ABSTRACT
There is a distinct group among the patients with unexplained infertility who are found to have enhanced humoral immune response. Recent studies focus on the expression of stress proteins being an important factor in the stages of gametogenesis, fertilization, implantation, early embryonic development and pregnancy. Increased expression of stress proteins and immune response against them was found in tissues exposed to stress. There is not enough data linking infertility to expression and immunity of α-crystallins in patients with endocrine diseases. The aim of this work was to study anti-α-crystallin antibodies in patients with polycystic ovary syndrome (PCOS), Graves’ disease, autoimmune thyroiditis, diabetes mellitus and obesity. Sera samples from 169 women with endocrine disorders (PCOS (n = 68); Graves’ disease (n = 26); autoimmune thyroiditis (n = 32); diabetes mellitus (n = 10); and obesity (n = 33)) were tested by ELISA. The statistical analysis was performed using SPSS program. The concentration of anti-α-crystallin antibodies is significantly elevated in the PCOS group compared to the control group (p = 0.021). The frequency of positive sera in the same group of patients is significantly higher compared to the control group (p = 0.029). In all other groups, no statistically significant elevation was observed. Elevated concentrations of anti-α-crystallin antibodies found in patients with PCOS suggest that the increased production of anti-α-crystallin antibodies in women with PCOS is most probably caused by failure of the immune tolerance and the induction of immune response. This is most likely due to an increased expression of this stress protein as a result of oxidative stress and chronic inflammation.
Introduction
Infertility is diagnosed in 10%–15% of couples from European populations, of which 25% are with unexplained aetiology, 20% are with ovulation disorders, 15% with tubal abnormalities, 30% with male factor infertility and 10% with pelvic inflammatory disease [Citation1]. There is a distinct group of the patients with unexplained infertility who have enhanced humoral immune response (approximately a quarter of them in the Bulgarian population) [Citation2]. Antibodies found to play a role in these cases, which include anti-sperm antibodies [Citation2], anti-zona antibodies [Citation3], anti-ovarian antibodies [Citation4], anti-2 GPI antibodies and anti-phosopholipid antibodies [Citation5,Citation6], but they cannot alone explain the reproductive problems in all cases. On the other hand, many endocrine disorders can cause female infertility: polycystic ovary syndrome (PCOS), autoimmune thyroiditis, hyperthyroidism, obesity. The mechanisms are still poorly understood and hormone disturbances do not seem to be the sole causative factor for infertility in all cases. Recent studies focus on the expression of stress proteins as an important factor for gametogenesis, fertilization, implantation, early embryonic development and pregnancy, and the immune response against them [Citation7]. Heat shock proteins (HSPs) are a large family of highly conservative proteins that are ubiquitously found in all organisms. HSPs are multifunctional proteins present in all cell compartments and interacting with a number of other molecules. Some HSPs are only found in physiological conditions and act as chaperones, have cytoprotective role, mediate cell signalling, act in the mechanisms of innate and acquired immunity. Other HSPs are expressed as a result of stress (temperature changes, free oxygen radicals, infections, ischaemia, shock, etc.) in order to protect cell proteins from the effect of the stress factor [Citation8].
According to Kampinga et al. [Citation9], in mammals, the following types of HSPs exist: HSPH (HSP110), HSPC (HSP90), HSPA (HSP70), DNAJ (HSP40), HSPB (small HSP), HSPD/E (HSP60/HSP10) and CCT (TRiC). The HSPB family consists of 11 members including α-crystallins. Among them, Hsp27, αA- and αB-crystallin are studied in most detail. Alpha-crystallin is a major lens water-soluble protein, comprising up to 40% of total lens protein [Citation10]. It has αA- and αB-subunits. Alpha-A is abundant in eye lеns and is found at low level in skeletal muscle, liver, spleen, adipose tissue, while αB-crystallin is ubiquitous, abundant in eye lens and high levels are found in heart and muscle [Citation11]. Similar to most HSPs, α-crystallins have chaperone-like function, but αB-crystallin is also expressed in a number of pathologies, such as neurological diseases, tumours [Citation12], infertility [Citation13], etc. Pathological conditions or diseases may relate to inadequate expression of αB-crystallin or to the production of serum antibodies against αB-crystallin [Citation14]. Alpha-crystallins had long been pointed out as an example of antigens with high organ and low species specificity, which remain in an immunological isolation and present potential autoantigens. After their presence was proven in extraocular tissues [Citation11], immunological response against them can be explained with increased αB-crystallin expression and leakage in the biological fluids due to different pathological conditions. This raises the question about the expression of αB-crystallin and immune response against it in patients with endocrine disorders linked to infertility.
