ABSTRACT
Simple sequence repeat (SSRs) markers derived from Malus were used to assess their transferability in the analysis of the genetic diversity and relationship of some Pyrus species. All studied microsatellite loci produced fragments and were polymorphic among the studied samples. Fifty-one allelic variants were detected at eight SSR loci, ranging from five (MS14h03, CH02b10 and CH03g06) to nine (CH02h11a), with a mean of 6.37 alleles per locus. A relatively high level of diversity was recorded among the studied accessions. The average of Shannon's index (I) was 1.04. The Dice genetic similarity coefficient ranged greatly and ‘Khoj 1’ (a member of P. communis) and ‘Shinko’ (a cultivar belonging to P. pyrifolia) had the lowest (0.21) values, while ‘Chojuro’ and ‘Nijisseiki’, two cultivars belonging to P. pyrifolia, had the highest genetic similarity (0.97) among the studied samples. Bayesian cluster analysis and principal coordinates analysis plot separated the P. pyrifolia samples from other species, and formed three distinct groups, indicating high genetic differences between P. pyrifolia and other studied pear species. Several private alleles were observed in some of the studied species that would be of great importance for species identification. Furthermore, in 23 samples, more than two alleles were observed in the CH04e03 locus, indicating presence of locus duplication in these samples. Our observations suggest that, for this locus, at least two homologous chromosomes or genomic regions may be presented in the genome of some pear samples.
Introduction
Pear (Pyrus spp.), apple (Malus domestica Borkh.) and quince (Cydonia oblonga M.) are three main commercial species in the Rosaceae family, subfamily Pomoideae. With the advent of new molecular analysis tools, more precise phylogenetic analyses were carried out on different species and some of the previous classifications were revised. According to the new classification, pome-bearing fruits are placed into subfamily Spiraeoideae, tribe Pyreae [Citation1]. At least 22 species have been identified in genus Pyrus, among them a few have been domesticated and widely used for fruit production [Citation2,Citation3]. The Pyrus containing subfamily, Spiraeoideae, has 17 chromosomes and most pear cultivars are diploid (2n = 34). However, there are few reports indicating some Pyrus species including Pyrus communis and Pyrus × bretschneideri are polyploid [Citation4]. In addition, it is reported that speciation among pear species occurred without variation in the chromosome number [Citation4].
It is believed that Pyrus is originated and domesticated from the region spanning from China to Asia Minor [Citation5]. In comparison with other fruit species of Rosaceae, pear has been less subjected to molecular investigations and its genetic resources are neither completely recognized nor exploited. This is somewhat due to the wide crossing has occurred among different species of this genus, which finally resulted in the high heterozygosity and low phenotypic diversity among various Pyrus species and subsequently made the estimation of genetic diversity in this genus difficult [Citation6].
Different molecular markers have been used to evaluate genetic variability in Pyrus spp. [Citation7,Citation8]. Simple sequence repeats (SSRs), a popular type of molecular markers, are versatile tools and have potential to be used in genetic diversity studies, germplasm characterization, cultivar discrimination, identification of progenies from different crosses, detection of duplications and genetic fingerprinting among different plants. Characteristics such as high abundance in the genome, co-dominant inheritance, multi-allelic nature as well as high reproducibility make SSRs one of the most efficient and highly used molecular markers in different plant studies [Citation7]. Apple-derived microsatellite markers have been successfully used to assess their transferability and polymorphism in 36 pear samples from five different species [Citation6]. After that, extensive screening of larger sets of apple SSR markers on different Pyrus species has established the usefulness of these markers for genetic diversity evaluation and mapping of Pyrus spp. [Citation7–14].
Situated in the range of the center of origin and diversity of Pyrus spp., Iran is a rich source of germplasm and genetic variability of this genus, which includes native, introduced and wild forms of Pyrus, mainly resulting from hybridization and natural seed propagation. Assessment of genetic diversity is the basic ingredient for plant breeding, and conservation of diverse genetic materials is a valuable task that guarantees future plant breeding progress. In the present study, eight SSR primer pairs developed from apple were used to assess their transferability and to evaluate the genetic diversity and relationship of 47 accessions of five Pyrus species.
