?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The aim of this study was to investigate the economic burden of Chronic Obstructive Pulmonary Disease (COPD)-related hospitalizations on a macro and micro level. Macro analysis was performed on the rate of DDD utilisation of COPD medicines, time of their inclusion in the reimbursement lists and the number of hospitalizations. On a micro level, a study of 426 patients with COPD was conducted to investigate the exacerbation and hospitalization rate relative to pharmacotherapy. A regression model, descriptive analysis, and Kruskal–Wallis non-parametric analysis were conducted. New drugs enter the market relatively quickly but are slow to be introduced into prescribing practices. The medicines’ utilization in Bulgaria has increased from 20 to 21 million DDD for all therapeutic groups, especially for long-acting beta-agonists/long-acting muscarinic antagonists (LABA/LAMA) fixed-dose combinations. A marked increase in hospitalization rates was observed despite lower numbers of registered and monitored COPD patients nationally. However, the hospitalization rates due to exacerbations remained around 18–19%. Variation was observed in the number of hospitalizations for the studied period: increasing during the period 2013–2014 and decreasing over the following two years. This trend was also observed for the number of health insured COPD patients. The hospitalization costs increased from 5.2 to 5.7 million BGN. A statistically significant decrease in the number of hospitalizations for LAMA/LABA patients vs. LABA/inhaled corticosteroids (ICS) patients was observed. Overall, frequent hospitalizations increased the total cost of COPD therapy. Patients on new medicines with improved inhaler devices seem to suffer fewer hospitalizations.
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a socially significant disease with more than 65 million people diagnosed as moderate or severe worldwide. COPD has caused approximately 3 million deaths in 2015 [Citation1]. The disease is estimated to become the third leading cause of death by 2030 [Citation2], making it among the most significant and deadly diseases that have to be tackled. This is attributed to the continually aging European population, as a higher incidence rate of COPD is reported in elderly patients, as well as increased exposure of people to risk factors such as tobacco smoke and air pollution [Citation3].
According to the latest update from the Global Initiative for COPD – GOLD 2017, COPD is defined as a ‘common, preventable and treatable disease that is characterised by persistent respiratory symptoms and airflow limitation that is due to airway and/or alveolar abnormalities usually caused by significant exposure to noxious particles or gases’ [Citation4], with frequently occurring exacerbations: events of acute onset, characterized by worsening in respiratory symptoms beyond the day-to-day variations and that may warrant a change in regular medication of the COPD patient. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) has identified four stages of COPD: GOLD 1 (mild), GOLD 2 (moderate), GOLD 3 (severe) and GOLD 4 (very severe) [Citation5]. The progression of the disease is characterized with more frequent exacerbations and hospitalizations [Citation6].
COPD is also associated with significant economic impact as the overall direct costs of treatment accounts for 38.6 billion euro in the European Union. Exacerbations have a major impact on both the severity of the disease and the costs. Positive correlation is observed between the progression of the disease, its severity and related therapeutic costs. A higher exacerbation rate leads to utilisation of more healthcare resources, more hospitalizations and increased hospital costs [Citation7, Citation8]. The impact of COPD exacerbations and related hospitalizations in Eastern Europe, especially in Bulgaria, still remains uncertain, and the allocation of resources is unclear. This could lead to increased social and economic burden of the disease both on patients and society [Citation9, Citation10]. In Bulgaria, there are still no official data on the prevalence and incidence of COPD, but some regional epidemiological studies and expert opinions suggest that the average prevalence varies from 6 to 14.9% depending on the region [Citation9, Citation11]. To our knowledge, the burden of exacerbations and related hospitalizations in Bulgaria has not yet been studied either.
The current treatment standard of COPD is inhaled therapy. GOLD recommends several classes of medicines for the treatment and management of COPD: β2-agonists, namely long-acting β2-agonists (LABA) such as salmeterol, formoterol and indacaterol, which improve the breathlessness [Citation12] and the health status [Citation13]; antimuscarinic agents, namely long-acting antimuscarinic antagonists (LAMA) such as tiotropium, aclidinium, glycopyrronium and umeclidinium; combination therapy of inhaled bronchodilators (short- or long-acting), most notably the combination of LABA and LAMA bronchodilators; as well as inhaled corticosteroids in combination with a long-acting agent usually prescribed in moderate-to-severe and severe stages of the disease.