It is known that HSP expression and immunity are intimately related to gametogenesis, fertilization and embryo development [Citation7]. Expression of HSP60 and HSP70 has been investigated, as well as the humoral immune response against them, but data on the participation of the sHSPs including αA- and αB-crystallin are scarce. Our previous investigation has proved high concentration of anti-α-crystallin antibodies in the sera of women with unsuccessful IVF, unexplained infertility, habitual and single spontaneous abortions. These findings suggest that there may be an immunological conflict present between the mother and the conceptus caused by these antibodies. Expression of some stress proteins has been investigated in cases of endocrine disorders coupled with infertility. There is information of the protective role of HSP72 in insulin resistance in patients suffering from diabetes mellitus type 1. In such cases, immune response has been increased against HSP60 and HSP70 [Citation15]. Increased concentration of HSP72 antibodies has been reported in Graves’ disease and their role in pathogenesis has been discussed [Citation16]. A correlation between circulating HSP70 and PCOS has been proposed [Citation17]. There are not enough data concerning the expression of αB-crystallins and immune response against them in patients with endocrine disorders linked to infertility.
The aim of this work was to study concentration of anti-α-crystallin antibodies in patients with PCOS, autoimmune thyroiditis, diabetes mellitus type 1 and obesity.
Materials and methods
Patients
Blood samples (sera) from 169 women (aged between 18 and 59, mean age: 34.9) with different endocrine disorders were tested. Informed consent was obtained from all patients. The sera were stored in aliquots at −20 °C until tested. Patient groups were formed based on diagnoses including PCOS (n = 68); Graves’ disease (n = 26); autoimmune thyroiditis (n = 32); diabetes mellitus type 1 (n = 10); and obesity (n = 33). A control group was formed from age-matched healthy female blood donors (n = 12) without reproductive (WHO criteria, 2014), immunological disorders, endocrine disorders and proven fertility (aged between 19 and 55, mean age: 34.58).
Polycystic ovary syndrome (PCOS)
The PCOS was diagnosed by Rotterdam criteria, which state that PCOS is present if any two out of the following three criteria are met: oligoovulation–anovulation, excess androgen activity or polycystic ovaries (determined by gynaecologic ultrasound, and after exclusion of other androgen excess disorders [Citation18,Citation19].
Graves’ disease
Graves’ disease was diagnosed when hyperthyroidism was present with levels of thyroid-stimulating hormone (TSH) less than 0.4 mIU/L and the presence of TSH-R Ab (TSH receptor antibodies).
Autoimmune thyroiditis
The autoimmune thyroiditis was diagnosed when autoantibodies (antithyroglobulin and/or thyroperoxidase (TPO) antibodies were present and by ultrasound characteristic of the gland's parenchymа.
Diabetes
Diabetes was diagnosed according to the American Diabetes Association 2010.
Obesity
Patients with body mass index more than 30 kg/m2 were considered as obese.
Enzyme-linked immunosorbent assay (ELISA)
As an antigen we used α-crystallins isolated by gel chromatography of porcine eye lens extract as described in previous articles [Citation20]. The purity of the α-crystallin fraction was demonstrated by polyacrylamide gel electrophoresis – 12% running gel.
