?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
Microbial fuel cells (MFCs) are known for their capability to directly convert organic substrates into electricity by the biochemical activity of specific microorganisms. Availability of a proper terminal electron acceptor is crucial for this process. Free radicals, with their one or more unpaired electrons, are extremely reducible and could be considered as electron acceptors in terms of cathodic processes in MFC. During this reduction, free radicals could be transformed in the same manner as they are transformed by antioxidants. The present study investigated this opportunity by utilization of 2,2-diphenyl-1-picrylhydrazyl (150 μmol/dm3 methanol solution) as a free-radical molecule. During the studied process, over 90% radical neutralization was observed in less than 16 hours. The results obtained demonstrate for the first time the potential of MFC type bio-electrochemical systems to serve as a free-radical scavenging tool and to provide antioxidant and anti-radical activity. In this way, this study opens a completely new field of research and application of bio-electrochemical systems.
| Abbreviations | ||
| MFC | = | microbial fuel cell; |
| DPPH | = | 2,2-diphenyl-1-picrylhydrazyl; |
| PEM | = | proton exchange membrane. |
Introduction
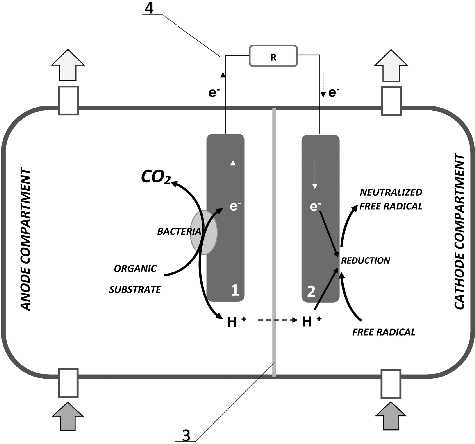
The concept of microbial fuel cells (MFCs) is not new [Citation1]. The first MFC reactors were demonstrated in the late nineteenth century [Citation2,Citation3]. Recently, they have been widely popularized as a technology which involves microbial biochemical and respiratory systems to generate electricity, while utilizing a variety of substrates [Citation4–13]. The typical MFC consists of two separate compartments containing the anode and the cathode. The electrodes are connected by an external electric circuit and divided by a proton exchange membrane (PEM) [Citation14]. The microbial species capable of such electrogenic activity oxidize the substrates through a cascade of redox reactions by exporting the resulting electrons and protons out of the cell, respectively, by transferring them to the anode (and then by an external electric circuit to the cathode) and, through a PEM, to the cathodic compartment [Citation15–17].
The availability of a suitable electron acceptor in the cathodic reactions is crucial for the process. Generally, oxygen (in solution or as a gas if air cathodes are used) is accepted as a universal electron acceptor. However, many studies involve other chemical compounds with oxidative properties as an electron acceptor involved in the cathodic processes. Probably, the most popular among them is ferricyanide [Citation18].
In recent years, MFCs are intensively studied in regard to their application both as an energy-yielding reactor and a biotechnological approach with potential use in wastewater treatment and waste management. Besides this, a variety of alternative applications are also studied. The reductive potential of the cathode seems to be an interesting prospect in the MFCs research and application. It is well known that the cathodic reactions are the most limiting stage in terms of overall system performance [Citation17,Citation19,Citation20]. However, they also open up a variety of possibilities which could further enlarge the application range of MFCs. Recently, several studies reported different processes based on the chemical transformation and reduction in the cathodic chamber, such as copper removal and recovery, decolourization of different dyes and electrochemical denitrification [Citation21–23].
A whole new field of bio-cathode applications was revealed with the recent discovery of microbes that are able to gain energy and catalyse certain reactions by accepting electrons from an electrode. One of the most ambitious goals pursued in this direction is the potential use of MFC technology and bio-cathodic processes in CO2 fixation and chemosynthesis of valuable organic compounds [Citation24].
In this study, we developed and tested the concept of using MFC and the reductive potential of cathodic processes as a non-reagent free-radical scavenging tool. The main idea is based on the electron and proton transfer via the electrode to the reactive molecules (free radicals) in the catholyte, which actually play the role of terminal electron acceptors in the cells’ electrochemistry.
In recent years, the role of free-radical generation and oxidative stress as negative processes affecting living cells on the molecular level has been widely investigated. The activity of radicals is directly associated with cell ageing and a number of pathological processes, including neoplastic growth, metabolic and neurovegetative disorders [Citation25].
Along with the natural metabolic processes, the main source of free radicals in the human body is food and the products obtained after its oxidation during storage and processing. That is why the modern concept of food and quality of life is associated with effective antioxidation and free-radical scavenging. Control of oxidation and elimination of the resulting free radicals is a critical factor in the production and storage of biologically active substances. The bio-electrochemical free-radical scavenging method proposed in this study could be a step further in advancing the technology in this field.
