 0.05 was considered to indicate significant difference.
Results and discussion
Experimental neuropathic pain in rats
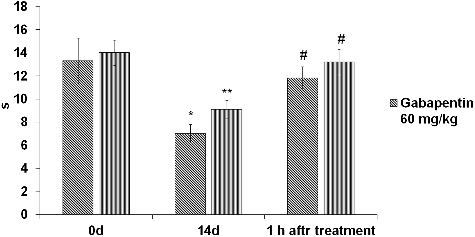
The results showed that the mechanical and thermal withdrawal threshold of ipsilateral paw of CCI rats decreased significantly on day 14 after surgery ((A,B), ). Oral administration of gabapentin (60 mg/kg) or pregabalin (30 mg/kg) increased significantly the paw withdrawal threshold (PWT) responsive to tactile (von Frey) or mechanical (paw pressure) stimuli ((A,B)).
Figure 1. Effect of gabapentin and pregabalin on tactile (A) and mechanical (B) hyperalgesia in rat model of CCI-induced neuropathic pain.
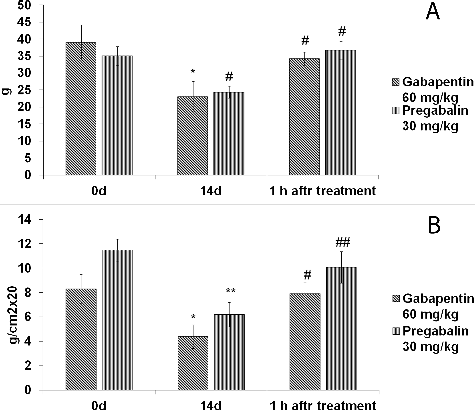
Figure 2. Effect of gabapentin and pregabalin on thermal hyperalgesia in rat model of CCI-induced neuropathic pain.
The effects of gabapentinoids on thermal hyperalgesia are shown in . The paw withdrawal latencies were similar in the groups treated with gabapentin (60 mg/kg) or pregabalin (30 mg/kg). The drugs significantly attenuated the thermal hyperalgesia in the plantar heat test (P < 0.05). The administration of gabapentin and pregabalin also attenuated the ipsilateral mechanical weight-bearing deficits in the rat CCI pain model (P < 0.05).
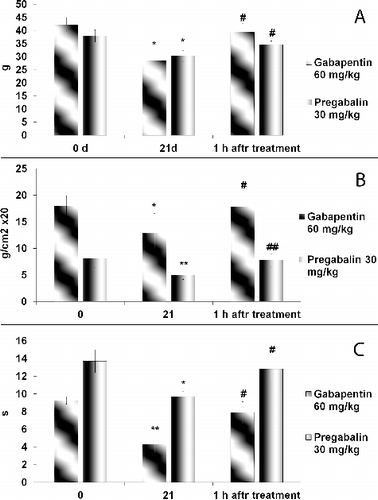
The presence of neuropathy (significant mechanical and thermal hypersensitivity) in diabetic rats was evaluated on day 25 after induction of diabetes (). The administration of gabapentin (60 mg/kg) significantly increased the PWT. The decreased PWT in diabetic rats was restored after application of pregabalin. Moreover, no significant decrease in the weight bearing was observed in the diabetic rats and neither of the two studied compounds altered the baseline ratio. The results demonstrated that gabapentinoids might significantly attenuate the neuropathic pain behaviour in both animal models of peripheral neuropathy.
Figure 3. Effects of gabapentin and pregabalin on mechanical (A, B) and thermal (C) hyperalgesia in rat model of STZ-induced diabetic neuropathy.
The mechanisms of post-incisional nociception are distinct from those of other pain conditions [Citation16]. Acute gabapentin (60 mg/kg) treatment was found to markedly alleviate thermal hyperalgesia in incision model of postoperative pain in diabetic rats. Incision of the plantar surface of the hind paw produced a significant reduction in weight bearing and in PWT, and gabapentin could not reduce the tactile or mechanical hyperalgesia.