Materials and methods
Plant materials
Forty-seven cultivars and genotypes in the Pyrus genus were used to test the cross-transferability of eight SSRs loci from the Malus genus ( and ) [Citation15–17]). Genomic DNA was extracted from fresh leaves according to the protocol described by Yamamoto et al. [Citation18]. The polymerase chain reaction (PCR) was carried out in a final volume of 15 µL containing 2.5 µL DNA template (10 ng/µL), 1.5 µL 10X PCR buffer, 0.45 µL MgCl2 (50 mmol/L), 0.3 µL deoxyribonucleoside triphosphates (dNTPs; 10 mmol/L), 0.4 µL of each of the two primers (10 pm), 0.1 µL Taq DNA polymerase (5 U/µL) and 9.35 µL sterile distilled water. PCR reaction was programmed as: one cycle of 4 min at 94 °C as initial denaturation, followed by 35 cycles, each of which consisted of a denaturation step at 94 °C for 1 min, an annealing step at specific annealing temperature of each primer for 1 min, and an extension step at 72 °C for 1 min, followed by final extension at 72 °C for 7 min. Before loading, 5 µL of formamide loading buffer was added to each PCR product. Then samples were denatured (5 min at 94 °C) and kept on ice. Later, 2–3 µL of the amplified products were loaded onto a 6% (0.4-mm thick) polyacrylamide gel. Electrophoresis was run at a constant 65 W at 50 °C for 1–2 h depending on allelic size.
Table 1. Different Pyrus accessions and their species used in this study.
Table 2. SSR primer sequences that were used to study Pyrus genetic diversity.
Data analysis
Amplified products from microsatellites were scored as allelic data for each locus. Polymorphic Information Content (PIC) was computed by CERVUS software, version 2.0 [Citation20]. The Dice genetic similarity coefficient values were used to visualize genetic relationships among the accessions using NTsys 2.2 software [Citation21]. Similarly, principal coordinate analysis (PCoA) of the germplasm set was performed based on the genetic similarity matrix using GenAlEx 6.5 [Citation22]. Nei's genetic distance [Citation23] was calculated using POPGENE version 1.31 [Citation24] and a dendrogram was constructed using Mega software ver. 6 according to Nei's matrix [Citation25].
STRUCTURE software ver. 2.3.4 was used to analysis genetic structure of accessions [Citation26]. The software was preset with a burn-in period of 10,000 interactions and a posterior number of Markov Chain Monte Carlo (MCMC) of 100,000 permutations. The number of subpopulations of germplasm (K value) were considered from 1 to 6, and 10 independent run of STRUCTURE were performed for each K [Citation27]. The results of the STRUCTURE analysis were preceded with the Structure Harvester [Citation28] to find the optimum number of K using the delta K (ΔK) method.
Results and discussion
SSR transferability and genetic diversity
All of the apple-derived SSR markers successfully amplified fragments in all studied pear species. The species and genera transferability of SSR primers are well-documented in several close genera [Citation29–32]. Yamamoto et al. [Citation6] observed that SSRs from apple produced discrete amplified fragments in pear accessions and reported that these loci can be utilized for the evaluation of genetic diversity in Pyrus spp. It was also reported that SSR markers from apple could be mapped in the genetic linkage maps of pear [Citation33]. Moreover, Liebhard et al. [Citation16] stated that some of the apple SSRs could be successfully used in a series of species in different genera in the subfamily Pomoideae, including Cydonia and Pyrus. In addition, Yamamoto et al. [Citation34] showed that SSRs isolated from apple and pear could be used for cultivar identification as well as evaluation of genetic diversity and relationships in quince cultivars. In fact high level of co-linearity and sequence conservation among different pome-bearing fruits in the subfamily Spiraeoideae enable researchers to use primers developed from the more studied genera such as Malus for genetic assessment in the less studied ones such as Cydonia and Pyrus.
