ABSTRACT
The chestnut gall wasp, Dryocosmus kuriphilus Yasumatsu (Hymenoptera: Cynipidae) is one of the most important insect pests of chestnut. The aim of this study was to isolate and characterize bacteria from D. kuriphilus to obtain new microbial agents for both biological control and other biotechnological applications. D. kuriphilus larvae were collected from chestnut fields located in Bursa and Yalova provinces of Marmara Region of Turkey during May–July 2014. Four bacterial isolates were obtained from D. kuriphilus. According to their morphological, biochemical and molecular properties, these isolates were identified as Staphylococcus saprophyticus (Dk1), Paenibacillus sp. (Dk2), Pseudomonas flourescens (Dk3) and Paenibacillus sp. (Dk4). To the best of our knowledge, this is the first study on the bacterial flora of D. kuriphilus. In our study, the potential of these isolates as a biological control agent against different hazardous pests and other possible biotechnological applications of importance were discussed under the light of literature.
Introduction
Insects are considered the most successful group of animals, in terms of both diversity and survivability in various ecological niches [Citation1]. The microflora of an insect's body contains many important bacterial species of biotechnological importance. These bacteria utilize various organic polymers and may belong to the groups of methanogenic and nitrogen-fixing bacteria [Citation2,Citation3]. The gut microflora of insects has also been reported to contribute to important processes, such as the synthesis of pheromones and vitamins, degradation of pesticides and protection against pathogen attack [Citation4,Citation5]. That is why both the total microflora and the gut microflora of insects are a good source of novel isolates of potential biotechnological importance. Furthermore, the insect gut is estimated to contain 10-fold more microbes than the total cells of the insects and 100-fold more microbial genes than animal genes [Citation1,Citation6].
The chestnut gall wasp, Dryocosmus kuriphilus Yasumatsu (Hymenoptera: Cynipidae), is one of the most important pests of chestnuts worldwide. It is native to China and has been detected in various countries from Asia to America and Europe. Reported for the first time in Turkey in 2014, it is currently present in Yalova and Bursa Regions [Citation7]. Considered as a major pest of Castanea species worldwide, D. kuriphilus causes galls and can reduce the fruit production.
Up to now, chemical substances such as methamidophos, omethoate and dichlorvos have been used to control this pest [Citation8]. However, chemical control has hazardous effects on the environment. Although there are a lot of biological control studies on the use of Torymus sinensis Kamijo (Hymenoptera, Torymidae) as a classical biological control agent against D. kuriphilus, to date, the isolation and characterization of bacteria from D. kuriphilus has remained a neglected area of research.
Recently, there has been an increasing interest in the discovery of more effective and pathogenic bacterial control agents against harmful insects. The aim of this study was to isolate and characterize bacteria from D. kuriphilus to obtain new microbial agents to be used for biological control. Other potential biotechnological applications of the bacterial isolates have also been discussed. To the best of our knowledge, this is the first study to investigate the bacterial flora of D. kuriphilus.
Materials and methods
Collection of insects
D. kuriphilus larvae were collected from chestnut fields located in Bursa and Yalova provinces of Marmara Region of Turkey during May–July 2014. The larvae were collected in aseptic conditions and immediately transported to the laboratory.
Isolation of bacteria
Living and dead larvae were distinguished by macroscopic examination. The larvae were surface-sterilized in 70% alcohol and then washed three times in sterile distilled water and homogenized in nutrient broth media (Merck, Darmstadt, Germany) by using a glass tissue grinder. Suspensions were diluted (from 10−1 to 10−8) and 0.1 mL of each suspension was plated on nutrient agar. Plates were incubated at 30 °C for 2–3 days. After the incubation period, the plates were examined and bacterial colonies were selected. The colonies were then purified by subculture on plates.
Identification of bacterial isolates
Bacterial isolates were examined and identified based on their morphological (cell morphology, endospore formation and mobility) and biochemical properties (Gram staining, oxidase and catalase tests). In addition, the biochemical properties of the bacterial isolates were also determined using the Analytical Profile Index API 20E, API 50CH and API STAPH test systems (BioMerieux, Marcy l'Etoile, France).