Neuropathic pain in diabetic patients
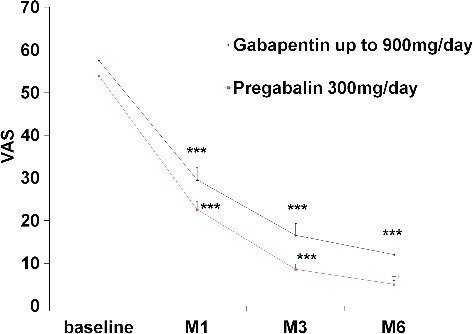
The study included 62 patients with type 1 diabetes hospitalized for a specified period satisfying the matching criteria. The patients received gabapentin (300 mg/d, n = 32) or pregabalin (900 mg/d, n = 30) for at least six months. Gabapentinoids were well tolerated and did not necessitate discontinuation of the treatment due to unexpected and/or serious adverse reactions. Only two patients at the initial stage (3.3%) and four patients at the titration phase (6.7%) of pregabalin treatment had to discontinue the therapy due to appearance of strong ADRs (vertigo and sleep disorders), being hence excluded from the study. The data demonstrated a significant reduction in VAS scores at the end point (). Improvements of month pain scores were observed at month 1 and remained significant throughout the study period (P < 0.001). The efficacies of gabapentin and pregabalin in the relief of neuropathic pain were similar. The reduction of pain intensity was significant in patients treated with pregabalin for six months (P < 0.05). At the six-month point, there was also significant difference between the effects of pregabalin and gabapentin (P < 0.05).
Pregabalin and gabapentin are recommended for initial treatment of neuropathic pain in the guidelines of the National Institute for Clinical Excellency (NICE). The improvement in the patients’ activities and quality of life in response to pregabalin treatment correlated well with the degree of pain relief. The data suggested that, relative to the baseline pain score, the treatment with pregabalin at doses of 300 mg/d or gabapentin at doses of 900 mg/d is effective and well tolerated in patients diagnosed with moderate diabetic polyneuropathy. The short-term treatment with gabapentin and pregabalin produced similar pain relief, though the six-month therapy with pregabalin proved to be more effective and better tolerated by the patients.
The therapeutic effect of gabapentinoids in neuropathic pain is mediated through binding to the α2δ1 subunit of voltage-dependent calcium channels. Pregabalin has an increased binding affinity for the α-2-δ protein and is reported to be a more potent analgesic in neuropathic pain compared to gabapentin [Citation17,Citation18]. Pregabalin was found to have distinct pharmacokinetic advantages over gabapentin. Unlike gabapentin, the absorption of pregabalin is not saturable and the drug has a linear pharmacokinetic profile [Citation17]. The higher bioavailability of pregabalin permits lower dosing, thus decreasing the risk of dose-related ADRs [Citation19,Citation20]. NICE clinical guidelines recommend amitriptyline (off-label), duloxetine, gabapentin or pregabalin as safe and cost-effective options for treatment of neuropathic pain. There is no evidence that one is clinically superior to another [Citation13].
A growing number of clinical trials reveal the efficacy of perioperative or postoperative use of gabapentanoids. The pharmacology of the plantar incision model of postoperative pain is distinct from that of inflammatory and neuropathic pain models [Citation21]. The present study demonstrated a partial efficacy of gabapentin in the incisional pain model, which suggests involvement of multiple, not fully understood mechanisms of action of gabapentin. Gabapentinoids could inhibit the pain transmission and central sensitization modulating the calcium-induced release of glutamate in the nociceptive pathways. Some evidence indicates that their antinociceptive mechanisms may involve activation of descending adrenergic pain-inhibiting pathways in brain and spinal cord [Citation22]. Gabapentinoids have also been shown to act on the inflammatory mechanisms. Gabapentin may, for example, decrease the expression of pro-inflammatory cytokines as well as upregulate the expression of anti-inflammatory cytokine interleukin-10 [Citation23].
Conclusions
Our study shows that Gabapentin and Pregabalin attenuate the mechanical, tactile and heat hypersensitivity in rats. The effect was comparable in rats with CCI of the sciatic nerve or STZ-induced diabetic neuropathy. Similarly, Gabapentin and Pregabalin were effective analgesics (VAS score) in the patients with diabetic neuropathy. Pregabalin manifested a higher efficiency than gabapentin in long term treated patients only. The comparable effects of gabapentinoids in animal models of neuropathic pain and in patients with diabetic neuropathy suggest the involvement of similar pathophysiological mechanisms.
Disclosure statement
The authors declare no conflicts of interest.
Additional information
Funding
References
- Fernandez VE, Abdi S. Painful diabetic peripheral neuropathy. In: Smith H, editor. Current therapy in pain. 1st ed. Philadelphia (PA): Saunders; 2009. p. 250–255.