Inhaler technology plays a major role in therapy effectiveness. The inhalation technique and particle size are important. Next generation devices pulverize micro-particles and allow easier access to lower level alveolar pathways, improving the health status and reducing the incidents with exacerbations, especially in chronically ill patients in advanced age [Citation14, Citation15].
Recently, LABA, LAMA and fixed-dose combinations of LABA/LAMA or inhaled corticosteroids (ICS)/LABA were included in the GOLD guidelines and were introduced in the European pharmaceutical market. The new inhaler technology is considered to provide better drug delivery for patients and to improve the control of COPD [Citation16–18].
In this setting, a reduction in the COPD hospitalization rate could be expected. This prompted our interest to explore the COPD hospitalization burden in Bulgaria and to compare it with the utilization of different therapies and inhalers that have been available on the Bulgarian market for the past four years. In our opinion, identifying such a possible relationship could improve the decisions in the choice of therapy, which in turn could reduce both the economic and social burden of the disease.
The objective of this study was to perform macro–micro analysis on the COPD hospitalization burden in the Bulgarian health care system. We tried to explore the speed of adoption of new COPD medicines in the reimbursement practice, their penetration on the market and in everyday therapy prescribing, as well as their influence on hospitalizations as a measure of long-term effectiveness of the new medicines.
Materials and methods
Information for the analysis was gathered from officially available healthcare databases, as well as data gathered from a representative ambispective national study [Citation19]. We compared the access to and utilisation of COPD medicines on a national level to the data obtained from the national representative study. Therefore, we characterized our approach as macro–micro analysis.
The macro analysis was performed for the period 2013–2016 and the micro analysis included information from the years 2014–2015.
Macro analysis
The Database of the National Council on Prices and Reimbursement on Medicinal Products (NCPRMP) was used to obtain information about the inclusion of new COPD medicines in the positive drug list (PDL) and changes in the rate of reimbursement enacted in 2013–2016 [Citation20]. Medicines were classified according to their international non-proprietary name (INN) of the active substance and their date of inclusion in PDL. INNs that were included in the PDL after 2013 were considered as ‘new’ because of the significant improvement to their nebulizing devises.
The data obtained from the National Health Insurance Fund (NHIF) include:
– number of health insured patients with COPD who have been registered for monitoring;
– number of hospitalizations due to COPD exacerbations;
– cost of hospitalizations [Citation21];
– the reimbursed cost for COPD medicines per INN.
The utilisation of COPD medicines on a national level was analysed through calculation of the number of defined daily doses per INN paid by the NHIF for the period 2013–2016 [Citation22]. Changes in the utilisation were analysed as average annual variation rate (AAVR) calculated with the following formula:
The number and percent of hospitalization were calculated for 2013–2016.
The cost of hospitalizations was calculated by multiplying the unit tariff of hospitalization for the particular year with the number of hospitalized patients [Citation22].
The cost is measured in National currency (Bulgarian Leva – BGN) at the exchange rate of 1 BGN = 0.95 Euro.
Micro analysis
An ambispective observational study of real-life therapy on a representative cohort of 426 patients with COPD was performed to investigate their exacerbation and hospitalization in relation to the prescribed pharmacotherapy. The detailed methodology of the study was explained elsewhere, but in general, the representative cohort from 17 country sites was selected and observed by pulmonologists for current and past COPD therapy in 2014–2015, with a lot of information of patients’ characteristics regarding severity of disease, exposure to risk, smoking habits, hospitalizations, exacerbations and cost variables [Citation11, Citation23].
The data about the exacerbations and related hospitalizations were systematized according to the severity of the disease and COPD medicines used.
Statistical analysis
Descriptive statistics of the included variables were used. The correlation between the number of hospitalizations and the number of DDDs for each therapeutic group was assessed through least square regression analysis with MedCalc based on the following formula:
The correlation between the number of hospitalizations and their costs for the observed period was also statistically assessed by least square regression analysis with MedCalc.
On the micro level, the following correlations were investigated: age, sex and choice of therapy impact on the number of exacerbations, and whether therapies affected the number of hospitalizations in the different stages of COPD. Kruskal–Wallis analysis of non-parametric test was also used.
