ABSTRACT
An alkaline lipase strain of Pseudomonas aeruginosa HFE733 was isolated from soil samples of domestic waste. The alkaline lipase from P. aeruginosa HFE733 was purified and characterized. The enzyme was purified 9.97-fold by means of ammonium sulphate precipitation, Sephadex G-25 and diethylaminoethyl (DEAE) cellulose chromatography. Purified alkaline lipase protein was analyzed by sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE), the molecular mass was 51.0 kDa. The enzyme exhibited maximum activity at 40 °C and pH 8.5. The enzyme is stable at pH 7.0–8.5. It was remarkably activated by some metal ions and chemical reagents, such as Fe3+, Al3+, β-mercaptoethanol, cysteine, DL-dithiothreitol (DTT), Tween 80 and Triton X-100, but suppressed by sodium dodecyl sulfate (SDS) at 10 mmol/L. The P. aeruginosa HFE733 strain and lipase exhibited remarkable potential for biodegradation of oil and organics (measured as chemical oxygen demand (COD)). We demonstrated that it can be used for biodegradation of food wastewater from restaurants.
Introduction
Lipases known as triacylglycerol acylhydrolases (E.C. 3.1.1.3) are ubiquitous carboxylic ester hydrolases that can catalyze hydrolysis of the long chain triglycerides to fatty acids, diacylglycerol, monoacylglycerol and glycerol [Citation1]. However, in non-aqueous medium, lipases synthesise esters from glycerol and long-chain fatty acids [Citation2]. Lipases are largely produced from bacterial strains like Pseudomonas alcaligenes [Citation3], Pseudomonas aeruginosa [Citation4–7], Pseudomonas fragi [Citation8], Bacillus subtilis [Citation9] etc. and fungi like Penicillium expansum [Citation10], Trichoderma [Citation11], Penicillium chrysogenum [Citation12], Aspergillus [Citation13] etc. Besides hydrolysis activity, lipases also display interesterification, esterification, aminolysis and alcoholysis activity [Citation14], which has contributed to their wide use in pharmaceuticals, paper manufacture, textile, food, detergents and cosmetics manufacture industries [Citation15,Citation16].
In recent years, more and more food wastewater is discharged in China, causing increasingly serious environmental pollution. Oil films on water surfaces prevent the diffusion of oxygen from air into water, which leads to the death of many aquatic species. Biodegradation of lipid-rich wastes has long been carried out [Citation17,Citation18]. Furthermore, many reports have described the use of lipolytic enzymes in wastewater treatment [Citation4,Citation19]. Microbial strains and lipases could have an impact on reducing the fats and oil content in food wastewater, and may help in reducing severe pollution problems caused by wastewater.
In this study, we report the isolation of an alkaline lipase strain of Pseudomonas aeruginosa HFE733 from soil samples of domestic waste. We purified and characterised the alkaline lipase from P. aeruginosa HFE733, and applied P. aeruginosa HFE733 strain and its lipase for treatment of restaurant wastewater.
Materials and methods
Chemicals
Ethylene diamine tetraacetic acid (EDTA), DL-dithiothreitol (DTT), glutathione (GSH) and sodium dodecyl sulfate (SDS) were purchased from Sigma Chemical Co. (St. Louis, MO, USA).
Microorganism
Lipase-producing strains were isolated from soil samples from sites of stacking domestic waste. The microorganisms were grown on Gauze-agar (peptone 10 g/L, yeast 5 g/L, sodium chloride 10 g/L, agar 20 g/L) at 30 °C. One of these strains that produced significantly highest level of lipase was identified as Pseudomonas aeruginosa HFE733 by biochemical tests and 16S rRNA sequence analysis.
Production of lipase
The optimized fermentation medium was composed of: olive oil 5.92 mL/L, yeast extract 34 g/L, cane sugar 12.5 g/L, copper sulfate 0.4 g/L and manganese sulfate 0.2 g/L. The pH of the medium was adjusted to 7.0. Erlenmeyer flasks (250 mL) containing 50 mL sterile culture medium were inoculated with 5 mL inoculums (6.2 × 108 CFU/mL). The flasks were incubated at 30 °C for 60 h on an orbital shaker. The microbial suspension was centrifuged at 6000 g for 10 min, and the clear supernatant was assayed for lipase activity.
