ABSTRACT
Drumstick (Moringa oleifera Lam.) is an industrially important tree species. Due to the availability of an efficient regeneration system, the present study was undertaken to develop a reliable and stable Agrobacterium tumefaciens-mediated transformation system for the genetic improvement of drumstick. A. tumefaciens strain EHA105, harbouring the plasmid pCAMBIA1301 containing the hygromycin phosphotransferase II and ß-glucuronidase genes driven by the CaMV 35S promoter, was used to establish a transformation system in drumstick. The use of vacuum infiltration-assisted Agrobacterium infection (excluding a pre-culture step) and co-cultivation for two days significantly increased the transformation frequency. Genomic integration and transgene expression were confirmed by β-glucuronidase (GUS) assays, polymerase chain reaction (PCR), Southern blot analysis and real-time quantitative PCR (RT-qPCR). This transformation protocol provides a basis for the future development of genetic engineering techniques to improve the performance of drumstick.
Introduction
Moringa oleifera Lam., commonly known as drumstick tree, belongs to the monogeneric family Moringaceae [Citation1]. This species is indigenous to northern India and some African countries and has currently spread to many tropical and subtropical regions due to its multiple uses [Citation2]. Drumstick is considered a fast-growing and drought-tolerant tropical perennial tree with a long history of traditional medicine and culinary uses [Citation3–5]. It may also become an important species for biofuel production [Citation6,Citation7] and has been used in a variety of industrial materials [Citation8,Citation9]. Despite its importance, limited breeding and selection efforts to improve drumstick have been reported, and a limited number of cultivars have been released [Citation2]. Drumstick production is also affected by many biotic factors and abiotic stresses [Citation2]. Thus, more attention should be given to improve its nutritional value and biofuel conversion ability.
Genetic improvements of forest species are limited by their long life cycles, self-incompatibility and other factors [Citation10–12]. However, several potential uses of biotechnology to improve tree species have been recognised. The basis for genetic transformation is plant regeneration. In recent years, several efficient tissue culture and regeneration systems have been developed for drumstick [Citation13–17]. Due to the fast development of sequencing technologies, the draft genome sequence of drumstick was released in 2015 [Citation18], which indicated the beginning of its genetic breeding era. Therefore, the establishment of an efficient genetic transformation platform for drumstick has become relevant. However, limited reports are available in genetic transformation of drumstick. To our knowledge, only one genetic transformation protocol for drumstick has been reported so far [Citation19].
We describe a reliable and efficient protocol for vacuum infiltration-assisted Agrobacterium tumefaciens-mediated transformation (AMT) of drumstick using nodal explants and a hygromycin (Hyg) selection system to select for transformants in the callus and regenerated plant stages. Transgene integration and inheritance were verified using histochemical GUS staining and molecular analyses.
Materials and methods
Plant material and culture conditions
Nodals from the plantlets of three drumstick clones, M-2, M-5 and M-17 (obtained from a genotype screening experiment), were used to optimise the transformation conditions. Nodal explants were incubated in shoot induction medium (SIM) (Murashige and Skoog (MS) basal medium + 1.0 mg/L 6-menzylaminopurine (BA) + 0.05 mg/L naphthaleneacetic (NAA) + 30 g/L sucrose). Regenerated shoots were excised from mother tissues and rooted in rooting medium (RM) (MS basal medium + 0.05 mg/L NAA). All media were adjusted to pH 5.8‒6.0 using 1 mol/L NaOH or 1 mol/L HCl solution and autoclaved at 121 °C for 15 min. Cultures were maintained under a 12-h photoperiod using cool-white light and at a temperature of 25 °C.
Hygromycin sensitivity detection
Nodal explants were cultured on SIM containing a combination of Hyg (5‒35 mg/L) and cefalexin (Cef; 150 mg/L) for 4 weeks. Explant survival was evaluated at the end of this period. Regenerated shoots were excised from mother tissue and rooted in RM containing a combination of Hyg (5‒25 mg/L) and Cef (150 mg/L) for 2 weeks. Experiments were performed three times, and 15 explants or shoots were used in each treatment.