Sera were tested using indirect enzyme-linked immunosorbent assay (ELISA). Flexible U-bottomed 96-well assay plates (Costar, Cambridge, MA, USA) were coated with 10 μg/mL antigen, 50 μL/well and incubated 1 h at room temperature (RT) and at 4 °C overnight. Plates were washed three times with 0.05% Tween20/PBS. Non-specific binding was reduced by incubating with 0.02% Tween 20/PBS for 2 h at RT. Plates were washed again. Serum dilutions of 1:20 were made in PBS, 50 μL/well were added to each well (in three wells per sample) and incubated for 2 h at RT. After another washing, the plates were incubated for 1 h at RT with rabbit-peroxidase-conjugated anti-human IgG (gamma chain specific, SIGMA), diluted 1:10 000, used as a second antibody. The reaction was developed using ex tempore prepared solution of o-phenylenediamine in citrate buffer, pH 5.0 containing 0.015% H2O2 and stopped 20 min later with 10% H2SO4. The optical density (OD) was measured at 492 nm on ELISA reader (Varioscan, Thermo electron corporation, Finland), with the instrument absorbance set to zero on wells where only the second antibody was present. All experiments were performed in duplicate. Sera of patients with lens cataract and high titre of anti-crystallin antibodies were applied as positive controls.
Statistical analysis
Statistical analysis was performed using SPSS 15 statistical program and non-parametric methods. The control (negative) group consisted of women without reproductive problems. As positive, we considered cases with OD > median value (control group) + 2 SD (standard deviation); when OD value was lower, the test is considered negative. We used Mann--Whitney test in order to compare OD values of each patient group and the negative control group. Fisher's exact test was used to compare frequencies of positive sera in patient groups opposed to the control group. The level of significance of null hypothesis was set at 0.05.
Results and discussion
Alpha-crystallins are water-soluble antigens/proteins of the lens, which remain in immunological isolation due to the formation of an impermeable lens capsule at an early stage of embryonic development, which does not allow contact between them and the immune system. Therefore, acquisition of immune tolerance would be impossible. However, recent studies have reported expression of αB-crystallin in small quantities in extraocular tissues [Citation11], and we have proved their expression in extraocular tissues in human embryos in previous studies [Citation21]. This could explain the tolerance to water-soluble lens antigens, of which α-crystallins are the most immunogenic [Citation22]. In certain pathologies, such as inflammation, hypoxia, metaplasia, etc., increased release of stress proteins could occur, which would lead to overwhelming the immune tolerance and strong immune response. Increased concentration of anti-α-crystallin antibodies does not necessary mean that the antibodies take direct part in immune pathogenesis, although detecting the increase in antibody levels in diseases coupled with infertility could prove to be a useful tool for better understanding of the correlation between the specific disease and infertility. In our study, we searched for anti-α-crystallin antibodies in sera of women with various endocrine diseases and infertility using ELISA. The ELISA plates were coated with porcine α-crystallin on order to detect patient anti-α-crystallin antibodies.
The usage of porcine α-crystallin as antigen was based on the strong antigenic similarity between human and porcine lens crystallins. This similarity was demonstrated by Trifonova et al. [Citation23], where they used water-soluble extracts of human and porcine lenses. Indirect ELISA of both antigens showed antigenic identity, which makes the porcine crystallins an adequate substitute for human crystallins.
The concentration of anti-α-crystallin antibodies in ELISA is proportional to the OD. The results from sera testing (by ELISA) of patients suffering from endocrine diseases (Graves’ disease, autoimmune thyroiditis, diabetes mellitus and obesity) are presented in and . The concentration of anti-α-crystallin antibodies and the frequency of positive sera were not statistically increased, compared to the control group (P > 0.05). However, statistical analysis of sera of patients suffering from autoimmune thyroiditis showed that the increase in the frequency of positive ones against α-crystallins was statistically at the border of significance (p = 0.059). There might be a connection between autoimmune thyroiditis and ovarian dysfunction [Citation1], which could explain the immunity against α-crystallins in the patients with autoimmune thyroiditis. Further tests of a larger group of patients are needed to determine whether the increased frequency of positive for anti-α-crystallins antibodies is statistically significant.
Table 1. Levels of anti-α-crystallin antibodies and frequency of positive sera among patients with endocrine disorders.
Figure 1. Concentration of anti-α-crystallin antibodies evaluated by OD (optical density at 492 nm wavelength) among patients with endocrine disorders (Graves’ disease, autoimmune thyroiditis, diabetes mellitus, obesity and PCOS) compared to the control group presented in box plots. In the endocrine disorder groups with Graves’ disease, autoimmune thyroiditis, diabetes mellitus and obesity, there is no statistically significant increase in concentration of anti-α-crystallin antibodies in the patients’ sera, compared to the control group (P > 0.05, Mann–Whitney test). In the PCOS group, there is statistically significant increase in concentration of anti-α-crystallin antibodies in the patients’ sera, compared to the control group (P < 0.05, Mann–Whitney test).