Lipase activity assay
Lipase activity was determined by an olive oil emulsion method [Citation20]. The substrate was prepared by mixing 50 mL of olive oil with 150 mL of polyvinyl alcohol solution (2%, w/v) to obtain emulsion. The reaction mixture consisting of 5.0 mL of phosphate buffer (0.25 mol/L, pH 7.5), 4 mL of the substrate emulsion above and a 1.0 mL crude enzyme extract was incubated for 15 min at 40 °C in a vessel. After 15 min, the reaction was stopped, 15 mL 95 wt% ethanol reagent was added, and the amount of free fatty acids released in the reaction was titrated with 0.05 mol/L of sodium hydroxide solution in the presence of phenolphthalein indicator. In addition, a blank experiment for comparison without adding crude enzyme was carried out by the above-described assay procedure. One unit (1 U) of lipase activity is defined as the amount of enzyme required to release 1 μmol free fatty acids per minute under the assay conditions. The relative activity presented in the figures is the rate (%) of enzyme activity at other test conditions relative to the highest enzyme activity at one test condition.
Purification and determination of the molecular weight of lipase
The culture supernatant was subjected to 80% saturation of ammonium sulphate and after overnight preservation at 4 °C, the precipitated enzyme solution was separated by centrifugation (9408 g, 15 min), and then was dissolved in phosphate buffer (0.25 mol/L, pH 7.5). Then the enzyme was desalinated at 0.5 mL/min with phosphate buffer (0.25 mol/L, pH 7.5) by a Sephadex G-25 column (0.9 × 30 cm). The enzyme was concentrated by ultrafiltration membrane (Millipore, USA) using centrifuge (6000 g, 25 min). Finally, the partially purified enzyme was passed through a diethylaminoethyl (DEAE) cellulose column (3 × 20 cm) and active enzyme was eluted by Tris-HCl (pH 8.5) with a gradient of NaCl (0.1–0.6 mol/L) at a flow rate of 2 mL/min. The fractions with enzyme activity were collected, concentrated by lyophilization and used for further experiments.
The enzyme from the DEAE cellulose column was loaded onto sodium dodecyl sulfate polyacrylamide gel (12%) electrophoresis (SDS-PAGE) following the method of Laemmli [Citation21]. The molecular weight of the lipase was determined by comparing its mobility with the medium range marker proteins (14.3–97.2 kDa). Protein concentration was measured following the method of Lowry et al. [Citation22].
Effects of temperature on enzyme activity and stability
The effect of temperature on lipase activity was determined by performing an enzymatic hydrolysis reaction for 15 min at various incubation temperatures (30, 35, 40, 45, 50 and 55 °C). To assay the thermostability, the residual enzyme activities were measured after pre-incubating the enzyme at different temperature (30, 40, 50, 60 and 70 °C) for 1, 2, 3 and 4 h without substrate.
Effects of pH on enzyme activity and stability
The optimal pH of lipase was determined by performing reactions in 50 mmol/L sodium acetate–acetic acid (pH 5.0–5.5), phosphate buffer (pH 6.0–7.5), Tris-HCl (pH 8.0–8.5) and glycine-NaOH (pH 9.0–10.0), respectively. The effect of pH on lipase activity was investigated by performing enzymatic hydrolysis reactions at various pH levels (5.0, 5.5, 6.0, 6.5, 7.0, 7.5, 8.0, 8.5, 9.0, 9.5 and 10.0) for 15 min. Stability of the enzyme at different pH was studied by incubating the enzyme (without substrate) at different pH ranging from 5.0 to 10.0 for up to 4 h at 30 °C. Then its activity was measured.