Polymerase chain reaction (PCR)
Bacterial colonies were inoculated into nutrient broth and incubated for approximately 18 h at 30 °C prior to DNA extraction. At the end of the incubation time, the bacterial cells were collected from the culture medium by centrifugation. Then, genomic DNA was isolated using a Genomic DNA Purification Kit (Promega, Madison, WI, USA,) in accordance with the manufacturer's recommendations. The oligonucleotide primers (Macrogen, Amsterdam, The Netherlands) of 27F (5′-AGAGTTTGATCMTGGCTCAG-3′ as forward) and 1492R (5′-GGYTACCTTGTTACGACTT-3′ as reverse) were used for amplification of the 16S rRNA genes. The PCR mixture contained 5 µL of 5× Taq DNA polymerase reaction buffer (Thermo Fisher SCIENTIFIC, Paisley, UK), 200 µmol/L of each deoxyribonucleoside triphosphate (dNTP), 10 pmol of primers, 1.5 U of Taq DNA polymerase (Thermo Fisher SCIENTIFIC, Paisley, UK), 3 mmol/L of MgCl2 and 5 µL of genomic DNA in a final reaction volume of 50 µL. The PCR was performed under the following conditions (T100™ Thermal Cycler, Bio-Rad, Hercules, CA, USA): 2 min initial denaturation at 94 °C; 35 cycles of denaturation (45 s at 94 °C), annealing (60 s at 55 °C) and extension (60 s at 72 °C) and a final extension at 72 °C for 10 min. Finally, the PCR products were analyzed by electrophoresis in 1% agarose gel and then visualized under ultraviolet (UV) light by staining with ethidium bromide. The PCR products were sequenced by RefGen Biotechnology Laboratory (Ankara, Turkey). The same primer pairs were used for sequencing. The obtained sequences were used for identification of the isolates and phylogenetic analyses. BLAST (Basic Local Alignment Search Tool) searches were performed using the NCBI (National Center for Biotechnology Information) GenBank database to confirm the isolate identification [Citation9].
The evolutionary relationships of the four bacterial isolates were evaluated. Cluster analyses of the sequences were performed using BioEdit (version7.09) with Clustal W followed by neighbour-joining analysis on aligned sequences performed with MEGA 6.0 software [Citation10]. The reliability of the dendrograms was tested by bootstrap analysis with 1000 replicates using MEGA 6.0.
Results and discussion
Recently, there has been an increasing interest in finding more effective and pathogenic bacterial control agents against harmful insects. Although there are a lot of biological control studies on D. kuriphilus to date, the isolation and characterization of bacteria from D. kuriphilus have been neglected. In the present study, four bacterial isolates, two spore-forming ones (isolates Dk2 and Dk4) and two non-spore-forming ones (isolates Dk1and Dk3), were isolated from D. kuriphilus. Some morphological and biochemical characteristics of these bacterial isolates are summarized in . In addition to conventional tests, API test results of bacterial isolates are given in –.
Table 1. Some morphological and biochemical characteristics of D. kuriphilus isolates.
Table 2. API 50CH test results for D. kuriphilus isolates.
Table 3. API 20E test results for D. kuriphilus isolates.
Table 4. API STAPH test results for the Dk1 isolate.
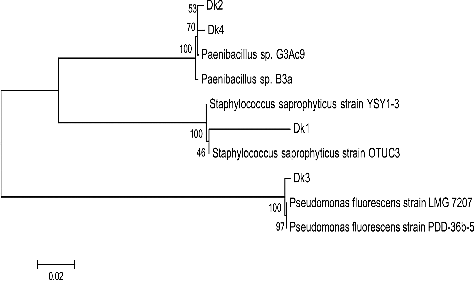
The partial 16S rRNA gene sequences generated in this study were deposited in the GenBank database under the following accession numbers: Dk1, KT377432; Dk2, KT377433; Dk3, KT377434 and Dk4, KT377435 (). A phylogenetic tree was constructed using the neighbour-joining method (). The isolates showed a similarity of 97%–99% compared with other species.
