?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Bioaccumulation by growing cells is a potential technique for the removal of heavy metals from wastewater. Cobalt bioaccumulation by Rhodopseudomonas palustris via different metabolic pathways was investigated for various pH levels, temperatures, co-cations and initial Co2+ concentrations. The distribution of cobalt and its chemical nature in R. palustris were examined by cell compartmentalization and X-ray diffraction analysis. Our results indicated that biomass and bioaccumulation capacity were superior under aerobic conditions in the dark at pH 6.5 and 30 °C. Cobalt removal efficiency and bioaccumulation capacity were, respectively, 84.9% and 287.18 mg/g for cultures with an initial Co2+ concentration of 160 mg/L. A pseudo-second-order kinetic model fits the removal process well (R2 = 0.9508–0.9913). Co2+ removal efficiency was not significantly influenced by Cu2+, Zn2+, Cd2+, Mn2+ or Fe2+ but was influenced by Ni2+. Cobalt was distributed between the cell surface, periplasmic space, membrane and cytoplasm. The periplasmic space and membrane accounted for 57.37% and 27.95% of Co2+ uptake, respectively. X-ray diffraction analysis showed that cobalt ions were predominantly deposited in the cell as cobalt phosphate octahydrate. These results demonstrate that R. palustris could be used to remove cobalt from industrial wastewater; it would therefore be beneficial to further study the mechanism of cobalt bioaccumulation in this organism.
Introduction
Pollution from heavy metals poses a significant threat to the environment and to human health due to the toxicity, persistence and non-biodegradability of various compounds that form the pollutants. Cobalt is an essential trace element and plays an important role in animals, yeasts, bacteria and plants [Citation1]. However, excess cobalt is often discharged by industrial activities such as mining, metallurgy, electroplating, painting, pigmenting and lithium ion batteries production [Citation2,Citation3]. Such discharges can result in serious health effects for animals and humans, including contact dermatitis, paralysis, lung irritation and bone defects. Exposure to ionizing radiation is associated with an increased risk of developing cancer [Citation4–Citation7]. The permissible limits of cobalt in irrigation water and livestock wastewater are 0.05 and 1 mg/L, respectively (Environmental Bureau of Investigation, Canadian Water Quality Guidelines) [Citation8]. A variety of technologies have been developed over the years to remove toxic cobalt from water, including chemical precipitation, ion exchange [Citation9], reverse osmosis [Citation10] and activated carbon adsorption [Citation11]. However, most of these technologies are expensive, inefficient, labour-intensive or unselective. Therefore, there is a great need for an alternative cobalt removal technology that is efficient and economical. Bioaccumulation by growing cells is a potential method for the removal of heavy metal from the environment [Citation12,Citation13].
In micro-organisms, the bioaccumulation of heavy metals arises from metabolism-independent extracellular adsorption (surface interaction of heavy metal ions with cell-surface functional groups) and metabolism-dependent uptake followed by intracellular accumulation. Intracellular accumulation is a more complex process and may involve the localization of metal ions within specific organelles, enzymatic detoxification and efflux pumps [Citation14]. In parallel, growing cells can cleave organo-metallic complexes, degrade organic contaminants such as dyes [Citation15] and 2,4-dichlorophenoxyacetic acid [Citation16] and take up other inorganic ions such as ammonium, nitrate and phosphate [Citation17]. Rhodopseudomonas palustris (R. palustris) is a purple photosynthetic bacterium and is widely found in soil, wastewater and activated sludge. R. palustris has extraordinary metabolic versatility, grows with or without oxygen and uses many alternative carbon and energy sources [Citation18]. Most importantly, many people have demonstrated that R. palustris exhibits high tolerances and enormous capacities for various metals [Citation19,Citation20]. The objective of this study is to investigate the effects of metabolic pathways, pH, temperature, co-cations and initial Co2+ concentrations on cobalt bioaccumulation in R. palustris. To understand the distribution of cobalt within R. palustris cells, cell compartmentalization and X-ray diffraction analysis were performed.
Materials and methods
Strains and growth conditions
R. palustris was from our laboratory and was cultured in enrichment medium: 4 g DL-malic acid, 1 g (NH4)2SO4, 0.12 g MgSO4.7H2O, 0.6 g K2HPO4, 0.9 g KH2PO4, 0.02 g sodium ethylenediaminetetraacetic acid (EDTA), 75 mg CaCl2.2H2O, 1 mg vitamin B1, 1 mg nicotinic acid, 15 mg biotin and 1 mL trace element solution dissolved in 1 L distilled water [Citation21]. The pH of the medium was adjusted to 6.5 with NaOH solution before autoclaving. R. palustris was incubated anaerobically at 30 C under 2000 lx illumination for 60 h.