Effect of metal ions and chemical reagents on lipase activity
The effects of metal ions on enzyme activity were determined by assaying the residual activity after the enzyme was incubated with 1 and 10 mmol/L of various metal ions: Na+ (NaCl), K+ (KCl), Mg2+ (MgCl2), Ca2+ (CaCl2), Mn2+ (MnSO4·H2O), Fe2+ (FeCl2), Co2+ (CoCl2), Cu2+ (CuSO4·5H2O), Zn2+ (ZnCl2), Ba2+ (BaCl2), Li+ (Li2SO4), Fe3+ (FeCl3), Pb2+ (Pb(NO3)2) and Al3+ (Al2(SO4)3·16H2O). No metal ion was used in the control sample solution. Some of the modifying reagents and amino acids (EDTA, DTT, β-mercaptoethanol, cysteine, GSH, SDS, Tween 20, Tween 80 and Triton X-100) were also tested to study their effect on lipase activity.
Biodegradation in food wastewater treatment
The food wastewater was collected from a restaurant in Wuhan China. In this experiment, 10% (v/v) fermentation broth (including cells and enzymes) was added to food wastewater and was cultured for 30 °C at 180 rpm. The oil concentration and chemical oxygen demand (COD) were determined, to analyze the degradation ability. Two control experiments were performed, in which no fermentation broth was added to restaurant wastewater: control 1, with incubation at 30 °C and 180 rpm; control 2, with stewing at 30 °C.
Oil concentration was determined according to the gravimetric method [Citation23]. Chemical oxygen demand (COD) was determined by dichromate titration [Citation23]. Sampling was carried out every 24 h. All the experiments were performed in triplicate, and the average was calculated.
Data analysis
The data are presented as mean values from triplicate experiments with standard deviation (±SD). Data analysis was performed using Origin 8.0.
Results and discussion
Microorganisms
One isolated strain of HFE733 showed the maximum lipase activity of 9.27 U/mL. Strain HFE733 was identified and deposited by the China Center for Type Culture Collection (CCTCC, Wuhan, China) with a culture collection number of CCTCC M 2015 735.16S rRNA were sequenced and subjected to phylogenetic analysis to further identify strain HFE733. Nucleotide-nucleotide BLAST in NCBI results showed that strain HFE733 has more than 99% sequence similarity with the first hit of Pseudomonas aeruginosa. Based on the 16SrRNA gene sequence, a phylogenetic tree was made (), and it showed 99.58% sequence similarity to P. aeruginosa (BAMA01000316). According to the results reported above, it is reasonable to identify strain HFE733 as P. aeruginosa.
Purification of the lipase from P. aeruginosa HFE733
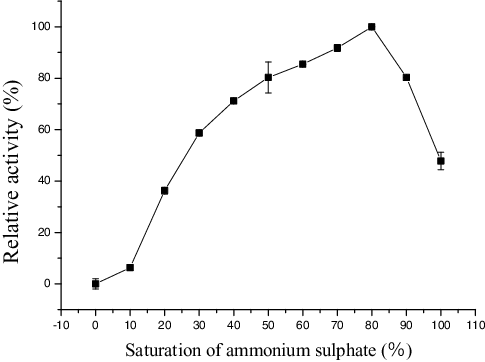
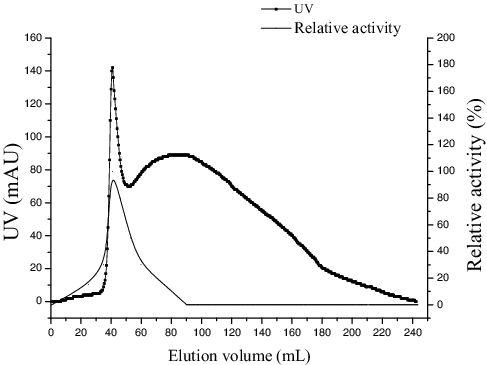
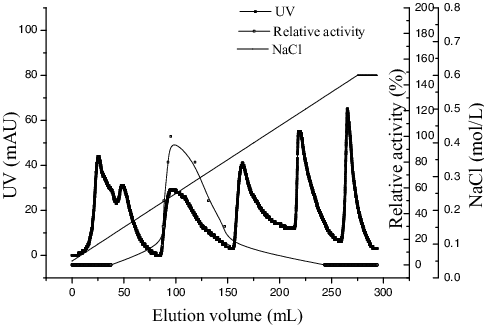
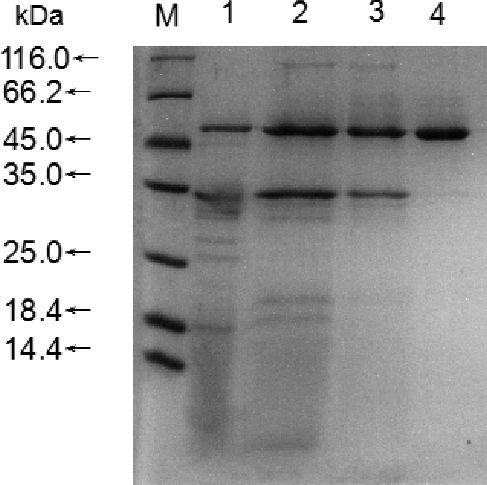
Purification of the enzyme was performed through the following steps: ammonium sulphate precipitation (), a Sephadex G-25 column () and DEAE cellulose column chromatography (). All purification steps are summarized (), which shows 9.97-fold purification with a recovery of 31.54% and specific activity of 79.25 U/mg protein.