Table 5. GenBank accession numbers and identification of bacterial isolates according to partial 16S rRNA gene sequences.
Figure 1. Neighbour-joining tree of bacterial isolates from D. kuriphilus. The dendrogram was constructed by MEGA 6.0 software based on the partial sequences of the 16S rRNA gene. Bootstrap values shown next to nodes are based on 1000 replicates. The scale on the bottom of the dendrogram shows the degree of dissimilarity.
Staphylococcus saprophyticus
The Dk1 isolate is Gram-positive, non-spore-forming, non-motile, cocci-shaped, catalase-positive and oxidase-negative, with cream-coloured colonies on nutrient agar. Based on the conventional tests, the API 20E, API 50CH, API STAPH, the morphological and biochemical characteristic and the partial 16S rRNA sequence analysis, this isolate was identified as Staphylococcus saprophyticus. Isolate Dk1 has been previously isolated from red imported fire ant larvae, Solenopsis invicta, and mealy bug, Rhizoecus amorphophalli [Citation11,Citation12].
One of the few reports on the potential of S. sapprophyticus as a biological control agent demonstrates that S. sapprophyticus can be used for the control of cattle tick Rhipicephalus (Boophilus) microplus (Acari: Ixodidae) [Citation13]. As pointed out by Miranda-Miranda et al. [Citation13,Citation14], under certain conditions, Staphylococci can cause a lethal infection in fully engorged ticks, but the underlying mechanism by which these bacteria infect the engorged females have yet to be clarified to better assess the potential of S. saprophyticus as an alternative method for control of the cattle tick and tick-transmitted diseases.
Apart from biological control, S. saprophyticus has been used in some other biotechnological applications as well. For example, Ilhan et al. [Citation15] report that S. saprophyticus can be used in the removal or recovery of heavy metal ions, especially lead and chromium ions, from industrial waste waters by biosorption. In another study, lysostaphin, a staphylococcal bacteriolysin with potential clinical applications, was produced and was shown to be potentially useful as a biopreservative in the food industry as well [Citation16].
S. saprophyticus is a good producer of slime (biofilm material). It is well known that a wastewater treatment system based on a trickling filter consists of a fixed bed of rocks containing slime material. That is why slime-producing bacteria might be useful in this type of wastewater treatment systems [Citation17,Citation18].
In addition to the above-mentioned biotechnological potential, some strains of S. saprophyticus (for example S. saprophyticus M36) have high activity of organic solvent-stable lipase [Citation19]. Organic solvent-stable lipase is used in various industries such as food, dairy, pharmaceutical, cosmetic, detergents, textile and biodiesel industry as well as in the synthesis of fine chemicals and new polymeric materials and in environmentally friendly industries [Citation20,Citation21].
Paenibacillus sp.
The Dk2 and Dk4 isolates are motile, Gram-positive, rod-shaped, catalase-positive, oxidase-negative, spore-forming, with cream-coloured colonies on nutrient agar. Based on the morphological and biochemical tests, the API system and 16S rRNA gene sequence analysis, these two isolates were identified as Paenibacillus sp. Some members of the genus Paenibacillus have been associated with insect mortality in one way or another [Citation22]. There are Paenibacillus species (e.g. P. popilliae, P. lentimorbus and P. larvae) that are pathogenic for insects. Isolates Dk2 and Dk4 have also been isolated from different insects in previous studies and have been investigated for pathogenic potential in insects [Citation23,Citation24]. In previous studies, Paenibacillus species have been used as biological control agents. For example, P. alvei strain K165 has been applied against the cotton black root rot pathogen, Thielaviopsis basicola [Citation25]; P. alvei strain K165, against Verticillium wilt of olive trees [Citation26]; P. polymyxa E681, in sesame seed pelleting for prevention of damping-off diseases and wilt [Citation27] and P. polymyxa cw, against tomato and red pepper powdery mildew [Citation28]. On the other hand, Paenibacillus species are some of the most industrially significant facultative anaerobic bacteria. Their natural habitat includes the soil, the rhizosphere and the roots of crop plants, and marine sediments [Citation29]. Although we have limited knowledge about their genomic information, the academic interest towards their ecological and biotechnological roles has increased in the last 20 years or so. For example, P. polymyxa has a lot of different features like nitrogen fixation, promotion of plant growth, solubilization of phosphorus, production of exopolysaccharides, hydrolytic enzymes, antibiotics, cytokinins and other valuable and useful compounds, such as optically active 2,3-butanediol. Other Paenibacillus strains boost the flocculation and enhance the soil porosity [Citation29].