Considerable levels of variability were observed among different samples. Variation among the accessions was observed within species as well as among species. Dice similarity matrices of pear genotypes were calculated from SSR data (). The Dice similarity coefficient among cultivars ranged greatly (0.21 to 0.97). An Iranian native individual from P. communis (Khoj 1) and a cultivar from P. pyrifolia (‘Shinko’) had the lowest similarity, while ‘Chojuro’ and ‘Nijisseiki’ (two cultivars belonging to P. pyrifolia) had the highest genetic similarity. The second lowest similarity was also recorded between ‘Khoj 1’ and ‘Knojoni’, two Iranian native samples belonging to P. communis, indicating high level of genetic differentiation between Iranian accessions. ‘Khoj 1’ also had the lowest mean of genetic similarity among studied samples (0.44). Most of the Khoj accessions in Iran were selected from the nature and are seedlings obtained from open pollination of different P. communis samples. Therefore, it would be concluded that cross-pollination between neighbour accessions might have great effect on the higher level of genetic diversity in Iranian pear compared with foreign cultivars.
Table 3. Dice similarity coefficient among 47 Pyrus accessions obtained from SSR markers. Numbers in the first column and the first line represent different accessions according to .
Different species in our investigation had different pattern of allele distribution. Among different samples in this study, P. mazandaranica produced the least number of alleles (11), while cultivar ‘Khoj 2’ from P. communis produced the highest number of alleles (19) (). Also among the species analysed in our study, P. salicifolia showed high level of heterozygosity in the analyzed loci, while P. mazandaranica was highly homozygous in most of the studied loci. We also identified some private alleles in different studied species (). Samples from P. communis had the highest number of private alleles (14 alleles), while samples of P. salicifolia and P. mazandaranica showed no private alleles using these SSR loci. The CH04e03 locus showed the highest number of private alleles (3 alleles in P. communis and 2 alleles in P. pyrifolia) and could be used for discrimination between P. communis and P. pyrifolia species, while the MS14h03 locus generated one private allele in P. bretschneideri and has the potential to be used as a specific marker for identification the members of this species. However, supplementary analysis with different accessions from these species is needed to support these observations. Identification of private alleles using different marker systems has been reported earlier in different Pyrus species [Citation15,Citation33–37].
Table 4. Number of alleles and private alleles observed in different species of Pyrus in this experiment.
The average number of produced alleles in each cultivar was compared between two groups of Iranian and introduced cultivars. The results showed that most markers produced a higher number of alleles in the Iranian cultivars compared to the introduced ones. These results demonstrated that the studied Iranian cultivars have more genetic variability, maybe because they have not been subject to breeding programmes and hence they may have high potential for utilization in crop improvement programmes.
The PIC values were higher than 0.50 for most SSR loci, which indicated that these SSR loci are highly polymorphic and suitable for diversity studies. In fact, subsets of loci that are highly informative, representative of the genome, robust and well defined are very useful for genetic diversity analysis. The high level of polymorphism observed at some of the apple SSR loci agrees with previous results in pear. For example, Yamamoto et al. [Citation6] observed 79 putative alleles using nine apple SSR markers in 36 pear samples belonging to different species, including hybrid accessions. The observed alleles for most SSRs loci were similar to previous results on pears and were discussed elsewhere [Citation15]. The pear is an allogamous plant with high level of self-incompatibility; therefore, it is not surprising to observe high level of heterozygosity in this genus. The pollination nature (allogamous or autogamous) has a prominent role in the observed heterozygosity in different species. On the other hand, allogamous plants are usually more diverse and have higher levels of heterozygosity compared with the autogamous ones.
Shannon's information index (I), an indicator for genetic diversity, was particularly high and varied from 0.38 (CH01d08) to 1.32 (CH02h11a, CH03g06 and CH04e03) with an average value of 1.04. The relatively high value of Shannon's index for some of the studied loci represents the effectiveness of these microsatellite loci to reveal the variation for Pyrus. Problems with the interpretation of the Shannon index have been noted for phenotypic traits [Citation38]. For our study and other investigations utilizing neutral polymorphic microsatellites, the Shannon index seems to provide a robust measure of diversity that can allow broad comparisons of multiple tests over time given the consideration of the nature of the examined groups like natural populations or various broad or narrow breeding populations. Our high number of alleles and high value of I are probably due in part to the broad diversity of our samples, different species, and use of accessions that had not been subject to breeding programmes. On the other hand, we analyzed samples from different species of Pyrus including wild types, which might have high level of diversity than cultivars belonging to a specific species.