Table 1. Purification of lipase from Pseudomonas aeruginosa HFE733.
The SDS-PAGE experiments showed that the enzyme reaches electrophoresis type of purity with a molecular mass of approximately 51.0 kDa () calculated using Syngene G: BOX (USA). This result is similar to the molecular mass reported for lipase from other P. aeruginosa strains, such as P. aeruginosa LX1 (56 kDa) [Citation5] and P. aeruginosas an-ai (54 kDa) [Citation6].
Figure 5. SDS–PAGE of purified lipase from Pseudomonas aeruginosa HFE733. Lane M, protein size marker; Lane 1, crude enzyme; Lane 2, enzyme purified after 80% ammonium sulphate precipitation; Lane 3, active fraction of Sephadex G-25 column; Lane 4, active fraction of DEAE cellulose anion-exchange column.
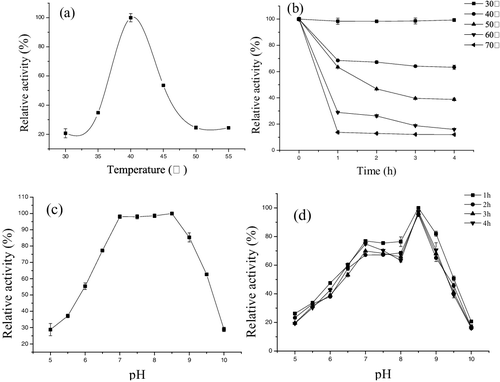
Effects of temperature on enzyme activity and stability
We studied the effect of temperature on lipase activity over the range 30–55 °C ((a)). The lipase exhibited the highest activity at 40 °C, which was similar to the corresponding enzymes from Burkholderia sp. ZYB002 [Citation24] and P. aeruginosa LX1 [Citation5]. The purified lipase was stable between 30 and 40 °C, retaining approximately 70% of its activity, but the thermal stability of the enzyme declined at 50–70 °C ((b)).
Effects of pH on enzyme activity and stability
The effect of pH on lipase activity is shown in (c). The lipase was active at pH 6.0–9.5 and exhibited the highest activity at pH 8.5, indicating that this lipase was an alkaline enzyme, and was stable at pH 7.0–8.5. After incubation in buffers within a pH range of 5.0–10.0 for 4 h, the enzyme retained more than 70% of its activity ((d)). In consistence with the present study, the activity of lipase produced by Halobacillus sp. AP-MSU 8 [Citation25] and Xanthomonas oryzae pv. oryzae YB103 [Citation26] was reported to reach a maximum at pH 9.0. Alkaline lipases are now popularly explored for a large variety of industrial purposes.
