ABSTRACT
Trichosporon cutaneum is able to metabolize high hydrophobic natural compounds such as fatty acids and also can be used as an effective biodegrader to remove a number of toxic aromatic compounds from the environment. However, its growth characteristics were poorly investigated and no yeast–mycelium dimorphism process has been established yet. In the present study, we provided first insights into the effect of nitrogen sources, carbon source, and amino acids together with pH and temperature on morphological switch of T. cutaneum B3. The results showed under close to neutral or weakly alkaline pH conditions, T. cutaneum B3 produced mostly yeast-like cells; while under acidic pH conditions, it produced mostly hyphal-like cells. Under buffered conditions, low nitrogen concentration (<0.2%) would facilitate T. cutaneum B3 to produce yeast-like cells, while the presence of relative high nitrogen concentration (>1%) would induce hyphal-like cells. Under non-buffered conditions, ammonium sulphate, diammonium phosphate, urea and N-acetylglucosamine may via alteration of environmental pH affect yeast–mycelium dimorphism transitions. Methionine, tryptophan and histidine invariably induce pseudohyphal or hyphal morphology. 25 and 28 °C can promote yeast-like cells growth, while cultivated at 37 °C can induce hyphal-like cells growth. Thus, the nitrogen source, alteration of environmental pH and temperature of cultivation played an important role in inducing yeast–mycelium dimorphism transition. Our study confirms the yeast–mycelium dimorphism process of T. cutaneum B3 and highlights that it seems to be a suitable yeast model for further molecular genetics investigation of dimorphism and applications in fermentation morphology engineering.
Introduction
Dimorphism is a peculiar characteristic of several yeast species and filamentous fungi, such as Candida albicans, Saccharomyces cerevisiae, Yarrowia lypolitica, Pichia fermentans, Schizosaccharomyces japonicus, Ustilago maydis, Ophiostoma floccosum, Ceratocystis ulmi, Mycosphaerella graminicola, which can switch between unicellular yeast and multicellular filamentous growth forms in response to changing environmental cues [Citation1–8]. Usually, in the yeast stage, mitotic divisions either by budding or fission to produce two independent cells, while in the filamentous stage, cells become elongated yet fail to abscise following cytokinesis, and remain attached to form chains of elongated pseudohyphal cells; the true hyphae are produced with long continuous tubes and septae separating each of the nuclei in these tubes [Citation2].
Although previous studies reported that the yeast–mycelium dimorphism transition of fungal might be functional and played an important role in their coping with nutrient starvation or other environmental stress, there is increasing evidence that dimorphism may be related to virulence and pathogenicity in several fungal pathogens of plants and animals [Citation3,Citation9–11]. For example, nutrient and nitrogen deprivation, exposure to air and acid pH can promote the yeast-to-hypha transition in U. maydis and show pathogenicity to corn [Citation12]. In C. albicans, alterations in pH, high temperature, nutrient deprivation and addition of serum or N-acetylglucosamine are the most commonly environmental cues which can induce its morphology to change from round budding cells to elongated hyphae or filamentous growth and also show pathogenic trait to mammals [Citation2,Citation13]. Moreover, under yeast-like morphology, the yeast Pichia fermentans DiSAABA 726 controls brown rot caused by Monilia spp. on apple fruit, while under pseudohyphal form, it shows pathogenic behaviour on peach fruit [Citation4,Citation14,Citation15]. Hence, in order to facilitate better control of morphology switch and further understanding of the dimorphic transitions mechanisms, a great number of investigations into the nutritional and environmental stimuli which can induce dimorphic transitions have been conducted. Previous investigations have documented that dimorphic transition is normally induced by a variety of environmental and nutritional factors including temperature [Citation9], quorum sensing molecules [Citation5], inoculum size [Citation16], the accessibility of nitrogen source [Citation14] and carbon [Citation17], the concentration of serum [Citation7], amino acid [Citation18], pH [Citation19] and so on. Despite many different environmental factors that can induce dimorphism transition, the signalling pathways involved in connecting external stimuli and cell morphological transformation are highly conserved even among distantly related fungi [Citation14,Citation20]. Signalling through both the nutrient-sensing cyclic adenosine monophosphate (cAMP)-protein kinase A (PKA) pathway and a mitogen-activated protein kinase (MAPK) pathway play an important role in morphogenesis or virulence in many fungi, including S. Cerevisiae [Citation1,Citation21] C. Albicans [Citation13,Citation22] and U. maydis [Citation23,Citation24]. However, the transcription factors or other stimulus which are involved in both signalling pathways can be different in different fungal species. In S. cerevisiae, both pathways converge on FLO11 promoter which encodes a cell surface protein required for pseudohyphal formation [Citation25]. Meanwhile, the FLO11 promoter is also controlled by numerous transcriptional regulators, including Rim101, Flo8, Ste12/Tec1, as well as Mss11 [Citation20]. In C. albicans, the transcription factors Cph1 and Efg1 are involved in the MAPK and cAMP signalling pathway, respectively [Citation26–28]. Unlike the cAMP-PKA signalling pathway which collaborates with the MAPK signalling pathway in S. cerevisiae and C. albicans, the signalling pathways act in the opposite way in U. maydis [Citation2]. The cAMP-PKA pathway represses hyphal growth, whereas the MAPK cascade acts as a positive effector of hyphal growth and mating [Citation29,Citation30]. Consequently, investigation into the nutritional and environmental stimuli in different dimorphic fungi species may help to understand the physiology characters and build foundations for elucidating the dimorphic biology mechanisms of the peculiar fungal models.