Genetic structure analysis
To elucidate the genetic relationship among Pyrus cultivars and genotypes, the genetic structure of the germplasm was evaluated using the STRUCTURE program. Bayesian model-based clustering clearly separated the accessions according to their phylogenetic relationships. Analysis with Structure Harvester showed a clear peak at K = 3 (ΔK = 5.68) indicating entire samples could be divided into three different groups (). Each accession is represented by a vertical line and classified based on its estimated membership probability (Q). According to K = 3, Japanese pear including all native and domesticated forms of P. pyrifolia were completely separated from other studied species, indicating a minimal gene flow between the two groups. Thirteen P. communis accessions, all native to Iran, were separated from other P. communis samples and formed a separate group. Some of these cultivars are commercially important, such as ‘Shahmive’, which is morphologically similar to the ‘Khoj’ genotype. ‘Shahmive’ is distributed in the central part of Iran and it is probable that this cultivar originated from ‘Khoj’ genotypes and has been propagated in this region as a new genotype. ‘Khoj’ genotypes belong to the P. communis species and are distributed in the forests of northern Iran. Interestingly, P. bretschneideri sample (44) was grouped with Iranian endemic cultivars such as ‘Dare Gazi’, ‘Konjoni’ and ‘Sebri’, which might be due to the gene flow and hybridization of this species with some Iranian genotypes of P. communis. Some members of this group are morphologically similar with Eastern pears. Similar to the Chinese species, ‘Dare Gazi’, an Iranian cultivar which is extensively cultivated in the East of Iran, is highly resistant to the Fire Blight disease. This cultivar showed high level of genetic differentiation form other P. communis genotypes. Chamberlain and Hubert [Citation39] reported that the phenotypical differences might have been determined by relatively few genes, which could not be reflected in the molecular results. In addition, two unknown samples of P. communis (23 and 12) were grouped with Iranian accessions indicating their higher genetic similarity with them.
Figure 1. Genetic structure of 47 pear accessions obtained from STRUCTURE analysis. (A) Results of Structure Harvester representing K = 3 as the best numbers of groups according to the delta K method. (B) Pattern of accession assignments into three different groups using the STRUCTURE model. Different colour of each vertical line represents the percent of membership (vertical values on the left of cluster) of each individual for three groups. Numbers at the bottom represent the samples based on .
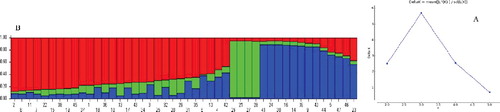
The largest group included 30 samples of P. communis. All of these samples were commercial cultivars and previously introduced to Iran. They were genetically related to each other and clearly separated from the Iranian accessions. However, some of the Iranian cultivars (such as ‘Peighambari’, ‘Felestini’ and ‘Beiroty’) were placed in the vicinity of introduced cultivars, indicating that they might have the same ancestors with foreign pear accessions. For instance, ‘Felestini’ and ‘Beiroty’ cultivars, as their names imply, might have originated from Palestine and Lebanon, respectively.
About 45% of the studied pear accessions shared more than 80% membership with one of the three main groups and the remaining 55% were admixed. The members of the biggest group (commercial pear) showed the highest degree of admixture, while the P. pyrifolia group had no admixture sample. P. mazandaranica which was grouped with introduced P. communis had the highest level of admixture with Iranian native samples. P. salicifolia was another accession with high degree of admixture but more similar with commercial P. communis samples. In the Iranian endemic group, ‘Konjoni’ and two unknown samples (12 and 23) had high level of admixture. ‘Beurre Hardy’, a commonly used interstock between pear scions and quince rootstocks, showed to be an admixture of P. communis and P. pyrifolia. Kumar et al. [Citation37] also made a similar observation and reported that ‘Beurre Hardy’ separated from the rest of the European pears and had higher genetic similarity with the P. pyrifolia group. All species of the Pyrus genus are intercrossable because there is no major barrier for hybridization in Pyrus. Interspecific hybridization and gene introgression have probably been involved in the evolution of the various species [Citation40]. However, the few hybrids found may indicate a reproductive barrier which reduces gene flow between the two species [Citation41]. Evaluation of genetic diversity and the fingerprinting of natural populations are important aspects in the management and utilization of plant collections which are very important as this plays a vital role in the survival and adaptability of species.