Binary vector and Agrobacterium preparation
A binary vector, pCAMBIA1301, containing hygromycin phosphotransferase II and ß-glucuronidase (gusA) genes driven by the CaMV 35S promoter was introduced into A. tumefaciens strain EHA105. The T-DNA region of pCAMBIA1301 contains a unique HindIII cleavage site.
A. tumefaciens strain EHA105 (1 mL) was inoculated into 100 mL yeast extraction broth medium [Citation20] containing 5 mg chloramphenicol and 5 mg kanamycin and grown with shaking (200 r/min) for 18 h at 28 °C. A. tumefaciens was collected by centrifugation (2500 × g, 5 min) and resuspended in SIM to an optical density at 600 nm (OD600) of 0.2.
Transformation procedure
To optimise the transformation protocol, different combinations of the pre-culture (0, 2, 4 or 6 days) and co-cultivation (1, 2, 3 or 4 days) periods were evaluated. Explants were infected with the A. tumefaciens suspension under normal atmospheric pressure or under 60 kPa vacuum infiltration for 30 min. After infection, A. tumefaciens-infected explants were blot-dried onto sterile filter paper and inoculated in the dark on co-cultivation medium. After co-cultivation, explants were washed with autoclaved double-distilled water five times. After blot drying, explants were transferred to selection medium. After approximately 4 weeks, putative transgenic shoots (more than 2 cm) were excised from mother tissues and rooted in RM.
GUS histochemical assay
GusA expression was analysed histochemically in tissues and plantlets as described by Jefferson [Citation21]. Samples were soaked in 500 mg/L X-Gluc solution overnight at 37 °C. The expression of GUS in tissues and plantlets was observed as blue staining.
Molecular characterisation by PCR, Southern blot and RT-qPCR assays
DNA was extracted from leaves according to the method described by Rogers and Bendich [Citation22]. The gusA gene was amplified from genomic DNA using forward (5′- GTCGCGCAAGACTGTAACCA-3′) and reverse (5′-CGGCGAAATTCCATACCTG-3′) primers. The expected length of the amplified product was 1081 bp. The PrimeSTAR Max DNA Polymerase (Takara, Beijing, China) was used and the thermocycling conditions were as follows: 95 °C for 5 min, followed by 95 °C for 1 min, 55 °C for 5 s and 72 °C for 10 s for 35 cycles and a final elongation step at 72 °C for 10 min (Bio-Rad, Hercules, USA). The products were analysed by 1.5% agarose gel electrophoresis.
For Southern blot analysis, 30 µg purified DNA were digested by restriction enzymes with one restriction site within the T-DNA region, and the resulting fragments were separated on 1.0% agarose gels prior to transfer to Zeta-Probe GT nylon membranes (Bio-Rad, Hercules, USA). DNA was fixed to nylon membranes by ultraviolet crosslinking. Hybridisation and membrane washing were conducted at 65 °C according to the manufacturer's instructions. The Prime-It RmT Random Primer Labeling Kit (Stratagene, Basel, Switzerland) was used to generate 32P-labeled probes targeting the gus gene.
RNA was isolated from regenerated shoots using the Simple Total RNA Kit (Takara, China). First-strand cDNA was synthesized from total RNA using the First-Stand cDNA Synthesis Kit and oligo-dT primer (Takara, China). Real-time quantitative PCR (RT-qPCR) was performed as described by Liu et al. [Citation23]. The forward primer 5′-GTGAGCGTCGCAGAACA-3′ and reverse primer 5′- GGCAACAAGCCGAAAGA-3′ were used for gus amplification. Actin was amplified as a reference gene using primers ACTf and ACTr [Citation24]. The PCRs were stopped after 40 cycles.
Data analysis
Data are presented as mean values with standard error (±SEM) from two independent experiments. Statistical analysis was carried out using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA). Significant differences among means were determined by Duncan’s multiple range tests. A P-value less than 0.05 was considered to indicate statistical significance.