Materials and methods
The present study employed 2,2-diphenyl-1-picrylhydrazyl (DPPH, Aldrich Chemistry) as a model free radical to investigate the dynamics of its neutralization in the cathodic compartment of dual chamber MFCs.
MFC construction and operation
The MFC used in this study was assembled as a cylindrical plastic reactor consisting of two chambers separated by Nafion® 424 perfluorinated proton exchange membrane (). The anode and cathode were made of carbon cloth with a circular shape and a diameter of 40 mm. The electrodes were connected with an external electric circuit with different load depending to the aims of each particular experiment. The total volumes of the cathodic and anodic compartments were 20 and 40 dm3, respectively.
Microorganisms and growth conditions
The electrogenic microorganisms were isolated from bottom sediments of Yasna Polyana Reservoir near Burgas, Bulgaria. The enrichment of the mixed culture was performed in anaerobic conditions by inoculation of 0.5 dm3 sediment in 20 dm3 Luria–Bertani (LB) nutrient medium (10 g/dm3 tryptone, 5 g/dm3 yeast extract and 5 g/dm3 NaCl, pH 7) containing 15 g/dm3 glucose. After 96 hours of cell growth, the enriched culture was suspended in fresh nutrient medium (LB without glucose to avoid fermentative metabolism) to a microbial concentration of 107 CFU/dm3 and loaded in the anode chamber of the MFC. The process was conducted at 18 ˚C.
DPPH assay
The changes in DPPH concentration were determined spectrophotometrically at 515 nm according to the method of Brand-Williams et al. [Citation26]. The effect of the treatment was expressed as percentage of the initial DPPH concentration (150 µmol/dm3). A control sample was prepared in the same manner but in abiotic conditions in order to evaluate any potential free-radical neutralization effects not related to the bio-electrochemical activity.
All measurements were made in three replicates and the data shown in the figures are mean values.
Results and discussion
In this study, to explore the potential of using MFC as a non-reagent free-radical scavenging tool, we performed three sets of experiments including a control sample in abiotic conditions and operational set-ups with 20 kΩ and 1 MΩ loads in the external circuit. The control sample lacked any dynamics and no neutralization was observed. In both experiments with working MFCs (each equipped with a different resistor in the external circuit), a high DPPH neutralization degree was achieved. However, there was a significant difference in the process kinetics (). Decreasing the resistance of the external circuit shifted the radical neutralization from first-order to zero-order kinetics behaviour. Zero-order kinetics is often called pseudo-zero order, since it is usually not a result of a particular molecular mechanism of the reaction, but an artefact of the conditions under which the transformation is carried out. One of the possible reasons for such behaviour is the scenario when the concentration of some of the reactants involved in the reaction is much greater than that of the others [Citation27].
Figure 2. DPPH concentration vs. time in abiotic control MFC and operational MFC under 20 kΩ and 1 MΩ resistance in the external circuit.
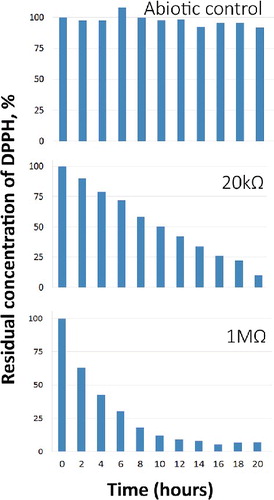
Besides the changes in the neutralization rate, this shift demonstrates some specifics in the molecular mechanisms involved in the scavenging of DPPH. Increasing the resistance practically affects the potential difference between the electrodes, which results in current change. In case of lower ohmic resistance, the overall process is characterized by lower potential difference. The average voltage obtained in these conditions (20 kΩ resistor in the circuit) was 24 mV. Contrary, maintaining higher resistance in the electric circuit limits the electron flow and keeps the voltage high (the average potential difference during the 1 MΩ experiments was 480 mV). Taking these effects into account, we could suggest that the preferred and more effective mechanism of DPPH neutralization in this particular case is the so-called ‘HAT’, or hydrogen atom transfer mechanism. The alternative mechanism is ‘SET’, or single electron transfer. It is usually known as a slower process, since it requires two steps of transformation. According to these results and the assumption made, in the MFC, the higher external circuit resistance contributes for the faster DPPH neutralization by providing a higher proton-motive force due to the higher potential difference. This results in more active transfer of protons from the anodic chamber to the cathode compartment via the ion exchange membrane of the reactor. Decreasing the ohmic resistance drops the potential difference and increases the current (and the electron flow) which obviously does not contribute to the neutralization. As a result, we have a lower proton exchange rate, which limits the process dynamics and kinetics by shifting it to the zero order. During the zero-order reactions, the neutralization rate is constant and independent of the DPPH concentration, making it possible to calculate just based on the experimental results. Conversely, first-order reactions are concentration-dependent in regard to the transformation rate. This dependence is expressed by the value of the so-called rate constant. In classic chemical kinetics, first-order reactions are expressed as follows:(1)
(1) where Ct is the concentration of DPPH (in its unreduced free-radical form) at any time; C0 is the initial concentration of DPPH and k is the rate constant. The rate constant itself is defined by the compound's half-life (t1/2) as
(2)
(2)
Based on the experimental data obtained (1 MΩ scenario), we determined a DPPH half-life of 3.5 hours and calculated the rate constant (k) to be 0.198 h−1.