The yeast, Trichosporon cutaneum, is an anamorphic fungi in the genus Trichosporon family Trichosporonaceae with versatile biotechnological potential, which can utilize a wide variety of carbon sources and also has an outstanding capacity to metabolize phenol and other various aromatic compounds [Citation31–35]. Due to these properties, the recent studies on T. cutaneum were focused more on bioconversion of various carbon source feedstocks such as cassava starch, pectin-derived carbohydrates, corn stover and corncob residues hydrolysate into microbial lipid [Citation35–39]. Meanwhile, studies on the fundamental physiology and morphology characters of T. cutaneum are still very limited [Citation33,Citation40]. To the best of our knowledge, although Depree et al. [Citation40] mentioned that T. cutaneum is able to form either yeast cells or filamentous form, inducing environmental and nutritional conditions are still unclear. Furthermore, this species belongs to the Basidiomycetes class and is distantly related to the Saccharomyces or Candida species, so it may show different and unusual features in its dimorphism and in environmental sensing which may also be used as an alternative model to study dimorphic transition. Hence, the aims of this work were (1) to provide a first insight into the nutritional and environmental stimuli that can induce T. cutaneum yeast–mycelium dimorphic transitions, and (2) to build foundations for future investigations on the regulatory mechanisms of dimorphism transition and applications in fermentation morphology engineering.
Materials and methods
Strain and culture conditions
The yeast strain T. cutaneum B3 was isolated from our previous mutation experiment and deposited in China Center for Type Culture Collection (NO. CCTCC M2010076). In this study, the strain was maintained on yeast extract peptone dextrose medium (YEPD) agar slants (2% glucose, 1% yeast extract, 1% peptone, pH 6.0) at 4 °C for short-term storage, and maintained in YEPD plus 20% glycerol at −80 °C for long-term storage.
The other culture media used were: YNB-based media (0.67% Yeast Nitrogen Base [with 0.5% ammonium sulphate], plus 0.2% or 1.17% glucoseor, xylose, fructose or N-acetylglucosamine, as the sole carbon sources, as required); and YCB-based media (1.17% Yeast Carbon Base, plus 0.02%, 0.2% and 1.0% yeast extract, peptone, urea, diammonium phosphate, ammonium sulphate or amino acids, as the sole nitrogen sources, as required). shows all the abbreviations of nutrient media used in this study.
Table 1. Abbreviations and compositions of nutrient media used in this study.
Phosphate buffer (0.2 mol/L disodium hydrogen phosphate, 0.2 mol/L sodium dihydrogen phosphate, pH 6.0) and citrate buffer (0.1 M citric acid, 0.1 mol/L sodium citrate, pH 6.0) were also added to the YCB-based culture media that contained 0.02%, 0.2% and 1.0% yeast extract, peptone, urea, or amino acids, ammonium sulphate and ammonium phosphate as required; and YNB-based media that contained 0.2% and 1.17% glucoseor, xylose, fructose or N-acetylglucosamine, as required. For solid culture, all of the media were used with 1.8% agar, unless otherwise specified.
Trichosporon cutaneum B3 was precultured overnight at 28 °C in YEPD and the cells were then rinsed thrice in sterile distilled water, inoculated on solid (5 × 106 cells) or liquid (5 × 106 cells m−1) media and incubated statically or in a rotary shaker at 180 rpm at 28 °C for 72 h or 96 h as required.
The effect of cultivate temperature on the morphology of T. cutaneum B3 was carried out on YEPD media, and the temperatures of cultivation were 25, 28 and 37 °C. Microscopic observations were made after 48 and 72 h of cultivation.
Morphology analysis
Cell morphology was determined by scraping the surfaces of the colonies and examining the cells by light microscopy or by growing cells in liquid media followed by microscopic analysis. The cells were photographed at 1000× magnification, using an Motic BA310 digital microscope with built-in 3.0 Mega Pixel Digital Camera (Ted Pella, Inc., Redding, CA, USA). Percentages of yeast-like cells and pseudohyphae were evaluated from more three-independent cultures for each growth condition and the images of representative cells are shown.
