?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.ABSTRACT
To investigate the effect and mechanism of Prunus tomentosa total flavone (PTTF) treatment on adjuvant arthritis (AA), the rat AA model was induced by a single intradermal injection of complete Freund's adjuvant (CFA) into the left ankle. The degree of lateral ankle swelling was measured by the foot volume method. The rats were sacrificed, the thymus and spleen were taken out and the organ index was calculated. Paraffin sections of the left ankle specimens were fabricated and the organization forms of the articular cartilage and synovium were observed. Kits were used to measure the serum nitric oxide (NO), NO synthase (NOS) and prostaglandin E2 (PGE2) content. After 18 days, the swelling degree of ankle joints in the dexamethasone group, the PTTTF 7.0 and 14 mg/kg groups were significantly lower than that in the model group (P < 0.05). The organ index in the dexamethasone group and the PTTF group were not statistically significantly different from that in the normal group (P > 0.05). Compared to the CFA model control group, the dexamethasone group and the PTTF each group had significantly less synovial proliferation and congestion. In these groups, the joint surface was smooth, with no obvious structural damage; the serum NO, NOS and PGE2 were significantly lower than in the control group (P < 0.01). The results indicated that PTTF at 7 and 14 mg/kg can effectively alleviate the secondary inflammatory response to AA in rats’ lateral ankle. The mechanism may be related to inhibition of the secretion of NO, PGE2 and NOS.
Introduction
Prunus tomentosa is widely used for the prevention and treatment of frostbite in Changbai Mountain vein plant fruit. It has been found that flavonoids have a wide range of biological activities, especially anti-inflammatory and immunomodulatory effects. Through the application of cherry wine, folk medicine experience suggests that there are active agents for effective treatment of frostbite in the alcohol extract. The total flavonoids content in the ethanol extracts of the cherry fruit was 78.3%, which indicated that the main active constituents were flavonoids. Reports on its anti-inflammatory effects have also been published. In recent years, research has shown that [Citation1] P. tomentosa total flavone (PTTF) has a significant anti-frostbite and anti-inflammatory effect. The experiments with the Croton oil ear swelling test in mice and the peritoneal capillary permeability test in mice showed that PTTF could inhibit the non-specific inflammatory reaction. However, its use as a therapeutic agent for rheumatoid arthritis (RA) and the mechanism thereof has not yet been systematically investigated. Rat adjuvant-induced arthritis (AA) is an important model of RA. This study successfully established a rat AA model. The effects of PTTF on the secondary regional inflammatory reaction and pathological changes were observed to evaluate the therapeutic effect of PTTF in RA and infer its mechanism of action.
Materials and methods
Model establishment and administration
The procedures were approved by the Medical Animal Ethics Committee, Beihua University.
After adaptive feeding for three days, 60 SD rats (males, clean grade, weight 170–190 g, supplied by Laboratory Animal Center, Jilin University; Certificate of qualification: SCXX-(JL)2003-0004) were randomly divided into six groups of 10 rats each. These groups were the blank control group, the complete Freund's adjuvant (CFA) model control group (a single intradermal injection of CFA into the left ankle), the dexamethasone group (dexamethasone injection, Hubei Tianyao Pharmaceutical Limited by Share Ltd; Natural Product Number H12020514), and the PTTF high-, medium- and low-dose groups. P. tomentosa (fruit part) was purchased in Jilin City, Northeast Asia Changbai Mountain Medicinal Plant Development Center, based on the source identified by the Department of Pharmacy, Beihua University. The PTTF was prepared in the lab.
To obtain the PTTF extract, P. tomentosa was mixed with water (1:20) and filtered after water immersion for 24 h. After removing the water, the rest was mixed with anhydrous ethanol at a proportion of 1:10. The ethanol reflux method was used to extract at a temperature of 60 °C. The residue was discarded after the solution was filtered; it then underwent rotary evaporation, and freeze drying. The finished product, PTTF, is a pale yellow powder.
The model group and the treatment groups were given mild ether after anaesthesia. Then, 0.1 mL CFA (FCA, SIGMA Company) was injected into the left ankle joint of each rat using a microsyringe, while the blank control group was injected with an equal volume of phosphate-buffered saline (PBS).
The blank group and the model group were given distilled water, and the dexamethasone group was given 5 mg/kg dexamethasone, equivalent to the clinical dose for humans.
After modelling, the rats were injected with high-dose (14 mg/kg), medium-dose (7 mg/kg) and low-dose (3.5 mg/kg) PTTF, respectively, once daily, continually for 7 days.