Effect of metabolic pathways on cobalt bioaccumulation
A 4 mL volume of growing cells in exponential phase (0.7--0.9 g dry cell/L) was suspended in 100 mL growth medium containing 80 mg/L Co2+, which was prepared by dissolving Co(NO3)2.6H2O in ultrapure water. R. palustris was cultured anaerobically in the light and in the dark. Other cells were cultured aerobically in illuminated and dark orbital shakers (Huamei Biochemical Instrument, QHZ-98B, China) at 120 rpm. The optical densities of bacterial cultures were determined at 600 nm using a UV/Vis spectrophotometer. OD600 measurements were then converted to corresponding dry cell weights. Residual Co2+ concentrations in supernatants were measured using an atomic absorption spectrophotometer (AAS, PerkinElmer AAnalyst 800, USA) following centrifugation (10,000 g, 10 min) and filtering through 0.22 μm cellulose acetate membranes. Co2+ removal efficiencies were calculated using the following equation:(1)
(1) where C0 and Ce are the initial and equilibrium metal concentration (mg/L), respectively.
Effect of pH and temperature on cobalt bioaccumulation
Cobalt bioaccumulation was investigated by adding R. palustris to medium containing 80 mg/L Co2+ and adjusted to various pH values (5.5–7.5) using NaOH solution. The effect of temperature on cobalt bioaccumulation was studied at pH 6.5 as noted earlier; the culture temperature varied between 20 and 40 °C.
Effect of co-cations on cobalt bioaccumulation
R. palustris was incubated within medium containing 80 mg/L Co2+ and equivalent concentrations of Ni2+, Fe2+, Cu2+, Zn2+, Cd2+ or Mn2+. Culture conditions were set to the optimal pH and temperature determined previously.
Effect of initial cobalt concentration on cobalt bioaccumulation
To assess the effect of Co2+ concentration on cell growth and metal uptake, experiments were performed with initial Co2+ concentrations ranging from 20 to 160 mg/L. Culture conditions were set to the optimal pH and temperature determined previously. A 2 mL volume of culture solution was withdrawn every 5 h. We monitored the dry cell weights and Co2+ concentrations in samples. After 80 h of cultivation, R. palustris was harvested via centrifugation and dried at 60 °C for 4 h to determine the bioaccumulation capacity of the organism. The Co2+ bioaccumulation capacity was calculated using the following equation:(2)
(2) where qe is the equilibrium Co2+ concentration in R. palustris (mg/g), X is the biomass of R. palustris (g dry cell/L) and C0 and Ce are the initial and equilibrium Co2+ concentrations (mg/L), respectively.
Cell compartmentalization
To determine the proportion of cobalt associated with surface exchange sites, the periplasmic space, the membrane fraction and the cytoplasm, a series of extractions were performed according to the modified method described by Kumar [Citation22]. Cells grown with 80 mg/LCo2+ were harvested at stationary phase, then washed twice with 0.03 mol/L Tris-buffer (pH 8.0) containing 2.5 mmol/L EDTA. Metals associated with exchange sites on the cell surface were extracted by re-suspending the cell pellet in 5 mmol/L Ca2+ as Ca(NO3)2 and incubating for 30 min with gentle shaking at 80 rpm [Citation23,Citation24]. The suspension was centrifuged at 10,000 g for 15 min, and cell pellets were treated with 200 μg/mL lysozyme and incubated for 30 min to generate spheroplasts. All subsequent steps were carried out at 0–4 °C. Spheroplasts were collected by centrifugation at 10,000 g for 15 min and were re-suspended in 0.03 mol/L Tris buffer (pH 8.1) containing 3 mmol/L EDTA. The supernatant consisted of the periplasmic fluid, including a peptidoglycan layer. The spheroplasts were disrupted with four 15 s bursts from an ultrasonic processor and centrifuged (2000 g, 10 min) to remove debris and unbroken cells. The resulting supernatant, which consisted of membrane and cytoplasm fractions, was centrifuged at 15,000 g for 150 min. The remaining pellet, which consisted of the membrane envelope, was then dissolved in 15 mL of 50% nitric acid for 30 min.