Pseudomonas fluorescens
The Dk3 isolate is Gram-positive, non-spore-forming, non-motile, rod-shaped, catalase- and oxidase-positive, with cream-coloured colonies on nutrient agar. Based on the applied battery of tests, it was identified as Pseudomonas fluorescens. P. fluorescens strains have been reported to produce metabolites with insecticidal properties, such as HCN and the lipopeptides viscosin and orfamide [Citation30,Citation31]. Interestingly, some of the plant root-colonizing pseudomonads are pathogenic to insects and have been proposed for potential exploitation as novel bioinsecticides against root-feeding insects (for review, see [Citation32]). Kuphferschmid et al. [Citation32] discuss the accumulated knowledge about the occurrence and the molecular basis of insecticidal activity in pseudomonads and how this trait could be used in novel root-based approaches for insect control in an integrated framework of pest management. P. fluorescens has been isolated from different insects in previous studies and has been investigated for pathogenicity in insects [Citation33,Citation34]. Although there is no literature on the use of P. fluorescens as a biological control agent against D. kuriphilus, it has been used as a biocontrol agent against some other hazardous organisms, especially phytopathogenic fungi and bacteria. Daval et al. [Citation35] report that P. fluorescens Pf29 can be used for control of the fungus Gaeumannomyces graminis var. tritici (Ggt) on wheat roots. On the other hand, some strains of P. fluorescens have also been used as biocontrol agents against Rhizoctonia solani in tobacco (Nicotiana tabacum) seed beds [Citation36], common scab symptoms in the field [Citation37] and three phytopathogenic fungi, Phythium ultimum, Macrophomina phaseolina and Pyricularia oryzae [Citation38].
Apart from biocontrol studies, P. fluorescens has been used in many other biotechnological applications such as biosynthesis of massetolide, a cyclic lipopeptide antibiotic [Citation39], detoxification and bioremediation of metals such as Al, Ga, and Ca [Citation40,Citation41], fluorescent pigment production [Citation42] and phenazine biosynthesis [Citation43]. All these examples of biological pest control and other biotechnological applications of the isolated bacterial strains and their closely related species strongly suggest that the four bacterial isolates from D. kuriphilus studied here might also possess such valuable properties.
Conclusions
To the best of our knowledge, there has been no report on the isolation and characterization of bacteria from D. kuriphilus until now. The four isolates identified as S. saprophyticus (Dk1), Paenibacillus sp. (Dk2 and Dk4) and Pseudomonas fluorescens (Dk3) belong to species and genera of known practical importance. The survey of relevant literature suggested that all four isolates have a promising potential for use in different biotechnological applications. Future studies should focus on investigation of their biological control properties and possibilities for biotechnological applications.
Disclosure statement
The authors declare that they have no competing interests.
References
- Krishnan M, Bharathiraja C, Pandiarajan J et al. Insect gut microbiome – an unexploited reserve for biotechnological application. Asian Pac J Trop Biomed. 2014;4:16–21.
- Nardi JB, Mackie RI, Dawson JO. Could microbial symbionts of arthropod guts contribute significantly to nitrogen fixation in terrestrial ecosystems. J Insect Physiol. 2002;48:751–763.
- Mrazek J, Strosova L, Fliegerova K et al. Diversity of ınsect ıntestinal microflora. Folia Microbiol. 2008;53:229–233.
- Reeson AF, Jankovic TK, Kasper ML et al. Application of 16 S rDNA–DGGE to examine the microbial ecology associated with a social wasp Vespula germanica. Insect Mol Biol. 2003;12:85–91.
- Özdal Ö, Özdal M, Algur ÖF et al. Isolation and identification of α-endosulfan degrading bacteria from insect microflora. TURJAF. 2016;4:248–254.