Effect of metal ions and chemical reagents on lipase activity
Among the different metal ions studied for their influence on alkaline lipase activity, the activity of the lipase was strongly influenced by the presence of metal ions (). The enzyme showed the maximum stimulation by Fe3+, similar to the lipase from Bacillus licheniformis MTCC 2465 [Citation27]. The enzyme activity was also strongly stimulated by Al3+. However, the lipase activity was inhibited by the other tested metal ions (). The inhibitory nature of metals has been thought to be due to interaction of methal ions with the charged side chain groups of surface amino acids, thus influencing the conformation and stability of the enzyme [Citation28].
Table 2. Effect of metal ions on activity of lipase from Pseudomonas aeruginosa HFE733.
The effects of inhibitors and surfactants on lipase activity are presented in . The enzyme was activated by β-mercaptoethanol and cysteine. This may be explained by β-mercaptoethanol counteracting the oxidative effect of the S–S bond formed between two cysteine residues [Citation29]. The addition of DTT also had a strong stimulation on enzyme activity, indicating that the thiol group could be essential for the activity of this enzyme. The lipase was stable in the presence of EDTA, suggesting that no metals are in or close to the active site of the enzyme. The addition of GSH resulted in non-remarkable stimulation or inhibition, suggesting that the sulfhydryl group of the enzyme is stable.
Table 3. Effect of various reagents on the activity of lipase from Pseudomonas aeruginosa HFE733.
Surfactants are known to reduce the interfacial tension between oil and water and to increase the lipid–water interface area, which enhances the rate of lipase catalyzed reactions in return [Citation7,Citation30]. However, different surfactants had different effects on lipase activity. In this study, the lipase was inhibited by the non-ionic surfactants such as Tween 80 and Triton X-100, similar to the corresponding enzymes from Pseudomonas [Citation31] and Pseudomonas cepacia [Citation32]. The strong inhibition by 10 mmol/L SDS could be due to local conformation changes in the active site of the enzyme molecule, resulting in inhibition, partial reversible unfolding and subsequent inactivation.
Biodegradation in food wastewater treatment
As a next step in our study, we evaluated the effect of the P. aeruginosa HFE733 fermentation broth in oil-rich restaurant wastewater treatment. The results are shown in (a) and (b). It was found that the initial oil content and COD in the food wastewater were 4296.00 mg/L and 11 449.42 mg O2/L, respectively. After 6 days of treatment, they decreased to 195.79 mg/L and 1243.97 mg O2/L, respectively, whereas control 1 had 3179.06 mg/L and 4798.93 mg O2/L, respectively, and control 2 had 3825.72 mg/L and 7460.28 mg O2/L, respectively. After 6 days of treatment, the oil content decreased by 95.44% and COD, by 89.14%. These results suggested that the P. aeruginosa HFE733 fermentation broth was effective for oil removal and COD decrease, and can be used for food wastewater treatment. Similar studies have been carried out with other lipase-producing strains, such as P. aeruginosa SL-72 [Citation33] and Aspergillus awamori BTMFW032 [Citation34]. The crude lipase of P. aeruginosa SL-72 was added to wastewater contaminated with crude oil, resulting in degradation of 82.83% of the oil content and 86.39% reduction of COD after 7 days of treatment, slightly lower than the effect of the HFE733 fermentation broth. The lipase from Aspergillus awamori BTMFW032 was evaluated for the treatment of hospital wastewater containing ayurvedic oil; 91.4% of the oil was degraded, indicating a good degradation effect after the treatment.
Figure 7. Oil content (a) and COD (b) decrease for P. aeruginosa HFE733 fermentation broth, control 1 and control 2.
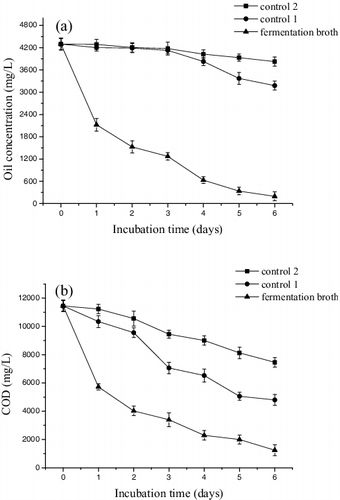
The oil removal and COD decrease in control 1 and control 2 may be due to the role of indigenous microorganisms. The fermentation broth contains strains and lipases, which may co-act to remove the oil and decrease the COD value. The alkaline lipase from P. aeruginosa HFE733 can hydrolyse fats and oils into glycerol and fatty acids, and glycerol and fatty acids can be utilized by P. aeruginosa HFE733 strain. We are now studying this interaction further to explore optimal pretreatment conditions.