The conventional approach to dealing with free radicals both in vivo and in situ is based on the activity of antioxidants. They are usually natural compounds including some vitamins, polyphenols and other plant-synthesized molecules. In living cells, there are several antioxidant enzymes that are key factors in oxidative stress protection [Citation28]. However, the application of chemical antioxidants is not always a suitable solution. In certain cases, adding an external compound to the products is not acceptable. This could be due to potentially negative influence on the organoleptic characteristics (in the case of food) or inadmissible change in the chemical and biological properties of the products subject to antioxidant protection (in the case of pharmaceuticals, ultra-pure and analytical chemicals, solutions of biologically active substances, etc.). Another drawback is related to the radical-chain reactions or pro-oxidant activities, which may occur in some cases after application of chemical antioxidants [Citation29].
In the alternative free-radical neutralization approach proposed here, the kinetic characteristics obtained are one of the advantages over the conventional chemical antioxidation. The antioxidant–free radical reactions are typically characterized by second-order kinetics where the half-lives of the free-radical compounds progressively increase during the process, which deteriorates the process dynamics [Citation30,Citation31].
In the MFC, the rate of free-radical scavenging was lower. This, however, was balanced by the deeper neutralization achieved by the MFC compared to the conventional chemical antioxidants [Citation25,Citation32]. Another advantage of the MFC approach to radical scavenging is that, after reaching maximum neutralization, the DPPH level remains stable, while it is well known that the processes employing chemical antioxidants are often reversible [Citation30]. The reversed reactions usually result in re-oxidation and re-activation of the radical molecules. This mechanism and scenario is expected to be strongly inhibited in the case of MFCs due to the permanent reductive conditions maintained in the cathodic chamber [Citation33,Citation34]. Thus, the bio-electrochemical free-radical scavenging method proposed here could offer a viable non-invasive alternative to the use of chemical antioxidants in a range of industrial applications.
Conclusions
To the best of our knowledge, this is the first report to demonstrate the potential of MFCs to be used as a radical-scavenging tool. The main advantage of this approach over the conventional antioxidant chemicals is the non-invasive nature of the process, which provides opportunity to perform antioxidant protection and free-radical neutralization without adding any reagents into the treated fluids or objects. Moreover, by electrochemical neutralization, the radical-chain reactions are inhibited and the overall process follows kinetic mechanisms of a lower order than in the cases where a chemical antioxidant is involved.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Rabaey K, Verstraete W. Microbial fuel cells: novel biotechnology for energy generation. Trends Biotechnol. 2005;23(6):291–298.
- Appleby J. From Sir William Grove to today: fuel cells and future. J Power Sources. 1990;29(1–2):3–11.
- Pant D, Bogaert GV, Diels L, et al. A comparative assessment of bioelectrochemical systems and enzymatic fuel cells. In: Arora R, editor. Microbial biotechnology: energy and environment. Wallingford (CT): CAB International; 2012. p. 39–57.
- He Z, Largus TA. Application of bacterial biocathodes in microbial fuel cells. Electroanalysis. 2006;18:2009–2015.
- Huang L, Regan JM, Quan X. Electron transfer mechanisms, new applications, and performance of biocathode microbial fuel cells. Bioresour Technol. 2011;102:316–323.
- Lovely DR. Bug Juice: harvesting electricity with microorganisms. Nat Rev Microbiol. 2006;4(7):497–508.
- Mohan SV. Harnessing bioelectricity through microbial fuel cell from wastewater. Akshay Urja. 2012;5(5):25–29.
- Mohan SV, Velvizhi G, Vamshi Krishna K, et al. Microbial catalyzed electrochemical systems: a bio-factory with multi-facet applications. Bioresour Technol. 2014;165:355–364.
- Palmore GTR, Whitesides GM. Microbial and enzymatic biofuel cells. In: Himmel ME Baker JO Overend RP, editors. Enzymatic conversion of biomass for fuels production. Oxford: ACS Symposium; 2009. p. 271–290.