Results and discussion
A. tumefaciens strain
Different A. tumefaciens strains exhibit different effects on plant transformation frequency and transgenic event quality [Citation25]. Do et al. [Citation26] made a comparison of three immature A. tumefaciens strains, AGL1, EHA101 and GV3101, regarding the infection of sorghum and found that AGL1 improves the transformation efficiency. Yu et al. [Citation27] developed an efficient transformation system using EHA105. LBA4404 was successfully used for the transformation of safflower [Citation18]. In the present study, strain EHA105 played a pivotal role in the transformation of drumstick. The vacuum infiltration technique enhanced the transformation efficiency, similar to previous results obtained in other plants, including sugarcane [Citation28], soybean [Citation29] and peace lilies [Citation27]. A suitable vector is also important for genetic transformation. The plasmid pCAMBIA 1301 is widely used for plant genetic engineering and has been applied to the genetic transformation of various plants, such as rice [Citation30], centipedegrass [Citation31] and peanut [Citation32].
Selection pressure of hygromycin in drumstick
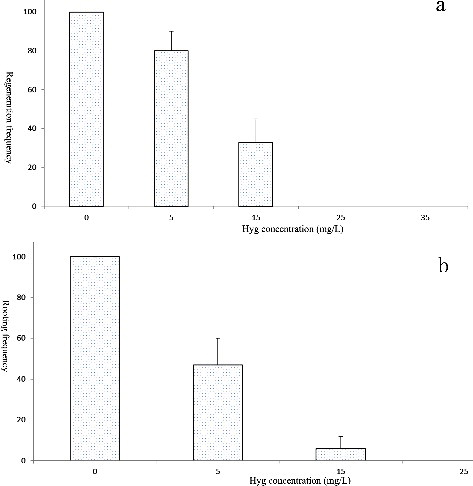
Determining the optimum concentration of a selection agent is crucial for the selection of true transformants; a lower or supra-optimal concentration will result in non-ideal results [Citation18]. Hyg is an ideal selection agent that has been widely used for the transformation of several tree species [Citation10,Citation12]. In this study, explants were cultured on selection medium containing different concentrations of Hyg for 4 weeks. All explants survived and grew rapidly in the absence of Hyg ((a)). The survival rate of explants significantly decreased as the Hyg concentration increased from 5 to 25 mg/L ((a)). At the highest dose of Hyg (25 mg/L), all explants turned brown in colour ((b)) and died after cultivation for 4 weeks. Thus, the minimum Hyg concentration necessary to suppress non-transformed explants at 4 weeks was 25 mg/L. The rooting frequency was 100% in the absence of Hyg; the minimum Hyg concentration required to suppress non-transformed explant rooting was 15 mg/L ((b)). Our data suggest that Hyg selection is efficient for drumstick transformation.
Optimisation of the pre-culture and co-cultivation durations
Optimisation of the pre-culture and co-cultivation durations in AMT is of foremost significance. Hence, different pre-culture and co-cultivation durations were tested. Among the various conditions, direct infection without pre-culturing and with a 2-day co-cultivation period evoked maximum GUS expression ( and ). Longer pre-culture and co-cultivation periods resulted in negative effects due to bacterial leaching. The infected explants were incubated in GUS staining solution, and the number of GUS-positive explants was counted. The frequency of GUS-positive explants was greater under the 60 kPa vacuum pressure condition than under normal atmospheric pressure (data not shown).
Table 1. Transient transformation frequency of drumstick explants after different duration of pre-culture.
Table 2. Transient transformation frequency of drumstick explants after different duration of co-cultivation.