Results and discussion
Preliminary studies of the morphology of T. cutaneum strain cultivated on YEPD
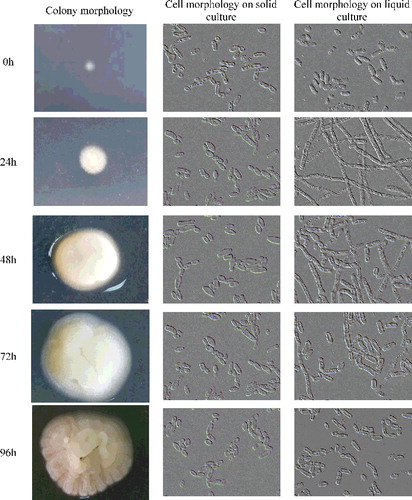
Trichosporon cutaneum B3 was cultivated on YEPD solid and liquid media and microscopic observations of cell morphology were made after 24, 48, 72 and 96 h of incubation. The colonies of the strain appeared from smooth with entire margin to rough and with filamentous margin and crepe morphology (). The liquid YEPD media can induce morphological switch, as they enabled the production of tenuous hyphae at 48 h, while after 72 h of incubation, the tenuous hyphae cells started to morph to short ones, the shorter, hyphal-like cells then switched to yeast form (). Although solid YEPD media can only induce a few hyphal form growth, most of them displayed yeast form growth (). Our present results were to some extend contradictory to the earlier report that T. cutaneum only assumed the yeast form when grown on liquid media, and in nature as a filamentous fungus growing in soil and leaf litter [Citation40]. It was noteworthy that the inducing environmental and nutritional conditions are still unclear. The changes observed on YEPD medium led us to investigate the effect of individual nutritional effectors on the cell morphology of T. cutaneum B3.
The nitrogen and carbon source dictates the morphology of T. cutaneum
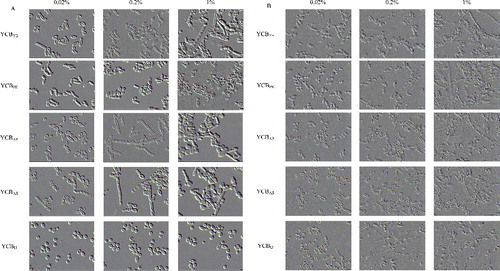
In order to identify the individual nutritional effectors of dimorphic transition, T. cutaneum B3 was plated onto a variety of culture media that differed according to the carbon and nitrogen sources (). For the majority of these culture media, T. cutaneum B3 produced mixtures of yeast-like cells and hyphae (). Some of the media tested led to the better segregation of these two morphologies. In particular, on YCBU (YCB plus 0.02%, 0.2% or 1% urea), YCBYE (YCB plus 0.02% or 0.2% yeast extract) and YCBPE (YCB plus 0.02% or 0.2% peptone), T. cutaneum B3 produced mainly yeast-like cells ((A)). On YCBYE (YCB plus 1% yeast extract) and YCBPE (YCB plus 1% peptone), YCBAS (YCB plus 0.02%, 0.2% or 1% ammonium sulphate) and YCBAP (YCB plus 0.2% or 1% diammonium phosphate), T. cutaneum B3 showed pseudohyphal/hyphal morphology or chlamydospores ((A)). Thus, as observed for other yeasts, T. cutaneum B3 produced mainly yeast-like cells on media containing readily assimilable nitrogen sources such as yeast extract and peptone or urea. In contrast, pseudohyphal or hyphal transition was seen with diammonium phosphate (YCBAP) or ammonium sulphate (YCBAS) or with high concentration nitrogen sources.
Table 2. Trichosporon cutaneum B3 cell morphology on the different culture media, according to the medium supplements.
Figure 2. Cell morphology of T. cutaneum B3 cultivated on YCB-based medium with 0.02%, 0.2% and 2% of the different of nitrogen sources. (A) Cultivation on non-buffered YCB-based medium and (B) cultivation on citrate-buffered YCB-based medium (pH 6.0). The cells were photographed at 1000× magnifications after 72 h growth on the medium and the inoculum was 100% yeast-like cells. YCBYE, YCB-based medium plus yeast extract; YCBPE, YCB-based medium plus peptone; YCBAP, YCB-based medium plus (NH4)2HPO4; YCBAS, YCB-based medium plus (NH4)2SO4; YCBU, YCB-based medium plus urea.
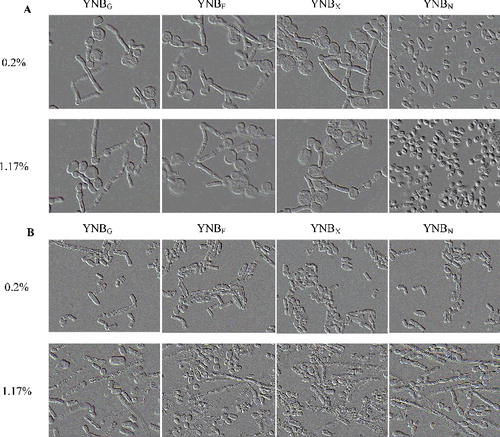
Considering that T. cutaneum B3 showed high potential in bioconversion of various carbon sources, we tested the effect of fructose, xylose, N-acetylglucosamine as compared with glucose on its growth and cell morphologies. It was observed that T. cutaneum B3 grew in YNB medium with all of those carbon sources ((A)). Nevertheless, growth rate of the T. cutaneum B3 in synthetic YNB medium containing different carbon sources was somewhat slower when compared to the one in YEPD medium without (NH4)2SO4. The YNB medium plus N-acetylglucosamine as sole carbon source can induce T. cutaneum B3 to morph into mainly yeast-like cells, in contrast the YNB medium plus fructose, xylose or glucose as sole carbon sources induced mostly pseudohyphal and chlamydospores morphology ((A)).