- Pant D, Bogaert GV, Diels, L, et al. A review of the substrates used in microbial fuel cells (MFCs) for sustainable energy production. Bioresour Technol. 2010;101:1533–1543.
- Rabaey K, Lissens G, Verstraete W. Microbial fuel cells: performance and perspectives. In: Lens P Westermann P Haberbauer M Moreno A, editors. Biofuels for fuel cells: renewable energy from biomass fermentation. London: IWA Publishing; 2005. p. 377–399.
- Wanga Y, Niua C-G, Zenga G-M, et al. Microbial fuel cell using ferrous ion activated persulfate as a cathodic reactant. Int J Hydrog Energy. 2011;36(23):15344–15351.
- Yuan Y, Chen Q, Zhou S, et al. Bioelectricity generation and microcystins removal in a blue-green algae powered microbial fuel cell. J Hazard Mater. 2011;187(1–3):591–595.
- Das S, Mangwami N. Recent developments in microbial fuel cells: a review. J Sci Ind Res. 2010;69:727–731.
- Mohan SV, Velvizhi G, Modestra JA, et al. Microbial fuel cell: critical factors regulating bio-catalyzed electrochemical process and recent advancements. Renew Sustainable Energy Rev. 2014;40:779–797.
- Pant D, ElMekawy A, Srikanth S, et al. Food and agricultural wastes as substrates for bioelectrochemical system (BES): the synchronized recovery of sustainable energy and waste treatment. Food Res Int. 2015;73:213–225.
- Pant D, Singh A, Bogaert GV, et al. Bioelectrochemical systems (BES) for sustainable energy production and product recovery from organic wastes and industrial wastewaters. RSC Adv. 2012;2:1248–1263.
- Logan BE, Hamelers B, Rozendal R, et al. Microbial fuel cells – methodology and technology. Environ Sci Technol. 2006;40(17):5181–5192.
- Logan BE, Regan JM. Microbial fuel cells – challenges and applications. Environ Sci Technol. 2006;40(17):5172–5180.
- Wang H, Ren ZJ. A comprehensive review of microbial electrochemical systems as a platform technology. Biotechnol Adv. 2013;31:1769–1807.
- Heijne AT, Liu F, van der Weijden R, et al. Copper recovery combined with electricity production in a microbial fuel cell. Environ Sci Technol. 2010;44(11):4376–4381.
- Mu Y, Rabaey K, Rozendal RA, et al. Decolorization of azo dyes in bioelectrochemical systems. Environ Sci Technol. 2009;43(13):5137–5143.
- Du H, Li F, Yu Z, et al. Nitrification and denitrification in two-chamber microbial fuel cells for treatment of wastewater containing high concentrations of ammonia nitrogen. J Environ Technol. 2016;37(10):1232–1239.
- Lovley DR. Powering microbes with electricity: direct electron transfer from electrodes to microbes. Environ Microbiol Rep. 2011;3(1):27–35.
- Valko M, Jomova K, Rhodes CJ, et al. Redox- and non-redox-metal-induced formation of free radicals and their role in human disease. Arch Toxicol. 2016;90(1):1–37.
- Brand-Williams W, Cuvelier ME, Berset C. Use of a free radical method to evaluate antioxidant activity. LWT. 1995;28:25–30.
- Pogliani L. Pseudo-zero-order reactions. React Kinet Catal Lett. 2008;93(2):187–191
- Carocho M, Ferreira ICFR. A review on antioxidants, prooxidants and related controversy: natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem Toxicol. 2013;51:15–25.
- Mohanakrishna G, Srikanth S, Pant D. Bioelectrochemical Systems (BES) for microbial electroremediation: an advanced wastewater treatment technology. In: Kaushik G, editor. Applied environmental biotechnology: present scenario and future trends. New Delhi: Springer India; 2015. p. 145–167.
- Bondet V, Brand-Williams W, Berset C. Kinetics and mechanisms of antioxidant activity using the DPPH free radical method. LWT. 1997;30:609–615.
- Mortensen A, Skibsted LH, Sampson J, et al. Comparative mechanisms and rates of free radical scavenging by carotenoid antioxidants. FEBS Lett. 1997;418(1–2):91–97.
- Otohinoyi DA, Ekpo O, Ibraheem O. Effect of ambient temperature storage on 2,2-diphenyl-1-picrylhydrazyl (DPPH) as a free radical for the evaluation of antioxidant activity. Int J Biol Chem Sci. 2014;8(3):1262–1268.
- Carr A, Frei B. Does vitamin C act as a pro-oxidant under physiological conditions? FASEB J. 1999;13:(9)1007–1024.
- Herbert V. Prooxidant effects of antioxidant vitamins. Introduction. J Nutr. 1996;126(4):1197S–1200S.