- Rajagopal R. Beneficial interactions between insects and gut bacteria. Indian J Microbial. 2009;49:114–119.
- Cetin G, Orman E, Polat Z. First record of the oriental chestnut gall wasp, Dryocosmus kuriphilus Yasumatsu (Hymenoptera: Cynipidae) in Turkey. Bitki Kor Bül. 2014;54:303–309.
- Yan YZ, Liu YS, Jiang DA et al. Study on techniques for integrated control of Dryocosmus kuriphilus Yasumatsu in North Hubei. Plant Protect. 1995;1:5–8.
- Altschul SF, Gısh W, Miller W et al. Basic local alignment search tool. J Mol Biol. 1990;215:403–410.
- Tamura K, Stecher G, Peterson D et al. MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2013;30:2725–2729.
- Peloquin JJ, Greenberg L. Identification of midgut bacteria from fourth instar red imported fire ant larvae, Solenopsis invicta buren (Hymenoptera: Formicidae). J Agr Urban Entomol. 2003;20:157–164.
- Sreerag RS, Jayaprakas CA, Ragesh L et al. Endosymbiotic bacteria associated with the mealy bug, Rhizoecus amorphophalli (Hemiptera: Pseudococcidae). Int Sch Res Notices. 2014:8. Article ID 268491.
- Miranda-Miranda E, Cossio-Bayugar R, Quezada-Delgado MR et al. Staphylococcus sapropiticus is a pathogen of the cattle tick Rhipicephalus (Boophilus) microplus. Biocontrol Sci Technol. 2010;20:1055–1067.
- Miranda-Miranda E, Cossio-Bayugar R, Quezdada-Delgado MR et al. Staphylococcus saprophyticus causa infeccion letal en la garrapata del ganado Rhipicephalus microplus [ Staphylococcus saprophyticus causes lethal infection in the cattle tick Rhipicephalus microplus]. In: Entomologia mexicana. Sociedad Mexicana de Entomologia; 2009. p. 104–108. Spanish.
- İlhan S, Nurbaş M, Kılıçarslan S et al. Removal of chromium, lead and copper ıons from ındustrial waste waters by Staphylococcus saprophyticus. Turk Electron J Biotechnol. 2004;2:50–57.
- Bastos MCF, Coutinho BG, Coelho MLV. Lysostaphin: a stapylococcal bacteriolysin with potential clinical applications. Pharmaceuticals. 2010;3:1139–1161.
- Hjelm E, Lundel-Etherden I. Slime production by Staphylococcus saprophyticus. Infect Immun. 1990;59:445–448.
- Sperling VP. Activated sludge and aerobic biofilm reactors. London: IWA Publishing; 2007.
- Fang Y, Lu Z, Fengxia LV et al. A newly ısolated organic solvent tolerant Staphylococcus saprophyticus M36 produced organic solvent-stable lipase. Curr Microbiol. 2006;53:510–515.
- Arpigly JL, Gaeger KE. Bacterial lipolytic enzymes: classification and properties. Biochem J. 1999;343:177–183.
- Hasan F, Ali Shah A, Hameed A. Industrial applications of microbial lipases. Enzyme Microb Technol. 2006;39:235–251.
- Enright MR, Mclnerney JO, Griffin CT. Characterization of endospore-forming bacteria associated with entomopathogenic nematodes, Heterorhabditis spp., and description of Paenibacillus nematophilus sp. nov. Int J Syst Evol Microbiol. 2003;53:435–441.
- Secil ES, Sevim A, Demirbag Z et al. Isolation, characterization and virulence of bacteria from Ostrinia nubilalis (Lepidoptera: Pyralidae). Biologia. 2012;67:767–776.
- Sezen K, Isci S, Muratoglu H et al. Identification and pathogenicity of bacteria from Gryllotalpa gryllotalpa L. (Orthoptera: Gryllotalpidae). Türk Biyo Müc Derg. 2013;4:89–108.