X-ray diffraction
X-ray diffraction patterns of dry, powdered samples of the metal-free control and Co2+-loaded (80 mg/L, 40 h) cells were taken with an Empyrean PANalytical Diffractometer operating at 40 kV using CuKα radiation in the 2θ range from 5° to 80°. Diffraction data from the control and the bacterial biomass were compared to data obtained from other standard cobalt compounds (Joint Committee on Powder Diffraction Standards).
Results and discussion
Effect of metabolic pathways on cobalt bioaccumulation
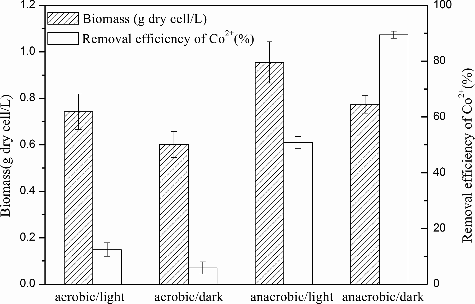
Under anaerobic conditions, the removal efficiencies of Co2+ by R. palustris were 12.45% and 5.81% in the light and in the dark, respectively. Under aerobic conditions, the removal efficiencies of Co2+ were 50.83% and 89.47% in the light and in the dark, respectively (). Removal efficiencies under aerobic conditions were higher than those under anaerobic growth; optimal bioaccumulation occurred under aerobic growth in the dark. R. palustris is the most metabolically versatile bacteria known and is capable of changing its metabolism in response to changes in light and oxygen availability [Citation18]. R. palustris has also been studied for its ability to biodegrade aromatic rings in the absence of molecular oxygen using an anoxic-sensitive enzyme [Citation25]. It has been suggested that the metabolic pathways that are active within the cell may significantly influence the mechanism of cobalt bioaccumulation.
Effect of pH on cobalt bioaccumulation
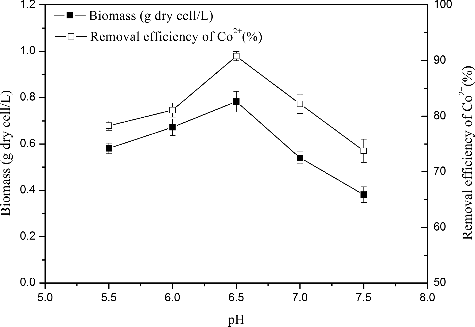
pH is considered an important environmental factor for bioaccumulation, as it affects cell growth, metal solution chemistry and the ionization state of the cell wall. shows that the maximum biomass and Co2+ removal efficiency (0.78 g and 90.8%, respectively) were obtained at a pH of approximately 6.5. also reveals the positive correlation between removal efficiency and biomass: removal efficiency increased with increasing biomass. High concentrations of H+ likely competed with cobalt ions at cell surfaces, inhibiting the approach of positively charged cobalt ions and decreasing removal efficiencies [Citation26].
Effect of temperature on cobalt bioaccumulation
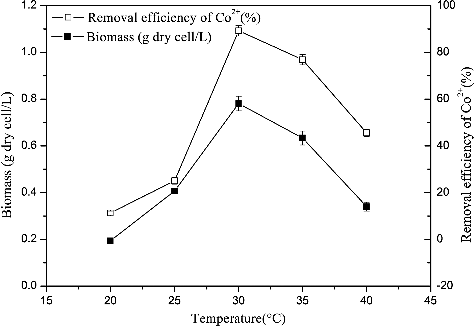
shows that, at 20 °C, R. palustris grew extremely slowly, exhibiting a removal efficiency of 11.27%. Similarly, R. palustris growth was also significantly inhibited at 40 °C: removal efficiency was 45.58% at this temperature. Maximum biomass (0.78 g dry cell/L) and Co2+ removal efficiency (89.25%) were observed at 30 °C. This result suggests that cobalt removal efficiencies are mainly due to the amount of organism present in the culture. Bioaccumulation processes that depend on cellular metabolism, such as active uptake, would be inhibited by low temperatures. In contrast, high temperatures could affect the integrity of cell membranes and hinder the compartmentalization of cobalt ions [Citation27].