PCoA analysis
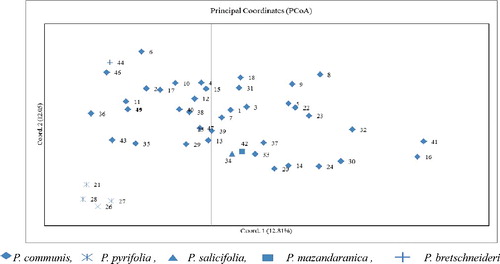
PCoA, which represents the relationship between individual Pyrus accessions, was used to characterize the subgroups of the germplasm set. A two-dimensional scatter plot involving all 47 accessions showed that the first two PCA axes accounted for 12.81% and 12.05% of the genetic variation among samples (). The PCoA result is in accordance with the clustering pattern produced by the STRUCTURE software. Four samples from P. pyrifolia, which formed a distinct group in the STRUCTURE cluster, were completely separated from members of other species, while samples from P. salicifolia, P. mazandaranica and P. bretschneideri species had higher similarities with P. communis than P. pyrifolia.
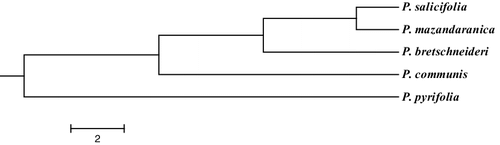
The results of clustering the species based on Nei's genetic identity confirmed the results of PCoA and Bayesian model-based clustering. According to Nei's genetic identity (), samples from P. pyrifolia were clearly separated from other species, while P. salicifolia and P. mazandaranica species were the most similar species and grouped together. In accordance with our observations, clear separation of P. communis samples from Asian pear based on genotyping by sequencing was reported recently [Citation37]. These authors indicated that P. pyrifolia samples are genetically different from P. communis and formed distinct cluster by Bayesian analysis while hybrids of these two species separated and comprised a distinct group [Citation37]. Members of these two species have high level of morphological differences. Allele distribution among these species is a confirmation for different clustering methods. On the other hand, P. pyrifolia and P. communis had high level of private alleles, indicating high level of genetic differentiation among these two species, while the allelic patterns of P. salicifolia and P. mazandaranica were highly similar and these two species had no private alleles.
Evidence of SSR duplication
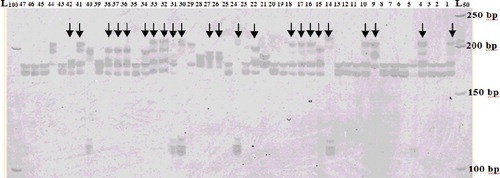
Among SSRs primers, CH04e03 loci amplified more than two alleles in some of the studied samples including some economically important and well-known cultivars such as ‘Beurre Diel’, ‘Bartlett’ and ‘Dare Gazi’ (). Our observations suggest that at least two homologous chromosomes or genomic regions exist for this locus in some of the studied pear genomes. Genome duplication creates extra copies of the chromosome set with the duplicated pairs in the genome defined as having paralogous relationships [Citation42]. Gene duplication has been known since the 1930 s [Citation43,Citation44] and reported among different organisms [Citation45,Citation46] including plant species [Citation47]. It is reported that genome-wide duplication that occurred > 50 million years ago in Pyreae increased the number of chromosomes from 9 to 17 [Citation48]. SSR duplication also has been reported in several other fruit crops [Citation17,Citation47,Citation49,Citation50]. High levels of genome duplications have been reported in hazelnut fruit tree using SSR primers [Citation47]. Gürcan [Citation47] used 90 polymorphic SSR loci to evaluate 25 hazelnut genotypes and observed multiple bands in 14 loci. Guilford et al. [Citation17] reported that approximately 25% of GA SSRs showed duplication in apple. Chavarriaga-Aguirre et al. [Citation49] found unexpected multiple bands in cassava at GA-repeat loci. Segmental gene duplication was also reported in the pear genome. Li et al. [Citation51] found a total of 70 collinear gene pairs in the pear genome, which might have resulted from whole-genome duplication in pear millions of years ago. Recently, Cao et al. [Citation52] observed high level of gene duplication in P. bretschneideri and reported that whole-genome and segmental duplication likely played key roles in expansion of the MYB gene family in pear. Therefore, it seems that segmental gene duplication has had a prominent role in the Pyrus development and indicates that gene duplications are common in different species of this genus.