Figure 3. Cell morphology of T. cutaneum B3 cultivated on YNB-based medium with 0.2% and 1.17% of the different of carbon sources. (A) Cultivation on non-buffered YNB-based medium and (B) cultivation on citrate-buffered YNB-based medium (pH 6.0). The cells were photographed at 1000× magnifications after 72 h growth on the medium and the inoculum was 100% yeast-like cells. YNBG, YNB-based medium plus glucose; YCBF, YNB-based medium plus fructose; YNBX, YNB-based medium plus xylose; YNBN, YNB-based medium plus N-acetylglucosamine.
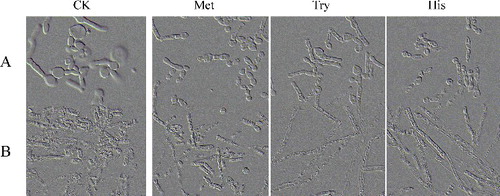
In a further experiment, the effect of some specific amino acids (considering that the YCB-based media contains 0.02% methionine, 0.02% tryptophan and 0.01% histidine) on the dimorphic transition was analysed in a YNB medium added with 1% glucose, initial pH 6.0. The results were observed similar to YNB medium plus fructose, xylose or glucose. All tested amino acids elongated the T. cutaneum B3 cell and led to transition into pseudohyphal and chlamydospores morphology, but to a lesser amount than diammonium phosphate (YCBAP) or ammonium sulphate (YCBAS) (), and the growth rate was also slow.
Figure 4. The cell morphology of T. cutaneum B3 cultivated on non-buffered and buffered YNB-based medium with 1% glucose as carbon source and 0.02% methionine or 0.02% tryptophan or 0.01% histidine as required. The cells were photographed at 1000× magnifications after 72 h growth on the medium and the inoculum was 100% yeast-like cells. A: non-buffered; B: citrate-buffered; CK: YNB-based medium contained 1% glucose without those three amino acids.
In sum, these results suggested that lower concentration of readily assimilable nitrogen sources can induce yeast-like cells growth in T. cutaneum B3. And this hypothesis, to some extent, is consistent with the known effects of nitrogen limitation on other yeast morphogenesis [Citation4,Citation41–43]. Although different concentration of yeast extract and peptone proved to be an effective inducer of dimorphism transition in T. cutaneum, their components responsible for this phenotypic switching have not yet been identified, which means that further research is needed. Interestingly, several studies have suggested that, in addition to their role as building blocks in protein synthesis, amino acids might also have morphogenetic activity. Donaton et al. [Citation44] reported that, under nitrogen starvation, the general amino acid permease Gap1 of S. cerevisiae acts as an amino acid sensor for activation of protein kinase A, a pathway which also controls pseudohyphal differentiation. Kim et al. [Citation18] reported that under the synthetic medium SD with 10 mmol/L of citrulline can strongly induce pseudohyphal morphogenesis in Candida parapsilosis. Maidan et al. [Citation45] in C. albicans and Xue et al. [Citation46] in Cryptococcus neoformans indicated that G-protein-coupled receptors are important for methionine-induced transition from yeast to hyphae cells. In this study, the three amino acids, i.e. methionine, tryptophan and histidine also can induce pseudohyphal and chlamydospores morphogenesis in T. cutaneum B3 as the similar effect attributed to YNB medium plus fructose, xylose or glucose ( and ).
The effect of pH on cell morphology of T. cutaneum
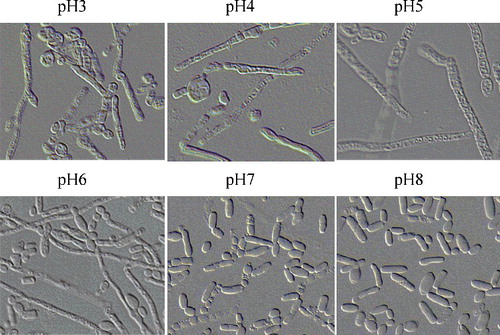
Earlier it was reported that altering the pH in the media can promote the transition yeast–mycelium dimorphism [Citation47,Citation48]. To determine whether the pH of the media is important for T. cutaneum B3 morphogenesis, yeast cells were inoculated on YEPD liquid media with different origination pH of 3.0, 4.0, 5.0, 6.0, 7.0 and 8.0. It was observed that at pH 3.0, 4.0 or 5.0 a larger amount of hyphal-like cells were obtained, at pH 6.0 with mixtures of yeast-like cells and hyphae cells, as compared to pH 7.0 or 8.0 with mostly yeast-like cells ( and ). Our results were contradictory to the studies on both Yarrowia lipolytica [Citation41] and C. albicans [Citation49], which under acidic pH conditions can induce yeast-like cells, whereas weakly alkaline pH conditions induced pseudohyphal cells. These different results obtained might be explained by the possibility of T. cutaneum species to use different pathways for morphogenesis transition under nutritional and environmental conditions.