Conclusions
In this study, to avoid the excess wastewater pre-treatment process and high cost, the P. aeruginosa HFE733 strain and lipase were added to lipid-rich food wastewater. We observed remarkable oil removal and COD decrease effects, showing important application potential. The purified lipase from P. aeruginosa HFE733 was an extracellular enzyme with a molecular mass of 51.0 kDa. The enzyme exhibited maximum activity at 40 °C and pH 8.5, and was stable over range of pH values (7.0–8.5). The purified enzyme was remarkably activated by several metal ions and chemical reagents, such as Fe3+, Al3+, β-mercaptoethanol, cysteine, DTT, Tween 80 and Triton X-100, but was suppressed in the presence of 10 mmol/L SDS. Further, the P. aeruginosa HFE733 strain and lipase showed they have promising potential for biodegradation as part of food wastewater treatment.
Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Carrière F, Thirstrup K, Hjorth S, et al. Cloning of the classical guinea pig pancreatic lipase and comparison with the lipase related protein 2. Febs Lett. 1994;338(1):63–68.
- Balan A, Ibrahim D, Abdul RR, et al. Purification and characterization of a thermostable lipase from Geobacillus thermodenitrificans IBRL-nra. Enzyme Res. 2012;2012(11):98–105.
- Chen H, Wu JP, Yang LR, et al. Improving Pseudomonas alcaligenes lipase's diastereopreference in hydrolysis of diastereomeric mixture of menthyl propionate by site-directed mutagenesis. Biotechnol Bioprocess Eng. 2014;19(4):592–604.
- Brahimi Horn MC, Mickelson CA, Gaal AM, et al. Lipolytic activity produced by Pseudomonas aeruginosa and Acinetobacter calcoaceticus strains grown in wool-scour effluent. Enzyme Microb Technol. 1991;13(9):740–746.
- Ji Q, Xiao S, He B, et al. Purification and characterization of an organic solvent-tolerant lipase from Pseudomonas aeruginosa LX1 and its application for biodiesel production. J Mol Catal B Enzym. 2010;66(3):264–269.
- Karadzic I, Masui A, Zivkovic L I, et al. Purification and characterization of an alkaline lipase from Pseudomonas aeruginosa isolated from putrid mineral cutting oil as component of metalworking fluid. J Biosci Bioeng. 2006;102(2):82–89.
- Ogino H, Nakagawa S, Shinya K, et al. Purification and characterization of organic solvent-stable lipase from organic solvent-tolerant Pseudomonas aeruginosa LST-03. J Biosci Bioeng. 2000;89(5):451–457.
- Santarossa G, Lafranconi PG, Alquati C, et al. Mutations in the “lid” region affect chain length specificity and thermostability of a Pseudomonas fragi lipase. Febs Lett. 2005;579(11):2383–2386.
- Chen H, Tian R, Ni Z, et al. Surface display of the thermophilic lipase Tm1350 on the spore of Bacillus subtilis. Extremophiles. 2015;19(4):799–808.
- Tang L, Su M, Yan J, et al. Lid hinge region of Penicillium expansum lipase affects enzyme activity and interfacial activation. Process Biochem. 2015;50(8):1218–1223.
- Roussel A, Amara S, Nyyssölä A, et al. A cutinase from Trichoderma reesei with a lid-covered active site and kinetic properties of true lipases. J Mol Biol. 2014;426(22):3757–3772.
- Kumar S, Negi S. Transformation of waste cooking oil into C18 fatty acids using a novel lipase produced by Penicillium chrysogenum through solid state fermentation. 3 Biotech. 2015;5(5):847–851.
- Guldhe A, Singh P, Kumari S, et al. Biodiesel synthesis from microalgae using immobilized Aspergillus niger whole cell lipase biocatalyst. Renew Energ. 2016;85:1002–1010.