Effect of co-cations on cobalt bioaccumulation
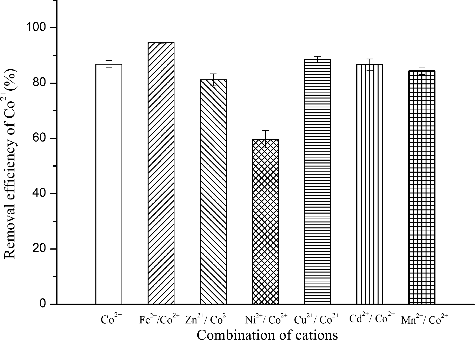
The removal efficiency of Co2+ by R. palustris was 86.8% when cobalt was the only metal ion present. However, Co2+ removal efficiencies varied if other cations were also present (). In the presence of Fe2+, Co2+ removal efficiency was slightly increased. Removal efficiencies were not significantly affected by the presence of Cu2+, Zn2+, Cd2+ and Mn2+. Interestingly, Co2+ removal efficiency decreased to 59.67% from 86.8% in the presence of Ni2+. Rhodococcus. rhodochrous (R. rhodochrous)) has been reported to behave similarly, showing a preference of Co2+ that is unaffected by Mn2+ and Zn2+ [Citation28]. Rashmi [Citation29] reported that a mutant fungus grown in iron-bearing medium before exposure to a simulated decontamination solution containing 0.033 μmol/L Co2+-EDTA exhibited increased or only marginally reduced(<10%) cobalt bioaccumulation capacity despite the presence of a high concentration of iron in the growth medium (initial[Fe/Co] = 2.5 × 103). The present data suggest that R. palustris may have evolved a specific mechanism to tolerate and accumulate high levels of cobalt in hostile environments.
Effect of initial cobalt concentrations on cobalt bioaccumulation
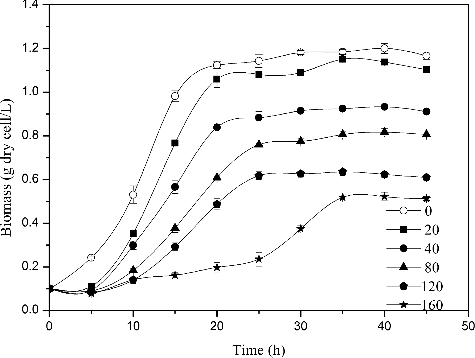
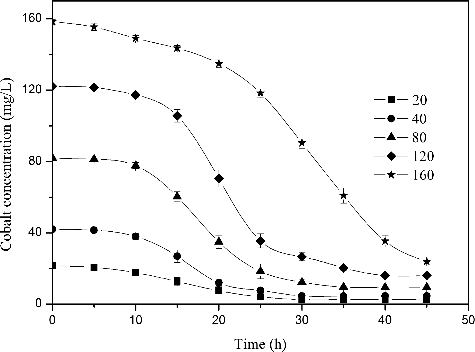
As the initial cobalt concentration was increased, the lag time of R. palustris also increased while the total biomass decreased under aerobic conditions in the dark (). In particular, cultures with an initial metal concentration of 160 mg/L exhibited a lag phase of 25 h and a clear decrease in biomass accumulation (0.5 g/L). This result implies that cell growth was strongly inhibited by the presence of 160 mg/L Co2+ in the culture medium. In contrast, in a culture containing 20 mg/L Co2+, both the growth curve and lag phase were similar to the control group, indicating no inhibition. The majority of cobalt ions were removed regardless of the initial Co2+ concentration (). More specifically, cultures with initial Co2+ concentrations of 20, 40, 80, 120 and 160 mg/L contained 2.5, 4.62, 9.53, 16.12 and 24.1 mg/L Co2+ following culture, respectively. These final Co2+ concentrations represented removal efficiencies of approximately 88.9%, 88.45%, 88.08%, 86.56% and 84.9%, and bioaccumulation capacities of 15.24, 37.95, 87.41, 185.61 and 287.18 mg/g, respectively. These data demonstrate that although Co2+ bioaccumulation capacity increases with increasing initial Co2+ concentration, Co2+ removal efficiency decreases with increasing Co2+ concentration.
Kinetics of cobalt removal by R. Palustris
To describe the dynamic removal of cobalt by R. palustris, correlation analysis parameters were conducted using a pseudo-first-order kinetic model (EquationEquation (3)(3)
(3) ) and a pseudo-second-order kinetic model (EquationEquation (4)
(4)
(4) ). These models have been widely applied to many organic material degradation systems and heavy metal removal processes [Citation30–32].