- Haanpää M, Treede RD. Diagnosis and classification of neuropathic pain. Pain Clin Updates. 2010 [cited 2017 Jan 6];18(7). Available from: http://www.iasp-pain.org/PublicationsNews/NewsletterIssue.aspx?ItemNumber=2083
- Wattiez AS, Barrière DA, Dupuis A, et al. Rodent models of painful diabetic neuropathy: what can we learn from them? J Diabetes Metab. 2012 [ cited 2017 Jan 6];S5:008. doi:10.4172/2155-6156.S5-008
- Challa SR. Surgical animal models of neuropathic pain: pros and Cons. Int J Neurosci. 2015;125(3):170–174.
- Finnerup NB, Attal N, Haroutounian S, et al. Pharmacotherapy for neuropathic pain in adults: a systematic review and meta-analysis. Lancet Neurol. 2015;14(2):162–173.
- Bril V, England J, Franklin GM, et al. Evidence-based guideline: treatment of painful diabetic neuropathy. Neurology. 2011;76:1758–1765.
- Tassone DM, Boyce E, Guyer J, et al. Pregabalin: a novel gamma-aminobutyric acid analogue in the treatment of neuropathic pain, partial-onset seizures, and anxiety disorders. Clin Ther. 2007;29:26–48.
- Alles SR, Smith PA. The Anti-allodynic gabapentinoids: myths, paradoxes, and acute effects. Neuroscientist. 2016;1:1–16.
- Rahimzadeh P. Gabapentinoids in pain setting. J Pain Relief. 2013 [ cited 2017 Jan 6];2:e114. doi:10.4172/2167-0846.1000e114
- Toth C. Pregabalin: latest safety evidence and clinical implications for the management of neuropathic pain. Ther Adv Drug Saf. 2014;5(1):38–56.
- Chang CY, Challa CK, Shah J, et al. Gabapentin in acute postoperative pain management. BioMed Res Int. 2014 [ cited 2017 Jan 6]; 2014:631756, 7 pages. doi:10.1155/2014/631756
- Pateland R, Dickenson AH. Mechanisms of the gabapentinoids and α2 δ‐1 calcium channel subunit in neuropathic pain. Pharmacol Res Perspect. 2016 [ cited 2017 Jan 6];4(2):e00205. doi:10.1002/prp2.205
- NCL JFC. Neuropathic pain prescribing guideline: gabapentin or pregabalin for neuropathic pain? [ Internet]. North Central London Joint Formulary Committee; 2015 [ cited 2016 Dec 16]. Available from: http://ncl-jfc.org.uk/uploads/3/4/5/0/34507515/6.1b_pregabalin_summary_for_jfc_march_2015.pdf
- Bennett GJ, Xie YK. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33(1):87–107.
- Jensen MP, Chen C, Brugger AM. Interpretation of visual analog scale ratings and change scores: a reanalysis of two clinical trials of postoperative pain. J Pain 2003;4:407–414.
- Deval E, Noël J, Gasull X. et al. Acid-sensing ion channels in postoperative pain. J Neurosci. 2011;31(16):6059–6066.
- Fudin J. How gabapentin differs from pregabalin. Pharmacy Times. c2006–2017 Intellisphere, LLC [ 2015 Sep 21; cited 2016 Dec 16]. Available from: http://www.pharmacytimes.com/contributor/jeffrey-fudin/2015/09/how-gabapentin-differs-from-pregabalin.
- Bockbrader HN, Wesche D, Miller R, et al. A comparison of the pharmacokinetics and pharmacodynamics of pregabalin and gabapentin. Clin Pharmacokinetics. 2010;49(10):661–669.
- Freynhagen R, Strojek K, Griesing T. et al. Efficacy of pregabalin in neuropathic pain evaluated in a 12-week, randomised, double-blind, multicentre, placebo-controlled trial of fble- and fixed-dose regimens. Pain. 2005;115(3):254–263.
- Wesche D, Bockbrader H. A pharmacokinetic comparison of pregabalin and gabapentin. J Pain. 2015;6(3 Suppl):S29.
- Whiteside GT, Harrison J, Boulet J. et al. Pharmacological characterisation of a rat model of incisional pain British J Pharmacol. 2004;141:85–91.
- Schmidt PC, Ruchelli G, Sean BA, et al. Perioperative gabapentinoids: choice of agent, dose, timing, and effects on chronic postsurgical pain. Anesthesiology. 2013;119:1215–1221.
- Kremer M, Yalcin I, Nexon L, et al. The antiallodynic action of pregabalin in neuropathic pain is independent from the opioid system. Mol Pain. 2016 [ cited 2017 Jan 6];12:1–12. Available from: http://journals.sagepub.com/doi/full/10.1177/1744806916633477