Conclusions
Eight microsatellites previously developed from the Malus genus were used to assess their transferability in different species of Pyrus. All of the SSR loci successfully amplified polymorphic fragments in Pyrus. Some of the endemic Iranian pears were investigated at the molecular level and were compared with introduced cultivars. The results showed a very high level of genetic variation in pear species and presented valuable information about Iranian pears and their relatedness to some economically important pear cultivars. According to STRUCTURE analysis, the entire germplasm was grouped into three main sub-sets, including P. pyrifolia samples; Iranian native P. communis; and commercial and introduced cultivars of P. communis. P. mazandaranica and P. bretschneideri samples were grouped with P. communis, which might be indicative of high crossability among these genera in Iran. Among the SSR markers, the CH04e03 locus amplified more than two alleles in some of the studied samples. Our observations suggest that at least two homologous chromosomes or genomic regions exist for this locus in some of the studied pear genomes. The results indicated a considerable level of genetic variation in most of the accessions which is very useful for starting pear-breeding programmes in Iran.
Acknowledgments
We are grateful to Dr. K. Arzani for providing Asian pear samples.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Potter D, Eriksson T, Evans RC, et al. Phylogeny and classification of Rosaceae. Plant Syst Evol. 2007;266:5–43.
- Bell RL. Pears (Pyrus). In: Moore JN, Ballington JR, editors. Genetic resources of temperate fruit and nut crops I. Wageningen (Netherlands): International Society for Horticultural Science; 1990. p. 655–697.
- Bell RL, Quamme HA, Layne REC, et al. Pears. In: Janick J, Moore.JN. editor. Fruit breeding, Vol 1. Tree and tropical fruits. New York (USA): Wiley; 1996. p. 441–514.
- Weeden N, Lamb RC. Genetics and linkage analysis of 19 isozyme loci in apple. J Am Soc Hort Sci. 1987;112:865–872.
- Vavilov NI. Origin and geography of cultivated plants; Vol. 15. Cambridge (UK:): Cambridge University Press; 1992.
- Yamamoto T, Kimura T, Sawamura Y, et al. SSRs isolated from apple can identify polymorphism and genetic diversity in pear. Theor Appl Genet. 2001;102:865–870.
- Yamamoto T, Kimura T, Shoda M, et al. Development of microsatellite markers in the Japanese pear (Pyrus pyrifolia Nakai). Mol Ecol Notes. 2002;2:14–16.
- Wunsch A, Hormaza JI. Characterization of variability and genetic similarity of European pear using microsatellite loci developed in apple. Sci Hort. 2007;173:37–43.
- Pierantoni L, Cho KH, Shin IS, et al. Characterization and transferability of apple SSRs to two European pear F1 populations. Theor Appl Genet. 2004;109:1519–1524.
- Volk GM, Richards CM, Henk AD, et al. Diversity of wild Pyrus communis based on microsatellite analysis. J Ame Soc Hort Sci. 2006;131:408–417.
- Brini W, Mars M, Hormaz JI. Genetic diversity in local Tunisian pears (Pyrus communis L.) studied with SSR markers. Sci Hort. 2008;115:337–341.