(3)
(3)
(4)
(4)
By separation of variables and integration with EquationEquation (5)(5)
(5) , the relationship between Co2+ concentration and removal time was obtained:
(5)
(5)
(6)
(6) where C0, C, t, k, k0, k1 and k2 are the initial concentration of cobalt, the concentration of cobalt at time t (mg/L), contact time (h), the rate constant of pseudo-first-order, constant and coefficient of C and C2 in a pseudo-second-order equation, respectively.
indicates that the pseudo-first-order kinetic model poorly fits the experimental data, with low correlation coefficients (R2 = 0.7848–0.9184). It is obvious that the pseudo-second-order kinetic model better fits the removal process. From , the values of q (2.2083, 4.3862, 8.871, 16.1875 and 20.2607 mg/L), which represented remaining cobalt concentrations, agreed closely with the experimental values (2.5000, 4.6154, 9.5300, 16.1154 and 24.1000 mg/L). In addition, the values of h (22.5324, 44.2804, 87.2258, 117.1875 and 153.83), which corresponded to the maximum amounts of cobalt available in the medium (20, 40, 80, 120 and 160 mg/L), also closely agreed with the experimental values. The values of p, which represented the maximum removal speed of cobalt, decreased from 0.2753 to 0.1871 h−1 as the initial concentration of cobalt increased. Values for k2 were negative (–0.0108, –0.0069, –0.0031, –0.0024 and –0.0014). Such negative values could be interpreted as the inhibitory effect of cobalt on R. palustris; the low absolute values of k2 suggest minimal inhibition. Values for k1, which are related to removal efficiencies, were positive (0.2672, 0.3358, 0.2979, 0.3201 and 0.2437). Finally, values for k0 were negative, which can be explained by competitions within the bacterial population for the limited amount of substrate in the medium.
Table 1. Pseudo-first-order model kinetic equations and kinetic parameters of the removal for different concentration of cobalt by growing R. palustris.
Table 2. Pseudo-second-order model kinetic equations and kinetic parameters of the removal for different concentration of cobalt by growing R. palustris.
Cell compartmentalization
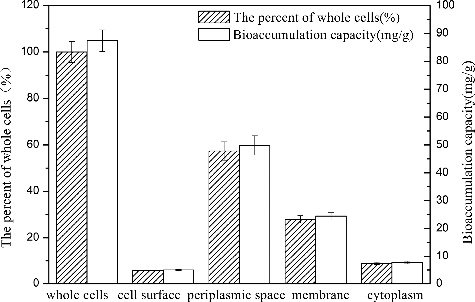
The amount of cobalt sequestration in R. palustris led us to investigate the compartmentalization of cobalt in various cell sectors. It was shown that, following Co2+ exposure (80 mg/L, 40 h), cobalt was distributed between the four cell compartments in R. palustris. Cobalt in the cell surface, periplasmic space, membrane fraction and cytoplasm represented 5.72%, 57.37%, 27.95% and 8.82% of the total cobalt taken up, respectively (). The result differed from a previous report, in which the removal of heavy metals was primarily attributed to the cell surface [Citation23]. Guo et al. [Citation33] reported similar results, noting that the majority of the removal and transformation of Cd (II) and Pb (II) ions by EB L14 occurred in the membrane fraction. Sar [Citation34] also reported that 88% of Ni2+ was restricted to the periplasm and cell membrane by Pseudomonas aeruginosa in combination with phosphoryl groups.
The distribution and uptake of Co2+ in R. palustris indicates that cobalt deposition primarily occurred at the membrane and in the periplasm, implying that intracellular accumulation plays a significant role in cobalt sequestration. Unknown components involved in Co2+ binding in the periplasmic space and membrane fraction of R. palustris remain. It seems that surface adsorption by electrostatic attraction and functional group binding with Co2+ only play minor role in cobalt uptake. Such peripheral sequestration of toxic cations exterior to the cytoplasm has been suggested to protect growing cells by limiting the amount of Co2+ transport into the cytoplasm.