Adjuvant joint swelling degree observation
Before and after model establishment for 3, 6, 9, 12, 15, 18, 21 and 24 days, measurements of the left joint swelling degree of rats were taken with a Toe Volume Meter. Before measurement, the rat ankle joints were labelled with the right amount of picric acid solution. During measurement, the hind limb of the rat was immersed in a cubage cup. Marking where the ankle joint and the surface overlap, the solution was suctioned to the level of the original liquid level. The imbibition volume was read, which is the measured foot paw volume.
Organ index determination
The rats were decapitated after the last measurement of the ankle joint swelling. The thymus and spleen were weighed with a BS210S electronic analytical balance (Sartorius Instrument System Co., Ltd.), and the Viscera index was calculated (thymus or spleen weight (g)/weight (100 g)) × 100%.
Joint tissue observation
The rat left ankle joint specimens were placed in 10% formalin, fixed, ethylene diamine tetraacetic acid (EDTA) decalcified and embedded in paraffin. They then underwent hematoxylin and eosin (HE) staining. The morphology of the articular cartilage and synovial tissue were observed under a BA310 type biological imaging microscope (Motic Company).
Serum nitric oxide (NO), nitric oxide synthase (NOS), prostaglandin E2 (PGE2) content determination
After the last measurement of joint swelling, blood was taken from the femoral artery and was collected in heparinized tubes (100 IU/mL) and centrifuged to obtain serum. Serum NO was measured using a Nitric Oxide Assay Kit (Griess Reagent, Beyotime Co., Ltd.), serum NOS was analysed with a Nitric Oxide Synthase Detection Kit (Sigma, America), and serum PGE2 was analysed with a Rat PGE2 detection Elisa Kit (Sigma, America). Assays were performed according to the manufacturers’ directions.
Statistical analysis
The data are presented as . SPSS20.0 software was used for statistical processing. In one-way analysis of variance, P < 0.05 was used to evaluate statistically significant differences.
Results and discussion
Effect of PTTF on the swelling degree of ankle joints in AA rats
Significant swelling occurred in the left ankle joint model of the rat model group compared to the blank group (P < 0.05), indicating that the model was successful. The model group reached a peak in the swelling at 18 d after modelling. There was no significant difference between the swelling degree in the model group and the dexamethasone group; the PTTF 3.5, 7 and 14 mg/kg dose groups (P > 0.05) in the model group and the dexamethasone group. In the first 18 days, the degrees of ankle joint swelling in the dexamethasone group and the PTTF middle- and high-dose groups were all significantly lower than that in the model group (P < 0.01, P < 0.05). The results are shown in .
Table 1. Effect of PTTF on the swelling degree of ankle joint in AA rats ()
Effect of PTTF on the organ index of AA rats
In the CFA model group, there was some degree of atrophy of the thymus, and there was a significant difference compared to the normal blank group (P < 0.05). There were no statistically significant differences between the thymus index in the blank group, the dexamethasone group and the PTTF 3.5, 7 and 14 mg/kg dose groups (P > 0.05), although in the PTTF groups, the index of the spleen was significantly higher (P < 0.05) than that in the normal blank control group ().
Table 2. Effect of PTTF on the organ index of AA rats ().
Pathological effects of PTTF in AA rats
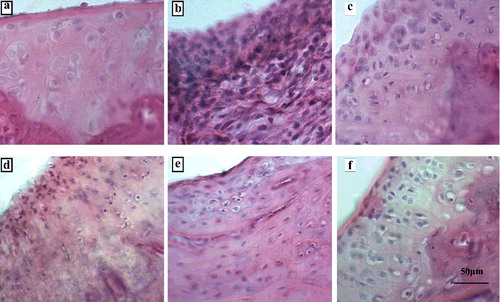
There was no proliferation of synovial cells, inflammatory cell infiltration or vascular proliferation in the normal ankle joints of the blank group; there were different degrees of damage to the articular cartilage surface of the AA rats. There were different degrees of congestion and oedema in the synovial membrane. Synovial hyperplasia could be observed under light microscope. There was vascular dilation and inflammatory cell infiltration in the synovial membrane. In the dexamethasone group and the 3.5, 7 and 14 mg/kg dose PTTF groups, the synovial hyperplasia and congestion were significantly reduced compared with the model group; the joint surface was relatively flat and there was no damage to the structure, as seen in .
Effect of PTTF on NO, NOS and PGE2 in the serum of AA rats
The serum NO, PGE2 and NOS content of the model group rats was significantly higher than that in the control group, whereas it was significantly lower in the dexamethasone group and PTTF (7 and 14 mg/kg) group rats than in the control group (P < 0.01). The data are presented in .
Table 3. Effect of PTTF on NO, NOS and PGE2 in serum of AA rats ().