- Wolko L, Antkowiak W, Lanartowicz E, et al. Genetic diversity of European pear cultivars (Pyrus communis L.) and wild pear (Pyrus pyraster (L.) Burgsd.) inferred from microsatellite markers analysis. Genet Resour Crop Evol. 2010;57:801–806.
- Santos A, Ramos-Cabrer AM, Díaz-Hernández MB, et al. Genetic variability and diversification process in local pear cultivars from northwestern Spain using microsatellites. Tree Genet Genomes. 2011;7:1041–1056.
- Queiroza A, Assunc A, Ramadasb I, et al. Molecular characterization of Portuguese pear landraces (Pyrus communis L.) using SSR markers. Sci Horti. 2015;183:72–76.
- Erfani J, Ebadi A, Abdollahi H, et al. Genetic diversity of some pear cultivars and genotypes using simple sequence repeat (SSR) markers. Plant Mol Biol Rep. 2012;30:1065–1072.
- Liebhard R, Gianfranceschi L, Koller B, et al. Development and characterization of 140 new microsatellites in apple (Malus x domestica Borkh.). Mol Breed. 2002;10:217–241.
- Guilford P, Prakash S, Zhu JM, et al. Microsatellites in Malus x domestica (apple): abundance, polymorphism, and cultivar identification. Theor Appl Genet. 1997;94:249–254.
- Yamamoto T, Iketani H, Ieki H, et al. Transgenic grapevine plants expressing a rice chitinase with enhanced resistance to fungal pathogens. Plant Cell Rep. 2000;19:639–646.
- Rechinger KH. Flora Iranica, no 66. Graz, Austria: Akademische Druck-und Verlagsanstalt; 1969.
- Marshall TC, Slate J, Kruuk LEB, et al. Statistical confidence for likelihood-based paternity inference in natural populations. Mol Ecol. 1998;7:639–655.
- Rohlf FJ. NTSYSpc numerical taxonomy and multivariate analysis system version 2.0 user guide. Setauket: Applied Biostatistics Inc.; 1998.
- Peakall R, Smouse PE. GenAlEx 6.5: genetic analysis in excel. Population genetic software for teaching and research—an update. Bioinformatics. 2012;8(19):2537–2539.
- Nei M. Genetic distance between populations. Am Nat. 1972;106:283–292.
- Yeh FC, Yang RC, Boyle TBJ, et al. POPGENE, the user-friendly shareware for population genetic analysis. Edmonton, Alberta (Canada:): Molecular Biology and Biotechnology Center, University of Alberta; 1997.
- Tamura K, Peterson D, Peterson N, et al. MEGA6:molecular evolutionary genetics analysis version 6.0. Mol Biol Evol. 2012;30:2725–2729.
- Pritchard, JK, Stepheens M, Donnelly P. Inference of population structure using multilocus genotype data. Genetics. 2000;155:945–959.
- Evanno G, Regnaut S, Goudet J. Detecting the number of clusters ofindividuals using the software structure, a simulation study. Mol Ecol. 2005;14:2611–2620.
- Earl DA, VonHoldt BM. STRUCTURE HARVESTER, A website and programfor visualizing STRUCTURE output and implementing the Evanno method. Conserv Genet Resour. 2012;4:359–361.
- Gürcan K, Mehlenbacher S. Transferability of microsatellite markers in the Betulaceae. J Am Soc Hortic Sci. 2010;135:159–173.
- Wang H, Walla JA, Zhong S, et al. Development and cross-species/genera transferability of microsatellite markers discovered using 454 genome sequencing in chokecherry (Prunus virginiana L.). Plant Cell Rep. 2012;31(11):2047–2055.
- Fan L, Zhang MY, Liu QZ, et al. Transferability of newly developed pear SSR markers to other Rosaceae species. Plant Mol Biol Rep. 2013;31:1271–1282.
- Drašnarová A, Krak K, Vít P, et al. Cross-amplification and multiplexing of SSR markers for Alnus glutinosa and A. incana. Tree Genet Genomes. 2014; [cited 2017 Nov 13];10:865. DOI:10.1007/s11295-014-0727-z.