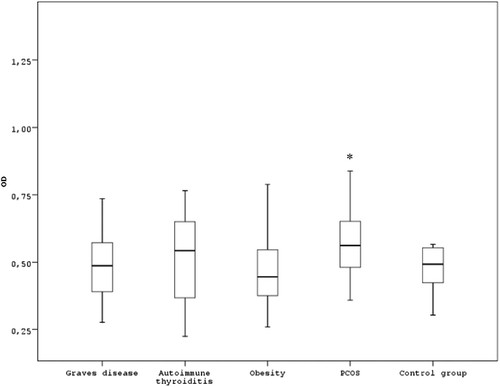
The obesity group showed the lowest concentration of anti-α-crystallin antibodies, but the standard deviation was high (). This might be caused by the heterogeneity of obesity. Further research is needed in order to determine the correlation between different types of obesity, anti-α-crystallin antibodies and reproductive failures.
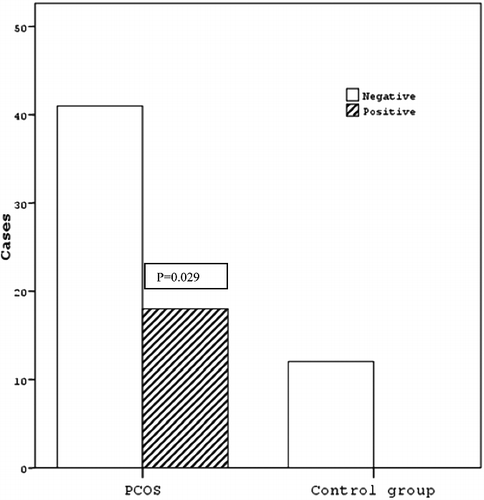
The results from the sera testing of women with PCOS are shown in and . The data show that the concentration of anti-α-crystallin antibodies is significantly elevated in the PCOS group compared to the control group (p = 0.021) (). From 59 women with PCOS, 15 showed OD higher than the median value (control group) + 2 SD, which is 25.4% of all cases (). The frequency of positive sera in the same group of patients is significantly higher, compared to the control group (p = 0.029) (). Failure of immune tolerance and the induction of humoral immune response against α-crystallin could be caused by prolonged oxidative stress as observed in PCOS. In the recent decades, oxidative stress is considered a potential trigger factor in the pathogenesis of PCOS [Citation24]. No analogous data were found in literature. Our study may be considered as a new piece in the immune puzzle of PCOS, which is considered by some authors an autoimmune disorder [Citation25], based on the ‘cocktail’ of proven autoantibodies.
Table 2. Levels of anti-α-crystallin antibodies and frequency of positive sera among patients with polycystic ovary syndrome (PCOS).
Figure 2. Frequency of positive and negative sera from PCOS group patients and control group. There is statistically significant higher frequency of patients’ sera positive for anti-α-crystallin antibodies, compared to the control group (P < 0.05, Fisher's exact test).
It is known that the promoter of αB-crystallin, besides constitutive expression with tissue-specific intensity, can mediate elevated expression induced by stress factors. High levels of expression of αB-crystallin have been found in tissues subjected to prolonged oxidative stress [Citation11].
Buteva-Hristova [Citation26] proved expression of α-crystallins in mice ovaries. Using immunoperoxidase reaction and anti-αB antibodies, she observed expression of αB-crystallins in follicles in different stages of maturation. The highest expression of αB-crystallins was demonstrated in the final stages of maturation (Graafian follicle).
The sHSP27 is overexpressed in oocytes derived from PCOS patients аnd leads to inhibition of oocytes maturation, but improves embryonic developmental potential [Citation27]. In women with PCOS, ovulation is often impossible, which leads to follicle arrest [Citation28]. This could possibly lead to an increased amount of stress proteins in the follicular fluid, which comes into contact with the immune system due to the inflammation processes accompanying PCOS [Citation17].