- Schoina C, Stringlis IA, Pantelides IS et al. Evaluation of application method and biocontrol efficacy of Paenibacillus alvei strain K165, against the cotton black root rot pathogen Thielaviopsis basicola. Biol Control. 2011;58:68–73.
- Markakis EA, Tjamos SE, Antoniou PP et al. Biological control of Verticillium wilt of olive by Paenibacillus alvei, strain K165. BioControl. 2016;61:293–303.
- Ryu CM, Kim J, Choi O et al. Improvement of biological control capacity of Paenibacillus polymyxa E681 by seed pelleting on sesame. Biol Control. 2006;39:282–289.
- Kim YK, Choi EJ, Hong SJ et al. Biological control of tomato and red pepper powdery mildew using Paenibacillus polymyxa CW. Korean J Pestic Sci. 2013;17:379–387.
- Lal S, Tabacchioni S. Ecology and biotechnological potential of Paenibacillus polymyxa: a mini review. Indian J Microbiol. 2009;49:2–10.
- Devi KK, Kothamasi D. Pseudomonas fluorescens CHA0 can kill subterranean termite Odontotermes obesus by inhibiting cytochrome c oxidase of the termite respiratory chain. FEMS Microbiol Lett. 2009;300:195–200.
- Jang JY, Yang SY, Kim YC et al. Identification of orfamide Aasan insecticidal metabolite produced by Pseudomonas protegens F6. J Agric Food Chem. 2013;61:6786–6791.
- Kupferschmied P, Maurhofer M, Keel C. Promise for plant pest control: root-associated pseudomonads with insecticidal activities. Front Plant Sci. 2013;4:1–17.
- Khan KI, Jafri RH, Ahmad M. Discovery and pathogenicity of Pseudomonas fluorescens against various species of termites. Punjab Univ J Zool. 2008;23:047–057.
- Sevim E, Celebi O, Sevim A. Determination of the bacterial flora as a microbial control agent of Toxoptera aurantii (Homoptera: Aphididae). Biologia. 2012;67:397–404.
- Daval S, Lebreton L, Gazengel K et al. The biocontrol bacterium Pseudomonas fluorescens pf29Arp strain affects the pathogenesis related gene expression of the take all fungus Gaeumannomyces graminis var. Tiritici on wheat roots. Mol Plant Pathol. 2011;12:839–854.
- Vallabhaneni SD. Biocontrol of Rhizoctonia solani in tobacco (Nicotiana tabacum) seed beds using Pseudomonas fluorescens. Agric Res. 2016;5:137.
- Arseneault T, Goyer C, Filion M. Pseudomonas fluorescens LBUM223 increases potato yield and reduces common scab symptoms in the field. Phytopathology. 2015;105:1311–1317.
- Goud M, Muralikrishnan V. Biological control of three phytopathogenic fungi by Pseudomonas fluorescens isolated from rhizosphere. Internet J Microbiol. 2008;7:6117 [ cited 2016 Oct 14]. Available from: http://ispub.com/IJMB/7/2/6117
- Bruijn I, Knok MJD, Waard P et al. Massetolide A biosynthesis in Pseudomonas fluorescens. J Bacteriol. 2008:2777–2789.
- Appanna V, Gazso L, Pierre M. Multiple metal tolerance in Pseudomonas fluorescens and its biotechnological significance. J Biotechnol. 1996;52:75–80.
- Lemire J, Auger C, Bignucolo A et al. Metabolic strategies deployed by Pseudomonas fluorescens to combat metal pollutants: biotechnological prospects. In: Mendez-Vilas A, editor. Current research technology education topics in applied microbiology and microbial biotechnology. Formatex; 2010. p. 177–187.
- Meyer JM, Abdallah MA. The fluorescent pigment of Pseudomonas fluorescenous: biosynthesis purification and physicochemical properties. J Gen Microbiol. 1978;107:319–328.
- Boronin AM, Movrodi DV, Ksenzenko VN et al. Characterization of genes involved in phenazine biosynthesis in plant growth- promoting Pseudomonas fluorescens [Abstract]. 5th International Symposium on Pseudomonas; 1995 Jul 1; Tsukuba, Japan; 1995. p. 70 ( Molecular Biology and Biotechnology).