- Yamamoto T, Kimura T, Shoda M, et al. Genetic linkage maps constructed by using an interspecific cross between Japanese and European pears. Theor Appl Genet. 2002;106:9–18.
- Yamamoto T, Kimura T, Soejima J, et al. Identification of quince varieties using SSR markers developed from pear and apple. Breeding Sci. 2004;54:239–244.
- Kato S, Imai A, Rie N, et al. Population genetic structure in a threatened tree, Pyrus calleryana var. dimorphophylla revealed by chloroplast DNA and nuclear SSR locus polymorphisms. Conserv Genet. 2013;14:983–996.
- Wolko L, Bocianowski J, Antkowiak W, et al. Genetic diversity and population structure of wild pear (Pyrus pyraster (L.) Burgsd.) in Poland. Open Life Sci. 2015;10:19–29.
- Kumar S, Kirk C, Deng C, et al. Genotyping-by-sequencing of pear (Pyrus spp.) accessions unravels novel patterns of genetic diversity and selection footprints. Hortic Res. 2017; [cited 2017 Nov 13];4:17015. DOI 10.1038/hortres.2017.15.
- Hennink S, Zeven AC. The interprestation of Nei and Shannon-Weaver within population variation indices. Euphytica. 1991;51:235–240.
- Chamberlain JR, Hubert JD. Molecular analysis of genetic variation. In: Chamberlain JR, editor. Calliandra calothyrsus: an agro-forestry tree for the humid tropics. Tropical Forestry Paper No. 40. Oxford (UK): Oxford Forestry Research Institute; 1998. p. 67–76.
- Bell RL, Hough LF. Interspecific and intergeneric hybridization of Pyrus. Hort Sci. 1986;21:62–64.
- Milne RI, Abbott RJ, Wolff K, et al. Hybridization among sympatric species of Rhododendron (Ericaceae) in Turkey: morphological and molecular evidence. Am J Bot. 1999;86:1776–1785.
- Doyle JJ, Egan AN. Dating the origins of polyploidy events. New Phytol. 2010;186:73–85.
- Zhang J. Evolution by gene duplication: an update. Trends Ecol Evol. 2003;18(6):292–298.
- Taylor JS, Raes J. Duplication and divergence: the evolution of new genes and old ideas. Annu Rev Genet. 2004;38:615–643.
- Messer PW, Arndt PF. The majority of recent short DNA insertions in the human genome are tandem duplications. Mol Biol Evol. 2007;24:1190–1197.
- Seyfert AL, Cristescu MEA, Frisse L, et al. The rate and spectrum of microsatellite mutation in Caenorhabditis elegans and Daphnia pulex. Genetics. 2008;178:2113–2121.
- Gürcan K. Simple sequence repeat marker development and use in european hazelnut (Corylus avellana L.). [dissertation]. Oregon (USA): Oregon State University; 2009.
- Velasco R, Zharkikh A, Affourtit J, et al. The genome of the domesticated apple (Malus domestica Borkh.). Nat Genet. 2010;42:833–839.
- Chavarriaga-Aguirre P, Maya M, Bonierbale M, et al. Microsatellites in cassava (Manihot esculenta Crantz): discovery, inheritance and variability. Theor Appl Genet. 1998;97:493–501.
- Kim NH, Choi HI, Kim KH. Evidence of genome duplication revealed by sequence analysis of multi-loci expressed sequence tag-simple sequence repeat bands in Panax ginseng Meyer. J Ginseng Res. 2014;38:130–135.
- Li M, Li L, Dunwell JM, et al. Characterization of the lipoxygenase (LOX) gene family in the Chinese white pear (Pyrus bretschneideri) and comparison with other members of the Rosaceae. BMC Genomics. 2014; [cited 2017 Nov 13];15:444. DOI:10.1186/1471-2164-15-444.
- Cao Y, Han Y, Li D. MYB transcription factors in Chinese pear (Pyrus bretschneideri Rehd.): genome-wide identification, classification, and expression profiling during fruit development. Front Plant Sci. 2016; [cited 2017 Nov 13];7:577. DOI: 10.3389/fpls.2016.00577.