Expression of α-crystallin and anti-α-crystallin antibodies has been proven in many oncological diseases. Cherneva et al. [Citation29] and Marinova et al. [Citation30], on the basis of investigation of patients suffering from different types of lung cancer, propose αB-crystallin as potential diagnostic and prognostic marker. PCOS is considered as a prerequisite for uterine cancer and levels of expression of stress proteins and immunity against them could also be used as biomarkers. However, further research is needed to clarify the role of α-crystallin and anti-α-crystallin antibodies to determine their possible role as biomarkers.
In literature, there is a discussion about the role of α-crystallin as autoantigens. The evaluation of αB-crystallin as autoantigen is based primarily on the presence of specific antibodies against this small HSP.
When examining the kinetics of the binding between the αB-crystallin and antibodies against it, Rothbard et al. [Citation31] determined that the complex that resulted from the reaction was stable, in comparison to the typical antigen–antibody reaction. They speculate that the role of the HSPs as autoantigens has to be re-evaluated. On the other hand, Papuc et al. [Citation32] propose the role of α-crystallins as potential autoantigens, since expression of antibodies against α-crystallins and other small HSPs was observed in healthy persons in low titre (designated as natural antibodies) and in patients suffering from Parkinson's disease. With progression of neurodegenerative disease, the concentration of IgG and IgM antibodies against αB-crystallin increases, which means that the disease progression is linked to the activation of the immune system. We speculate that autoantigen or not, αB-crystallin should not be underestimated as potential biomarker which can give us a better insight in the progression of different diseases, endocrine and reproductive disorders.
Conclusion
Elevated concentrations of anti-α-crystallin antibodies are found in patients with PCOS. Our results suggest that the increased production of anti-α-crystallin antibodies above the positive threshold in women with PCOS is caused by the failure of immune tolerance and induction of humoral immune response. This is most likely due to the increased expression of this stress protein as a result of oxidative stress and chronic inflammation. Further research is needed to evaluate α-crystallin as autoantigen and the potential of α-crystallins and anti-α-crystallin antibodies against them as biomarkers for reproductive failure coupled to endocrine disorders and other chronic diseases.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Unuane D, Tournaye H, Velkeniers B, et al. Endocrine disorders and female infertility. Best Pract Res Clin Endocrinol Metab. 2011;25:861–873.
- Dimitrova D, Kalaidzhiev S, Nakov L. Role of anti-sperm antibodies in immunologically induced human infertility. Obst Gyn. 2001;40(3):34–38. Bulgarian.
- Naz RJ, Menge AC. Antisperm antibodies: origin, regulation, and sperm reactivity in human infertility. Fertil Steril. 1994;61:1001–1013.
- Novosad J, Kalantaridou SN, Tong ZB, et al. Ovarian antibodies as detected by indirect immunofluorescence are unreliable in the diagnosis of autoimmune premature ovarian failure: a controlled evaluation. BMC Womens Health. 2003;3:2.
- Matsura E, Igarashi Y, Fujimoto M, et al. Heterogeneity of anticardiolipin antibodies defined by the anticardiolipin cofactor. J Immunol. 1992;148:3885–3891.
- Matsuura E, Igarashi Y, Yasuda T, et al. Anticardiolipin antibodies recognize beta 2-glycoprotein I structure altered by interacting with an oxygen modified solid phase surface. J Exp Med. 1994;179(2):457–462.
- Witkin SS, Linhares IM. Heat shock proteins and fertility. In: Asea AAA Pederson BK, editors. Heat shock proteins and whole body physiology. Vol. 5, Chapter 9. 2010. p. 151–162.
- Neuer A, Spandorfer SD, Giraldo P, et al. The role of heat shock proteins in reproduction. Hum Reprod Update. 2000;6(2):149–159.
- Kampinga HH, Hageman J, Vos MJ, et al. Guidelines for the nomenclature of the human heat shock proteins cell stress chaperones. 2009;14(1):105–111.
- Horwitz HJ, Alpha-crystallin. Exp Eye Res. 2003;76(2):145–153.
- Bakthisaran R, Tangirala R, Rao CM. Small heat shock proteins: Role in cellular functions and pathology. Biochim Biophys Acta. 2015:291–319.
- Cherneva R, Georgiev O, Рetrov D, et al. Alpha crystallin antibodies in NSCLC patients. C R Acad Bulg Sci. 2009;62(12):1615–1620.