Putative mechanism of action and therapeutic potential
RA is a common clinical systemic autoimmune disease. It results chiefly in the proliferation of synovial cells, a variety of inflammatory cell infiltration, cartilage tissue destruction and, ultimately, joint deformity and loss of function [Citation2]. Because the aetiology of the disease has not yet been fully clarified, a successful clinical treatment has still not been developed by Western medicine. Current treatments do not result in a satisfactory therapeutic effect, and long-term treatment brings about a variety of adverse reactions that many patients are unable to tolerate [Citation3]. In recent years, it has become a hot spot for RA therapy at home and abroad to find effective components in the treatment of RA with little adverse reactions and a wide range of drugs.
The rat AA model induced by complete adjuvant is a commonly used model of RA, which leads to a secondary inflammatory reaction caused by immune dysfunction [Citation4]. This study showed that PTTF at a dose of 7 and 14 mg/kg can effectively eliminate inflammation and improve the symptoms of RA. The determination of the organ index revealed no significant effect on the thymus, but the volume of the spleen was significantly increased. This indicates that PTTF stimulates some immune organs, which can improve RA and improve immunity without the adverse side effects of dexamethasone and other steroids, which can inhibit the immune organ function. The results from the pathological examination showed that PTTF can improve the pathological changes associated with the secondary inflammation of the lateral ankle and reduced the synovial cell hyperplasia and inflammatory cell infiltration, vascular proliferation and the inflammation process, resulting in effective inhibition.
NO, as an inflammatory mediator in the pathogenesis of RA, has an important role; NO is a multifunctional gaseous biological messenger to mediate a variety of biological phenomena by changing the metabolism of chondrocytes and synoviocytes. Cartilage proteoglycan degradation, which interferes with the extracellular matrix of cartilage cell function related to its regulatory role, leads to destruction of cartilage cells and repair function decline [Citation5]. At the same time, NO also increases the synovial microvascular permeability and the oxygen free-radical level, causing joint damage. NO can also affect fibroblasts like synovial cells induced by MMPs, which can increase the severity of joint injuries [Citation6]. NO is synthesized by NOS. A large amount of NO could be found in the synovial fibroblasts and chondrocytes of RA joints. In vivo, interleukin-1 (IL-1), tumour necrosis factor α (TNF-α) pro-inflammatory cytokine stimulation, over-expression of iNOS in macrophages and synovial cells occurs in RA synovial tissue, resulting in NO synthesis [Citation7]. NO promotes the release of IL-1 and TNF-α from macrophages, which leads to a vicious cycle, causing the RA clinical symptoms to worsen. PGE2 has a pronounced pro-inflammatory effect in the RA of the joint cavity, and local blood circulation increases significantly, which can cause local inflammatory reactions such as swelling, pain and/or exudation [Citation8]. This study showed that a 7 or a 14 mg/kg dose of PTTF can effectively reduce the secretion of NO, NOS and PGE2 in the serum of AA rats, suggesting that the reduction of NO, NOS and PGE2 secretion may be one of the therapeutic mechanisms of action of PTTF against RA.
Conclusions
In this study, 7 and 14 mg/kg PTTF were shown to inhibit the development of FCA side ankle inflammation in AA rats, and also to improve the immune function in rats. This is possibly because it decreases NO, NOS and PGE2 secretion in the serum of AA rats. The results from this study suggest that PTTF could be considered a promising and effective component for future treatment of RA.
Disclosure statement
The authors declare no conflict of interest.
References
- Zhang C, Shao S, Shi Y, et al. Effects on frostbite and inflammation dependability of Chang Bai Prunus tomentosa thumb total flavone. Chinese J Immunol. 2010;26(11):977–981.
- Yang J, Liu X, Li X, et al. Effect of Th17/Treg balance in rheumatoid arthritis. Chinese J Pharmacol Bull. 2013;29(8):1045–1048.
- Sun J, Han Q, Zhang F, et al. A review on treating RA. Clin J Chinese Med. 2013;1(9):117–118.
- Li P, Xie G, Song S, et al. Clinical manifestations and the main evaluation method on adjuvant-induced arthritis model in rats. Chinese J Immunol. 2012;28(5):453–457.
- Abd El-Rahman RS, Suddek G M, Gameil NM, et al. Protective potential of MMR vaccine against complete Freund's adjuvant-induced inflammation in rats. Inflammopharmacology. 2011;19(6):343–348.
- Wang D, Chang Y, Wei W, et al. Therapeutic effects of TACI-Ig on rat with adjuvant arthritis. Clin Exp J Immunol. 2011;163(2):225–234.
- Liu W, Zhang X. Receptor activator of nuclear factor-κB ligand(RANKL) /RANK/osteoprotegerin system in bone and other tissues. Mol Med J Rep. 2015;11(5):3212–3218.
- Ito I, Shunji Y, Kaoru Y, et al. A Novel JAK inhibitor, peficitinib, demonstrates potent efficacy in a rat adjuvant-induced arthritis model. J Pharmacol Sci. 2017;133(1):25–33.