GUS staining analysis
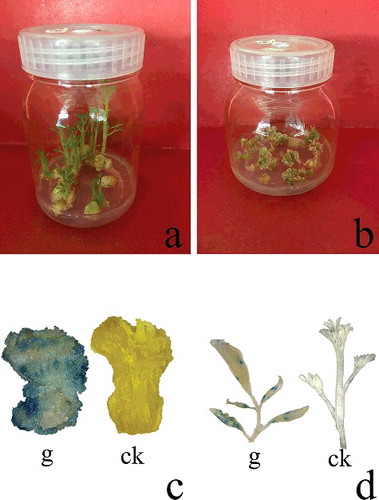
The efficiency of transformation was first evaluated by transient expression of gus in drumstick callus 1 week after inoculation. Twenty Hyg-resistant explants were randomly selected and used for GUS expression analysis. The gus gene was stably expressed in calli ((c)). Of the 135 regenerated plants, 37 were randomly selected to analyse the gus gene expression in leaf tissues. Of the 37 plants, 18 showed obvious blue GUS staining ((d)). No GUS expression was detected in the other 19 plants or non-transgenic controls. The efficiency was higher than that reported by Abbas [Citation19]. The plants negative for GUS expression may have been derived from untransformed cells that survived within the Hyg-resistant calli.
Molecular analysis
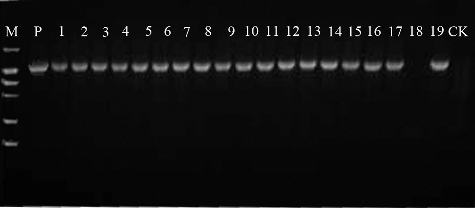
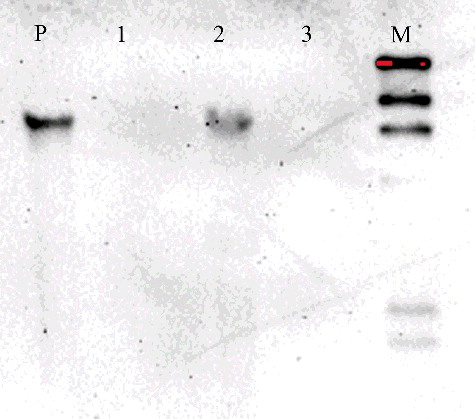
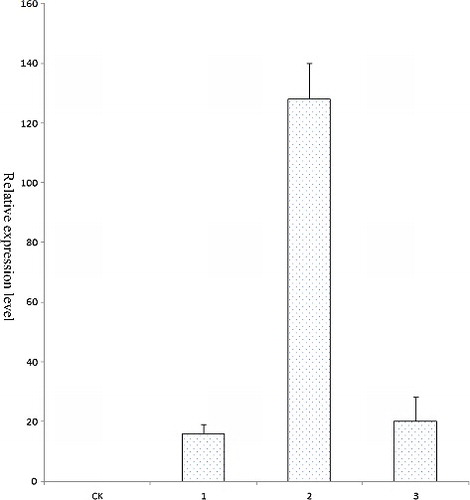
In this study, PCR, Southern blot analysis and RT-qPCR were used to determine the transformation efficiency. These analyses can reduce the probability of false-positives. Eighteen GUS-positive plants and one GUS-negative plant were randomly selected and subjected to PCR. DNA was extracted from the transformed and control plants. A 1081 bp gus-specific fragment was detected in all plants that tested positive for GUS in the GUS assay, whereas no amplification products were detected in the GUS-negative plant (). Three putative transformants that were both GUS-positive and PCR-positive were examined by Southern blot analysis to confirm transgene integration and copy number. Of these three plants, only one had T-DNA integration, containing one copy of the transgene (). PCR and Southern blot analysis revealed that the gus gene was integrated into the drumstick genome and revealed only one copy of integrated T-DNA. These low copy numbers in drumstick are similar to those in a number of other species, such as centipedegrass [Citation31], European chestnut [Citation33] and Populus [Citation12]. AMT may integrate several copies of the gene of interest into different parts of the plant genome. The gene insertion copy number may vary among different plant species. RT-qPCR of the transgenic shoots was also conducted. The expression of gus in drumstick was comparable to that of the reference genes (), indicating that the plasmid pCAMBIA 1301 had been introduced successfully into drumstick cells and that gus had been integrated into the genome. In the previous report, PCR and GUS histochemical assay were used to determine drumstick transformation efficiency [Citation19]. However, besides these two techniques, Southern blot and RT-qPCR were used in this study as well. These analyses can effectively reduce the number of false-positive transformants.