Figure 5. The cell morphology of T. cutaneum B3 cultivated on YEPD medium of different pH. The cells were photographed at 1000× magnifications after 72 h growth on the medium and the inoculum was 100% yeast-like cells.
Previous investigations have documented that the consumption of the carbon and nitrogen sources may led to production of organic acid or other metabolites and consequently change the pH in the media [Citation50,Citation51]. A similar phenomenon was also seen in the culture broth of T. cutaneum B3, the pH in some culture broth can be greatly changed during the cell cultivation (). The pH of YNB-based media (YNBG, YNBF and YNBX) and YCB-based media (YCBAP and YCBAS) dropped between 3.07 and 4.18 after 72 h of incubation. These results coincided with the previous report of strain of Ophiostoma cultured in OMM medium supplemented with ammonium sulphate [Citation52]. While the pH of YNBN and YCBU culture broth were increased to alkaline conversely. However, the pH of YCB-based media YCBYE and YCBPE culture broth were both kept rather constant after 72 h of cultivation. Therefore, it needed to be considered whether the influence of the above carbon and nitrogen sources on cell morphology of T. cutaneum B3 was due to the pH change in the media during cultivation. To evaluate this hypothesis, T. cutaneum B3 was inoculated into YCB-based liquid media containing yeast extract, peptone, urea, diammonium phosphate, ammonium sulphate at the same concentrations as previously used, with the addition of citrate buffer (pH 6.0) to keep pH rather constant. Both cell morphology and pH were monitored for up to 72 h.
Table 3. pH value of the non-buffered and buffered liquid culture media at the start (0 h) and end (72 h) of the T. cutaneum B3 culture broth.
The results obtained here showed that T. cutaneum B3 grew with mostly yeast-like cells in buffered YCBYE plus 0.02% or 0.2% yeast extract, but with some hyphal-like cells in buffered YCBYE plus 1% yeast extract, which was the same as in the non-buffered YCBYE ((A,B)). Similarly, both in buffered and non-buffered YCBPE, T. cutaneum B3 grew with mostly yeast-like cells. Apparently, in buffered YCBAS and YCBAP, T. cutaneum B3 grew with mostly yeast-like cells, contrary to non-buffered YCBAS and YCBAP that T. cutaneum B3 grew with pseudohyphal/hyphal morphology correspond to our previous results ((A,B)). However, in buffered YCBU, T. cutaneum B3 grew with mixtures of yeast-like cells and hyphal-like cells ((B)), these results contrary to non-buffered YCBU that induced T. cutaneum B3 grew with mostly yeast-like cells ((A)).
The same protocol was thus repeated on buffered and non-buffered YNB-based liquid media containing glucose, fructose, xylose, N-acetylglucosamine, methionine, tryptophan or histidine. Contrary to what was seen for non-buffered YNBG, YNBF, YNBX, YNBG+M, YNBG+T and YNBG+H media, on these buffered YNBG, YNBF and YNBX media T. cutaneum B3 grew with mostly tenuous pseudohyphal or hyphal morphology ((A,B)), while on buffered YNBG+M, YNBG+T and YNBG+H media mostly hyphal morphology was observed ((B)). However, in buffered YNBN, the cell morphology of T. cutaneum B3 was mixtures of yeast-like cells and hyphae cells, contrary to non-buffered YNBN, on which T. cutaneum B3 grew with only yeast-like cells ((B)). To further verify the influence of pH on the cell morphology of T. cutaneum B3, but not due to the citrate, the above same protocol was thus repeated and investigated on another buffer phosphate buffer (pH 6.0) to keep pH rather constant. The results were similar to what were seen for using citrate buffer (data not shown). According to our results illustrated in , and indicated that the dimorphic transition of T. cutaneum B3 induced by diammonium phosphate, ammonium sulphateammonium, urea and N-acetylglucosamine was also dependent on the their pH, as diammonium phosphate and ammonium sulphateammonium both can reduce the pH value in media to acidic and then induce hyphal growth, while urea and N-acetylglucosamine both can increase pH value in media to alkaline and then induce yeast-like cells growth.