X-ray diffraction analysis
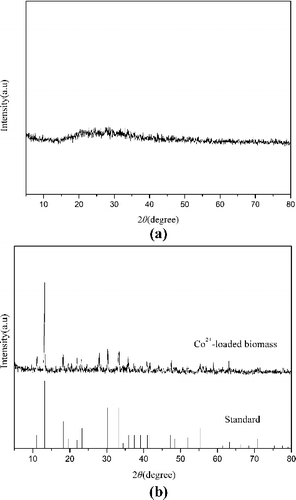
The chemical nature of cellular cobalt was ascertained via X-ray diffraction analysis of powdered bacterial biomass. The control biomass, which lacked any distinct peaks, is characterized by an amorphous nature ((a)). However, Co2+-loaded biomass exhibited several distinct peaks, indicating that cobalt crystals were deposited in the cells ((b)). In comparison with X-ray diffraction (XRD) standards available in databases, the diffraction pattern of Co2+-loaded biomass corresponded to that of cobalt phosphate octahydrate (Joint Committee on Powder Diffraction Standard (JCPDS) card 01–0121). Such observations strongly suggest that Co2+ interacted with phosphate groups in R. palustris to form cobalt phosphate octahydrate crystals. In a similar fashion, P. aeruginosa accumulated uranium due to precipitation of intracellular crystalline U-phosphate compounds [Citation35]. The formation of nickel phosphides and carbides was also reported, primarily in the cell envelope of P. aeruginosa, indicating the predominant role of the phosphoryl and carboxyl/carbonyl groups in cell wall/membrane components for metal sequestration [Citation34].
Conclusion
The optimal pH, temperature and metabolic pathway for cobalt bioaccumulation were 6.5, 30° C and aerobic growth in the dark, respectively. A pseudo-second-order kinetic model was able to describe such a cobalt removal system under optimal culture conditions. Cu2+, Zn2+, Cd2+, Mn2+ and Fe2+ did not significantly influence cobalt removal efficiencies, while Ni2+ did. Furthermore, cell compartmentalization analysis revealed that 85.32% of the accumulated cobalt was distributed between the periplasm space and the membrane, implying that intracellular accumulation plays a significant role in cobalt sequestration. The accumulated cobalt was determined to be cobalt phosphate octahydrate by X-ray diffraction. This study demonstrates that R. palustris has a great potential for the removal of cobalt from industrial wastewaters
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Odaka M, Kobayashi M. Cobalt proteins, overview. In: Kretsinger RH Uversky VN Permyakov EA, editors. Encyclopedia of metalloproteins. New York (NY): Springer; 2013. p. 670–678.
- Manohar DM, Noeline BF, Anirudhan TS. Adsorption performance of Al-pillared bentonite clay for the removal of cobalt(II) from aqueous phase. Appl Clay Sci. 2006;31(3–4):194–206.
- Schmidt T, Buchert M, Schebek L. Investigation of the primary production routes of nickel and cobalt products used for Li-ion batteries. Resour Conserv Recy. 2016;112:107–122.
- He M, Zhu Y, Yang Y, et al. Adsorption of cobalt(II) ions from aqueous solutions by palygorskite. Appl Clay Sci. 2011;54(3–4):292–296.
- Vilvanathan S, Shanthakumar S. Biosorption of Co(II) ions from aqueous solution using Chrysanthemum indicum: kinetics, equilibrium and thermodynamics. Process Saf Environ. 2015;96:98–110.
- Suh M, Thompson CM, Brorby GP, et al. Inhalation cancer risk assessment of cobalt metal. Regul Toxicol Pharmacol. 2016;79:74–82.
- Behl M, Stout MD, Herbert RA, et al. Comparative toxicity and carcinogenicity of soluble and insoluble cobalt compounds. Toxicology. 2015;333:195–205.
- Bhatnagar A, Minocha AK, Sillanpää M. Adsorptive removal of cobalt from aqueous solution by utilizing lemon peel as biosorbent. Biochem Eng J. 2010;48(2):181–186.
- Krishnan KA, Anirudhan TS. Kinetic and equilibrium modelling of cobalt(II) adsorption onto bagasse pith based sulphurised activated carbon. Chem Eng J. 2008;37(2):257–264.
- Kryvoruchko AP, Yurlova LY. Influence of some organic and inorganic additives on pressure-driven purification of waters containg cobalt. J Water Chem Technol. 2015;37(6):271–276.
- Rengaraj S, Moon S-H. Kinetics of adsorption of Co(II) removal from water and wastewater by ion exchange resins. Water Res. 2002;36(7):1783–1793.
- Huang F, Guo CL, Lu GN, et al. Bioaccumulation characterization of cadmium by growing Bacillus cereus RC-1 and its mechanism. Chemosphere. 2014;109:134–142.