- Vakhlu J, Kour A. Yeast lipases: enzyme purification, biochemical properties and gene cloning. Electron J Biotechn. 2006;9(1):69–85.
- Długołecka A, Cieśliński H, Bruździak P, et al. Purification and biochemical characteristic of a cold-active recombinant esterase from Pseudoalteromonas sp. 643A under denaturing conditions. Pol J Microbiol. 2009;58(3):211–218.
- Masomian M, Rahman RNZRA, Salleh AB, et al. A new thermostable and organic solvent-tolerant lipase from Aneurinibacillus thermoaerophilus, strain HZ. Process Biochem. 2013;48(1):169–175.
- Borja R, Banks C J. Response of an anaerobic fluidized bed reactor treating ice-cream wastewater to organic, hydraulic, temperature and pH shocks. J Biotechnol. 1995;39(3):251–259.
- Borja R, Banks C J, Garrido A. Kinetics of black-olive wastewater treatment by the activated-sludge system. Process Biochem. 1994;29(7):587–593.
- Zinebi S, Henriette C, Petitdemange E, et al. Identification and characterization of bacterial activities involved in wastewater treatment by aerobic fixed-bed reactor. Water Res. 1994;28(12):2575–2582.
- Vorderwülbecke T, Kieslich K, Erdmann H. Comparison of lipases by different assays. Enzyme Microb Technol. 1992;14(8):631–639.
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685.
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193(1):265–275.
- National Standard. The Standard on Industry Construction of Town in China: Methods for the examination of municipal sewage. Beijing, China: Ministry of Construction; 2004. CJ/T51–2004.
- Shu Z, Lin H, Shi S, et al. Cell-bound lipases from Burkholderia, sp. ZYB002: gene sequence analysis, expression, enzymatic characterization, and 3D structural model. BMC Biotechnol. 2016 [cited 2017 Jul 16];16(1):38. DOI: 10.1186/s12896-016-0269-6.
- Esakkiraj P, Prabakaran G, Maruthiah T, et al. Purification and characterization of halophilic alkaline lipase from Halobacillus sp. P Natl Acad Sci India B Biol Sci. 2016;86(2):309–314.
- Mo Q, Liu A, Guo H, et al. A novel thermostable and organic solvent-tolerant lipase from Xanthomonas oryzae pv. oryzae YB103: screening, purification and characterization. Extremophiles. 2016;20(2):157–165.
- Bora L. Purification and characterization of highly alkaline lipase from Bacillus licheniformis MTCC 2465: and study of its detergent compatibility and applicability. J Surfactants Deterg. 2014;17(5):889–898.
- Rahman RN, Baharum SN, Salleh AB, et al. S5 Lipase: an organic solvent tolerant enzyme. J Microbiol. 2006;44(6):583–590.
- Yang H, Shi P, Lu H, et al. A thermophilic β-mannanase from Neosartorya fischeri P1 with broad pH stability and significant hydrolysis ability of various mannan polymers. Food Chem. 2015;173:283–289.
- Shaoxin C, Lili Q, Bingzhao S. Purification and properties of enantioselective lipase from a newly isolated Bacillus cereus C71. Process Biochem. 2007;42(6):988–994.
- Gaoa XG, Cao SG, Zhang KC. Production, properties and application to nonaqueous enzymatic catalysis of lipase from a newly isolated Pseudomonas strain. Enzyme Microb Technol. 2000;27(1):74–82.
- Pencreac'h G, Leullier M, Baratti JC. Properties of free and immobilized lipase from Pseudomonas cepacia. Biotechnol Bioeng. 1997;56(2):181–189.
- Verma S, Saxena J, Prasanna R, et al. Medium optimization for a novel crude-oil degrading lipase from Pseudomonas aeruginosa SL72 using statistical approaches for bioremediation of crude-oil. Biocatal Agric Biotechnol. 2012;1(4):321–329.
- Basheer SM, Chellappan S, Beena PS, et al. Lipase from marine Aspergillus awamori BTMFW032: production, partial purification and application in oil effluent treatment. New Biotechnol. 2011;28(6):627–638.