Conclusively, by investigating the effect of ammonium salts as nitrogen source on yeast–mycelium dimorphism, we observed several differences between our results and previously published on other yeast. Sanna et al. [Citation4] reported that diammonium phosphate and ammonium sulphate can induce in P. fermentans mainly formation of yeast-like cells and pseudohyphae, respectively. Contradictory to the report by Sanna et al. [Citation4], the two salts (NH4)2HPO4 (diammonium phosphate) and (NH4)2SO4 (ammonium sulphate) which contain both equal molecular weight and equal amounts of N showed a similar effect on T. cutaneum dimorphism transition as nitrogen sources. Both of them induced formation of pseudohyphal at 1.52 mmol/L (i.e. plus 0.2%) in non-buffered YCBAP and YCBAS, while an opposite phenomenon was observed for buffered YCBAP and YCBAS which at the same concentration induced yeast-like cells (). The low pH value readings (3.97 and 3.09) were recorded both in non-buffered YCBAP and YCBAS after 72 h of incubation. In contrast, the pH values in buffered YCBAP and YCBAS culture broth kept relatively constant (5.59 and 5.62). In the non-buffered YCBAP and YCBAS, the similar trend of pH values changes was also recorded in the other concentrations of nitrogen sources (i.e. plus 0.02% and 1% diammonium phosphate or ammonium sulphate) (). Thus, it appears that the diammonium phosphate or ammonium sulphate-induced morphogenesis in T. cutaneum was very pH value dependent, as well as concentration dependent. In both citrate or phosphate buffered YCBAP and YCBAS, it produced mostly yeast-like cells under a relatively lower concentration (0.02% or 0.2%) of diammonium phosphate or ammonium sulphate. However, when 1% diammonium phosphate or ammonium sulphate was added, T. cutaneum B3 showed dimorphism transition from mostly yeast-like cells to a few pseudohyphal. As expected from the effect of different pH values (3.0–8.0) on morphogenesis in T. cutaneum under YEPD, low pH values (below 5.0) can also induce mostly hyphae, while the relative high pH values (over 5.0) induce mostly yeast-like cells in YCB and YNB-based media. This alteration of environmental pH effects on yeast–mycelium dimorphism transitions were also observed in the presence of urea and N-acetylglucosamine. Both of them alkalinized the extracellular environment in the non-buffered YNBN and YCBU media and T. cutaneum B3 showed yeast-like cells growth. However, an opposite phenomenon was observed in buffered YNBN and YCBU media, with the mixtures of yeast-like cells and hyphae growth.
Dimorphic transition in T. cutaneum is reversible
The yeast-like cells and pseudohyphae cells from YCBYE containing 0.02% yeast extract and YCBYE containing 1% yeast extract media, respectively, were separated with a micromanipulator and inoculated on both YCBYE containing 1% yeast extract and YCBYE containing 0.02% yeast extract media. This resulted in the yeast-like cells maintaining their morphology on YCBYE containing 0.02% yeast extract media but producing pseudohyphae on YCBYE containing 1% yeast extract media. Similarly, the pseudohyphae formed shifted to yeast-like cells on YCBYE containing 0.02% yeast extract media, while maintained their pseudohyphal morphology on YCBYE containing 1% yeast extract media (data not shown). Thus, as had been seen for dimorphic transition with other yeasts, T. cutaneum B3 showed a completely reversible morphology, which may be due to the need to rapidly adapt to nutritional and environmental conditions.
The effect of cultivation temperature on cell morphology of T. cutaneum
The results showed that 25 and 28°C can promote yeast-like cells growth, by contrast, 37 °C can induce the formation of hyphal-like growth (). Thus, as had been seen for other thermally dimorphic fungus, T. cutaneum B3 also showed morphological switch that was related to the temperature of cultivation. But the relationship between dimorphic transition and temperature of cultivation in T. cutaneum was the reverse of that described by Penicillium marneffei [Citation53] and Blastomyces dermatitidis [Citation54].
Conclusion
Based on our findings, we provided for the first time a direct evidence of the nutritional and environmental factors that induce yeast-like and pseudohyphal morphologies in T. cutaneum B3 under laboratory cultural conditions. The nitrogen source, alteration of environmental pH and temperature of cultivation all played an important role in inducing yeast–hyphal dimorphism transition. Our study confirms the yeast–mycelium dimorphism process of T. cutaneum B3 and highlights that it seems to be another suitable model yeast for further molecular genetic investigation of dimorphism and applications in fermentation morphology engineering.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Borges-Walmsley MI, Walmsley AR. cAMP signalling in pathogenic fungi: control of dimorphic switching and pathogenicity. Trends Microbiol. 2000;8:133–141.
- Sánchez-Martínez C, Pérez-Martín J. Dimorphism in fungal pathogens: Candida albicans and Ustilago maydis—similar inputs, different outputs. Curr Opin Microbiol. 2001;4:214–221.
- Nadal M, Gold SE. Dimorphism in fungal plant pathogens. FEMS Microbiol Lett. 2008;284:127–134.
- Sanna ML, Zara S, Zara G et al., Pichia fermentans dimorphic changes depend on the nitrogen source. Fungal Biol. 2012;116:769–777.