- Zhao MH, Zhang CS, Zeng GM, et al. Toxicity and bioaccumulation of heavy metals in Phanerochaete chrysosporium. Trans Nonferr Metal Soc China. 2016;26(5):1410–1418.
- Vargas-García MC, López MJ, Suárez-Estrella F, et al. Compost as a source of microbial isolates for the bioremediation of heavy metals: in vitro selection. Sci Total Environ. 2012;431:62–67.
- Gönen F, Aksu Z. Single and binary dye and heavy metal bioaccumulation properties of Candida tropicalis: use of response surface methodology (RSM) for the estimation of removal yields. J Hazard Mater. 2009;172(2–3):1512–1519.
- Roane TM, Josephson KL, Pepper IL. Dual-bioaugmentation strategy to enhance remediation of cocontaminated soil. Appl Environ Microbiol. 2001;67(7):3208–3215.
- Malik A. Metal bioremediation through growing cells. Environ Int. 2004;30(2):261–278.
- Larimer FW, Chain P, Hauser L, et al. Complete genome sequence of the metabolically versatile photosynthetic bacterium Rhodopseudomonas palustris. Nat Biotechnol. 2004;22(1):55–61.
- Deng X, Jia P. Construction and characterization of a photosynthetic bacterium genetically engineered for Hg2+ uptake. Bioresour Technol. 2011;102(3):3083–3088.
- Nunkaew T, Kantachote D, Nitoda T, et al. Characterization of exopolymeric substances from selected Rhodopseudomonas palustris strains and their ability to adsorb sodium ions. Carbohydr Polym. 2015;115:334–341.
- Weaver PF, Wall JD, Gest H. Characterization of Rhodopseudomonas capsulata. Arch Microbiol. 1975;105(1):207–216.
- Kumar M, Upreti RK. Impact of lead stress and adaptation in Escherichia coli. Ecotoxicol Environ Safe. 2000;47(3):246–252.
- Pabst MW, Miller CD, Dimkpa CO, et al. Defining the surface adsorption and internalization of copper and cadmium in a soil bacterium, Pseudomonas putida. Chemosphere. 2010;81(7):904–910.
- Salt DE, Pickering IJ, Prince RC, et al. Metal accumulation by aquacultured seedlings of Indian mustard. Environ Sci Technol. 1997;31(6):1636–1644.
- Egland PG, Pelletier DA, Dispensa M, et al. A cluster of bacterial genes for anaerobic benzene ring biodegradation. Proc Natl Acad Sci USA. 1997;94(12):6484–6489.
- Congeevaram S, Dhanarani S, Park J, et al. Biosorption of chromium and nickel by heavy metal resistant fungal and bacterial isolates. J Hazard Mater. 2007;146(1–2):270–277.
- Mishra A, Malik A. Recent advances in microbial metal bioaccumulation. Crit Rev Environ Sci Technol. 2013;43(11):1162–1222.
- Degen O, Eitinger T. Substrate specificity of nickel/cobalt permeases: insights from mutants altered in transmembrane domains I and II. J Bacteriol. 2002;184(13):3569–3577.
- Rashmi K, Sowjanya TN, Mohan PM, et al. Bioremediation of 60Co from simulated spent decontamination solutions. Sci Total Environ. 2004;328(1–3):1–14.
- Bai HJ, Zhang ZM, Yang GE, et al. Bioremediation of cadmium by growing Rhodobacter sphaeroides: kinetic characteristic and mechanism studies. Bioresour Technol. 2008;99(16):7716–7722.
- Luo W, Gu Q, Chen W, et al. Biodegradation of acetochlor by a newly isolated Pseudomonas strain. Appl Biochem Biotech. 2015;176(2):636–644.
- Prokkola H, Kuokkanen T, Vähäoja P, et al. Characterization and biodegradation rates of tall oil soaps in different water and soil environments. Water Air Soil Pollut. 2014;225(9):1–11.
- Guo H, Luo S, Chen L, et al. Bioremediation of heavy metals by growing hyperaccumulaor endophytic bacterium Bacillus sp. L14. Bioresour Technol. 2010;101(22):8599–8605.
- Sar P, Kazy SK, Singh SP. Intracellular nickel accumulation by Pseudomonas aeruginosa and its chemical nature. Lett Appl Microbiol. 2001;32(4):257–261.
- Choudhary S, Sar P. Uranium biomineralization by a metal resistant Pseudomonas aeruginosa strain isolated from contaminated mine waste. J Hazard Mater. 2011;186(1):336–343.