Conclusions
Drumstick has gained interest globally in recent years because of its unique industrial value. Therefore, a genetic transformation system which facilitates its improvement by genetic engineering and facilitates functional genomic analyses using molecular tools would be very important. In the present study, we describe a more efficient and reliable genetic transformation protocol for drumstick, which should open the door for biotechnological improvements of this important tree species and systematic functional genomic studies.
Disclosure statement
No potential conflict of interest is reported by the authors.
References
- Popoola JO, Obembe OO. Local knowledge, use pattern and geographical distribution of Moringa oleifera Lam. (Moringaceae) in Nigeria. J Ethnopharmacol. 2013;150(2):682–691.
- Leone A, Spada A, Battezzati A, et al. Cultivation, genetic, ethnopharmacology, phytochemistry and pharmacology of Moringa oleifera leaves: an overview. Int J Mol Sci. 2015;16(6):12791–12835.
- Anwar F, Latif S, Ashraf M, et al. Moringa oleifera: a food plant with multiple medicinal uses. Phytother Res. 2007;21(1):17–25.
- Fahey JW. Moringa oleifera: a review of the medical evidence for its nutritional, therapeutic, and prophylactic properties. Part 1. Trees Life J. 2005 [ cited 2017 Feb 19];1:5. Available from: http://www.tfljournal.org/article.php/20051201124931586
- Stohs SJ, Hartman MJ. Review of the safety and efficacy of Moringa oleifera. Phytother Res. 2015;29(6):796–804.
- Fotouo-M. H, Toit ESD, Robbertse PJ. Effect of storage conditions on Moringa oleifera Lam. seed oil: biodiesel feedstock quality. Ind Crop Prod. 2016;84:80–86.
- Nguyen HN, Gaspillo PAD, Maridable JB, et al. Extraction of oil from Moringa oleifera, kernels using supercritical carbon dioxide with ethanol for pretreatment: optimization of the extraction process. Chem Eng Process. 2011;50(11–12):1207–1213.
- Kituyi JL, Foulkes M, Worsfold P, et al. Efficiency of pre-treated Moringa oleifera, for the removal of Cd2+, and Zn2+, ions from wastewaters. Ecohydrol Hydrobiol. 2013;13(4):267–271.
- Sengupta ME, Keraita B, Olsen A, et al. Use of Moringa oleifera seed extracts to reduce helminth egg numbers and turbidity in irrigation water. Water Res. 2012;46(11):3646–3656.
- Fernando SC, Goodger JQD, Gutierrez SS, et al. Plant regeneration through indirect organogenesis and genetic transformation of Eucalyptus polybr actea, R.T. Baker. Ind Crop Prod. 2016;86:73–78.
- Li ZN, Fang F, Liu GF, et al. Stable Agrobacterium-mediated genetic transformation of London plane tree (Platanus acerifolia Willd.). Plant Cell Rep. 2007;26(5):641–650.
- Priti M, Igor K. Agrobacterium-mediated stable genetic transformation of Populus angustifolia and Populus balsamifera. Front Plant Sci. 2016 [ cited 2017 Feb 19];7:296. DOI: 10.3389/fpls.2016.00296.
- Förster N, Mewis I, Ulrichs C. Moringa oleifera—establishment and multiplication of different ecotypes in vitro. Gesunde Pflanzen. 2013;65(1):21–31.
- Gayathri M, Kumar PS, Prabha AML, et al. In vitro, regeneration of Arachis hypogaea L. and Moringa oleifera Lam. using extracellular phytohormones from Aphanothece, sp. MBDU 515. Algal Res. 2015;7:100–105.