- Berrocal A, Oviedo C, Nickerson KW et al., Quorum sensing activity and control of yeast-mycelium dimorphism in Ophiostoma floccosum. Biotechnol Lett. 2014;36:1503–1513.
- Bellou S, Makri A, Triantaphyllidou IE et al., Morphological and metabolic shifts of Yarrowia lipolytica induced by alteration of the dissolved oxygen concentration in the growth environment. Microbiology. 2014;160:807–817.
- Papp L, Sipiczki M, Holb IJ et al., Optimal conditions for mycelial growth of Schizosaccharomyces japonicus cells in liquid medium: it enables the molecular investigation of dimorphism. Yeast. 2014;31:475–482.
- Coelho MAZ, Belo I, Pinheiro R et al., Effect of hyperbaric stress on yeast morphology: study by automated image analysis. Appl Microbiol Biotechnol. 2004;66:318–324.
- Nemecek JC, Marcel W, Klein BS. Global control of dimorphism and virulence in fungi. Science. 2006;312:583–588.
- Klein BS, Tebbets B. Dimorphism and virulence in fungi. Curr Opin Microbiol. 2007;10:314–319.
- Gauthier GM. Dimorphism in fungal pathogens of mammals, plants, and insects. PLoS Pathog. 2015;11(2):e1004608.
- Martínez-Espinoza AD, Ruiz-Herrera J, León-Ramírez CG et al., MAP kinase and cAMP signaling pathways modulate the pH-induced yeast-to-mycelium dimorphic transition in the corn smut fungus Ustilago maydis. Curr Microbiol. 2004;49:274–281.
- Zhu Y, Fang HM, Wang YM et al., Ras1 and Ras2 play antagonistic roles in regulating cellular cAMP level, stationary-phase entry and stress response in Candida albicans. Mol Microbiol. 2009;74:862–875.
- Sanna ML, Zara G, Zara S et al., A putative phospholipase C is involved in Pichia fermentans dimorphic transition. Biochim Biophys Acta. 2014;1840:344–349.
- Giobbe S, Marceddu M, Scherm B et al., The strange case of a biofilm-forming strain of Pichia fermentans which controls Monilinia brown rot on apple but is pathogenic on peach fruit. FEMS Yeast Res. 2007;7:1389–1398.
- Nickerson KW, Atkin AL, Hornby JM. Quorum sensing in dimorphic fungi: farnesol and beyond. Appl Environ Microbiol. 2006;72:3805–3813.
- Palande AS, Kulkarni SV, León-Ramirez C et al., Dimorphism and hydrocarbon metabolism in Yarrowia lipolytica var. indica. Arch Microbiol. 2014;196:545–556.
- Kim SK, El BK, Ben MC. Amino acids mediate colony and cell differentiation in the fungal pathogen Candida parapsilosis. Microbiology. 2006;152:2885–2894.
- Heintz-Buschart A, Eickhoff H, Hohn E et al., Identification of inhibitors of yeast-to-hyphae transition in Candida albicans by a reporter screening assay. J Biotechnol. 2013;164:137–142.
- Ryan O, Shapiro RS, Kurat CF et al., Global gene deletion analysis exploring yeast filamentous growth. Science. 2012;337:1353–1356.
- Thevelein JM, de Winde JH. Novel sensing mechanisms and targets for the cAMP-protein kinase A pathway in the yeast Saccharomyces cerevisiae. Mol Microbiol. 1999;33:904–918.
- Román E, Arana DM, Nombela C et al., MAP kinase pathways as regulators of fungal virulence. Trends Microbiol. 2007;15:181–190.
- Gold S, Duncan G, Barrett K et al., cAMP regulates morphogenesis in the fungal pathogen Ustilago maydis. Genes Dev. 1994;8:2805–2816.
- Durrenberger F, Wong K, Kronstad J W. Identification of a cAMP-dependent protein kinase catalytic subunit required for virulence and morphogenesis in Ustilago maydis. Proc Natl Acad Sci. 1998;95:5684–5689.
- Rupp S, Summers E, Lo HJ et al., MAP kinase and cAMP filamentation signaling pathways converge on the unusually large promoter of the yeast FLO11 gene. EMBO J. 1999;18:1257–1269.
- Lo HJ, Köhler JR, DiDomenico B et al., Nonfilamentous C. albicans mutants are avirulent. Cell. 1997;90:939–949.
- Brown AJP, Gow NAR. Regulatory networks controlling Candida albicans morphogenesis. Trends Microbiol. 1999;7:333–338.
- Feng Q, Summers E, Guo B et al., Ras signaling is required for serum-induced hyphal differentiation in Candida albicans. J Bacteriol. 1999;181:6339–6346.
- Hartmann HA, Kahmann R, Bolker M. The pheromone response factor coordinates filamentous growth and pathogenic development in Ustilago maydis. EMBO J. 1996;15:1632–1641.
- Kahmann R, Basee C, Feldbrugge M. Fungal-plant signalling in the Ustilago maydis-maize pathosystem. Curr Opin Microbiol. 1999;2:647–650.