- Buteva-Hristova I, Trifonova N, Genov M, et al. Antibodies against alpha crystallins in women with reproductive failures. C R Acad Bulg Sci. 2009;62(2):285–291.
- Cherneva R, Georgiev O, Petrova D, et al. The role of small heat shock protein alpha-B crystallin in COPD pathogenesis. Int J Chron Obstruct Pulmon Dis. 2012;7:633–640.
- Whitham M, Febbraio MA. HSP and diabetes. In: Asea AAA Pederson BK, editors. Heat shock proteins and whole body physiology. New York, NY; Springer. Vol. 5, Chapter 1. 2010. p. 3–18.
- Prumell MF, Van Pareren Y, Bakker O, et al. Anti-heat shock protein (hsp)72 antibodies are present in patients with Graves’ disease (GD) and in smoking control group, Clin Exp Immunol. 1977;110:292–295.
- Gao H, Meng J, Xu M, et al. Serum heat shock protein 70 concentrarion in relation to Polycystic ovary syndrome in non-obese Chinese population. PloS One. 2013;8(6):e67729.
- Ben-Shlomo I, Younis JS. Basic research in PCOS: are we reaching new frontiers? Reprod Biomed Online. 2014;28(6):669–683.
- American Diabetes Association. Standards of medical care in diabetes. Diabetes Care. 2010;33(Suppl 1):S11–S61.
- Trifonova N, Mazhdrakova I, Stamenova M, et al. Anti α-, β- and γ- crystallins antibodies in sera of cataract patients. C R Acad Bulg Sci. 2000;53(7):107–110.
- Hristova I, Novachkov V, Petrov S, et al. Immunohistochemical study of Hsp27 in human embryos. C R Acad Bulg Sci. 2014;67(3):367–372.
- Izumi K, Kano M. Immunochemical studies on the soluble protein of the lens (on homologous immunity). Nippon Ganka Gakkai Zasshi. 1964;68(10):1121–1125.
- Trifonova N, Kalaydjiev S, Stamenova M, et al. Porcine eye lens crystallins: antigenic similarity with human crystallins, and tool for the detection of anti-lens crystallin antibodies. Graefe's Arch Clin Exp Ophthalmol. 2002;240:777–781.
- Zuo T, Zhu M, Xu W. Roles of oxidative stress in polycystic ovary syndrome and cancer (review article). Oxidative Med Cellular Longev. 2016;2016;14.
- Mobeen H, Afzal N, Kashif M. Polycystic ovary syndrome may be an autoimmune disorder. Scientifica (Cairo). 2016;2016:4071735.
- Buteva-Hristova I. Expression and meaning of small heat shock proteins in reproduction of an experimental model and in human [Ph.d. thesis]. Sofia; 2014. Bulgarian.
- Cai L, Ma X, Liu S, et al. Effects of upregulation of Hsp27 expression on oocyte development and maturation derived from polycystic ovary syndrome. PLoS One. 2013;8:e83402.
- Zhivkova R, Panevska M, Delimitreva S, et al. Investigation of cytokeratin and vimentin intermediate filaments in polycystic ovaries (PCOS) – presence and specific structure of Balbiani body in primordial follicles. Akush Ginekol (Sofiia). 2013;52:7–12.
- Cherneva R, Georgiev O, Petrov D, et al. Expression of small heat shock protein alpha-B crystallin in NSCLC. Comp Rend Acad Bulg Sci. 2009;62(11):1483–1488.
- Marinova D, Slavova Y, Titorenkov PL, et al. Immunohistochemical expression of Hsp27 and alphaB-crystallin in different histological subtypes of lung cancer. In: 22nd European Respiratory Society Annual Congress; Vienna; 2012:40(1213):1–5.
- Rothbard JB, Zhao X, Sharpe O, et al. Chaperone activity of aB-crystallin is responsible for its incorrect assignment as an autoantigen in multiple sclerosis. J Immunol. Available from: wwwjimmunol.org/cgi/doi/10.4049/jimmunol.1003934
- Papuc E, Kurys-Denis E, Krupski W, et al. Humoral response against small heat shock proteins in Parkinson's disease. 2015. 10(1). doi: 10.1371/journal.pone0115480