- Mathur M, Yadav S, Katariya PK, et al. In vitro propagation and biosynthesis of steroidal sapogenins from various morphogenetic stages of Moringa oleifera Lam. and their antioxidant potential. Acta Physiol Plant. 2014;36:1749–1762.
- Shahzad U, Jaskani MJ, Ahmad S, et al. Optimization of the micro-cloning system of threatened Moringa oleifera Lam. Pak J Agr Sci. 2014;51(2):459–467.
- Zhang JJ, Yang YS, Lin MF, et al. An efficient micropropagation protocol for direct organogenesis from leaf explants of an economically valuable plant, drumstick (Moringa oleifera Lam.). Ind Crop Prod. 2017;103:59–63.
- Yang T, Yan Z, Jing Z, et al. High quality reference genome of drumstick tree (Moringa oleifera Lam.), a potential perennial crop. Sci China. 2015;58(7):627–638.
- Abbas DE. Development of in vitro regeneration and Agrobacterium mediated transformation systems for Moringa oleifera plant. Egypt J Genet Cytol. 2014;43:99–111.
- Patial V, Krishna R, Arya G, et al. Development of an efficient, genotype independent plant regeneration and transformation protocol using cotyledonary nodes in safflower (Carthamus tinctorius L.). J Plant Biochem Biotechnol. 2016;25(4):421–432.
- Jefferson RA. Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Rep. 1987;5(4):387–405.
- Rogers SO, Bendich AJ. Extraction of total cellular DNA from plants, algae and fungi. Springer Netherlands. 1994: 183–190.
- Liu S, Su L, Liu S, et al. Agrobacterium rhizogenes-mediated transformation of Arachis hypogaea: an efficient tool for functional study of genes. Biotechnol Biotecnol Equip. 2016;30(5):869–878.
- Deng LT, Wu YL, Li JC, et al. Screening reliable reference genes for RT-qPCR analysis of gene expression in Moringa oleifera. Plos One. 2016 [ cited 2017 Feb 19];11(8):e0159458. DOI:10.1371/journal.pone.0159458.
- Zhi L, Teronde S, Meyer S, et al. Effect of Agrobacterium strain and plasmid copy number on transformation frequency, event quality and usable event quality in an elite maize cultivar. Plant Cell Rep. 2015;34(5):745–754.
- Do PT, Lee H, Mookkan M, et al. Rapid and efficient Agrobacterium -mediated transformation of sorghum (Sorghum bicolor) employing standard binary vectors and bar, gene as a selectable marker. Plant Cell Rep. 2016;35:2065–2076.
- Yu B, Liao F, Liu J, et al. Efficient regeneration and transformation of Spathiphyllum cannifolium. Plant Cell Tiss Organ Cult. 2016;127(2):325–334.
- Mayavan S, Subramanyam K, Jaganath B, et al. Agrobacterium-mediated in planta genetic transformation of sugarcane setts. Plant Cell Rep. 2015;34(10):1835–1848.
- Arun M, Subramanyam K, Mariashibu TS, et al. Application of sonication in combination with vacuum infiltration enhances the Agrobacterium-mediated genetic transformation in Indian soybean cultivars. Appl Biochem Biotechnol. 2015;175(4):2266–2287.
- Gao XL, Yang J B, Jing F X, et al. The detection of plasmid pCAMBIA1301 in transgenic rice by arrayed primer extension. Hereditas. 2005;27(2):271–278.
- Liu M, Lu S, Liu L, et al. Agrobacterium-mediated transformation of centipedegrass (Eremochloa ophiuroid es, [Munro] Hack.). Plant Cell Tiss Organ Cult. 2012;109:557–563.
- Chen MN, Yang QL, Wang T, et al. Agrobacterium-mediated genetic transformation of peanut and the efficient recovery of transgenic plants. Can J Plant Sci. 2015;95(4):735–744.
- Corredoira E, José MCS, Vieitez AM, et al. Agrobacterium-mediated transformation of European chestnut somatic embryos with a Castanea sativa, (Mill.) endochitinase gene. New Forests. 2016;47(5):669–684.