- Moon NJ, Hammond EG, Glatz BA. Conversion of cheese whey and whey permeate to oil and single-cell protein. J Dairy Sci. 1978;61:1537–1547.
- Anderson J J, Dagley S. Catabolism of aromatic acids in Trichosporon cutaneum. J Bacteriol. 1980;141:534–543.
- Sieńko M, Stępień PP, Paszewski A. Generation of genetic recombinants in Trichosporon cutaneum by spontaneous segregation of protoplast fusants. J Gen Microbiol. 1992;138:1409–1412.
- Gerginova M, Zlateva P, Peneva N et al., Influence of phenolic substrates utilised by yeast Trichosporon cutaneum on the degradation kinetics. Biotechnol Biotec Equip. 2014;281:33–37.
- Wang Y, Gong Z, Yang X et al., Microbial lipid production from pectin-derived carbohydrates by oleaginous yeasts. Process Biochem. 2015;50:1097–1102.
- Hu C, Wu S, Wang Q et al., Simultaneous utilization of glucose and xylose for lipid production by Trichosporon cutaneum. Biotechnol Biofuels. 2011;4:25.
- Yuan JY, Ai1 ZZ, Zhang ZB et al., Microbial oil production by Trichosporon cutaneum B3 using cassava starch. Chin J Biotechnol. 2011;27:453–460.
- Liu W, Wang Y, Yu Z et al., Simultaneous saccharification and microbial lipid fermentation of corn stover by oleaginous yeast Trichosporon cutaneum. Biores Technol. 2012;118:13–18.
- Gao Q, Cui Z, Zhang J et al., Lipid fermentation of corncob residues hydrolysate by oleaginous yeast Trichosporon cutaneum. Biores Technol. 2014;152:552–556.
- Depree J, Emerson GW, Sullivan PA. The cell wall of the oleaginous yeast Trichosporon cutaneum. J Gen Microbiol. 1993;139:2123–2133.
- Morales-Vargas AT, Domínguez A, Ruiz-Herrera J. Identification of dimorphism-involved genes of Yarrowia lipolytica by means of microarray analysis. Res Microbiol. 2012;163:378–387.
- Morschhäuser J. Nitrogen regulation of morphogenesis and protease secretion in Candida albicans. Int J Med Microbiol. 2011;301:390–394.
- Orlova M, Ozcetin H, Barrett L et al., Roles of the Snf1-activating kinases during nitrogen limitation and pseudohyphal differentiation in Saccharomyces cerevisiae. Eukaryot Cell. 2010;9:208–214.
- Donaton MC, Holsbeeks I, Lagatie O et al., The Gap1 general amino acid permease acts as an amino acid sensor for activation of protein kinase A targets in the yeast Saccharomyces cerevisiae. Mol Microbiol. 2003;50:911–929.
- Maidan MM, Thevelein JM, Van Dijck P. Carbon source induced yeast-to-hypha transition in Candida albicans is dependent on the presence of amino acids and on the G-proteincoupled receptor Gpr1. Biochem Soc Trans. 2005;33:291–293.
- Xue C, Bahn YS, Cox GM et al., G proteincoupled receptor Gpr4 senses amino acids and activates the cAMP-PKA pathway in Cryptococcus neoformans. Mol Biol Cell. 2006;17:667–679.
- González-López CI, Ortiz-Castellanos L, Ruiz-Herrera J. The ambient pH response rim rathway in Yarrowia lipolytica: identification of YlRIM9, and characterization of its role in dimorphism. Curr Microbiol. 2006;53:8–12.
- Selvig K, Alspaugh JA. pH response pathways in fungi: adapting to host-derived and environmental signals. Mycobiology. 2011;39:249–256.
- Biswas S, Van Dijck P, Datta A. Environmental sensing and signal transduction pathways regulating morphopathogenic determinants of Candida albicans. Microbiol Mol Biol Rev. 2007;71:348–376.
- Fang QH, Zhong JJ. Submerged fermentation of higher fungus Ganoderma lucidum for production of valuable bioactive metabolites-ganoderic acid and polysaccharide. Biochem Eng J. 2002;10:61–66.
- Shih IL, Tsai KL, Hsieh C. Effects of culture conditions on the mycelial growth and bioactive metabolite production in submerged culture of Cordyceps militaris. Biochem Eng J. 2007;33:193–201.
- Naruzawa ES, Bernier L. Control of yeast-mycelium dimorphism in vitro, in Dutch elm disease fungi by manipulation of specific external stimuli. Fungal Biol. 2014;118:872–884.
- Suwunnakorn S, Jr CC, Kummasook A et al., Role of the yakA gene in morphogenesis and stress response in Penicillium marneffei. Microbiology. 2014;160:1929–1939.
- Marty AJ, Gauthier GM. Blastomyces dermatitidis, septins CDC3, CDC10, and CDC12, impact the morphology of yeast and hyphae, but are not required for the phase transition. Med Mycol. 2013;51:93–102.