Results and discussion
Macro analysis
In total 20 INNs are included into the PDL with indication COPD, and 12 of them were introduced after 2013. The market access of COPD medicines is relatively fast. Most of the INNs entered the Bulgarian pharmaceutical market within one year after their marketing authorisation in Europe regardless of the procedure and authorizing body. The inclusion into the PDL ensures access to public funding and frequent prescribing by pulmonologists ().
Table 1. Year of EU marketing authorisation, inclusion in PDL and percentage of reimbursement.
A centralized procedure of marketing authorisation was applied by the European Medicines Agency towards half of the INNs that prove their innovativeness. A decentralized procedure by other European agencies was applied towards 2 INNs in 2014 and 2015 and a national procedure was applied for the remaining 8 INNs, mostly prior to 2010.
The level of reimbursement depends on the indications for which the medicines are included in the PDL. For COPD therapeutic indication the medicines are 75% reimbursed, and those which have indication for asthma and COPD obtained a 100% reimbursement rate.
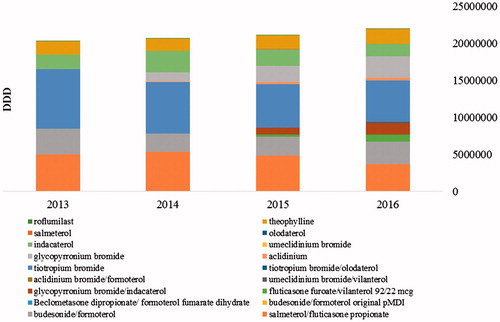
The utilisation of COPD medicines has increased over the observed period from 20 to 21 million of DDD for all therapeutic groups (). Most evident is the increase in the fixed-dose LABA/LAMA combination from 25.6 thousand to 1.7 million DDDs in 2014–2016. On the other hand, there was a decrease in the utilisation of ICS/LABA from 8.4 to 6.7 million DDDs and LABA INNs from 3.8 to 3.75 million DDDs ().
Table 2. Number of utilized DDDs per INNs and therapeutic groups.
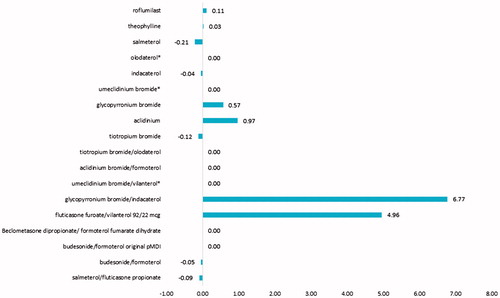
The AAVR per INN shows that, for the observed period, there was a significant increase in the DDD utilisation of glycopyrronium/indacaterol and fluticasone furoate/vilanterol (AAVR of 6.77 and 4.96, respectively), and increase in the DDD utilisation of glycopyrronium and aclidinium (AAVR of 0.57 and 0.97, respectively). A decrease in the DDD utilization was observed for salmeterol, indacaterol, budesonide/formoterol and salmeterol/fluticasone propionate ().
Figure 2. Average annual variation rate (AAVR) of the DDDs utilization for the observed period. *AAVR was not calculated due to lack of data for more than 1 year.
The number of hospitalizations shows a slow increase (from 13,602 for 2013 to 13,679 for 2016) despite the decreasing number of registered and monitored COPD patients at a national level (from 76,796 to 73,816). The hospitalization rate due to COPD exacerbations remained relatively stable, around 18–19%, but the economic burden as cost of hospitalizations increased from 5.2 to 5.7 million BGN. Some patients might experience more than one hospitalization but this is not evident from the national level databases.
Micro analysis
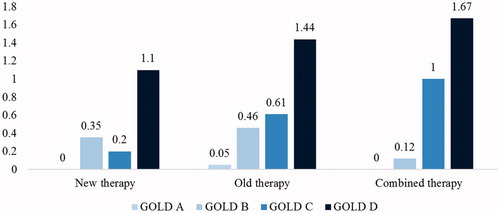
According to the indicator ‘prescribed therapy’ and the time of inclusion in PDL (), the patients recruited in the national study were divided into three subgroups: patients receiving ‘new’ therapy (new INNs introduced in the Bulgarian market after 2013), patients receiving ‘old’ therapy (INNs introduced in the Bulgarian market before 2013) and patients receiving ‘combined’ therapy (new INNs + INNs introduced before 2013).
The gender distribution of the patients according to the type of therapy, the severity of the disease and the hospitalizations is summarized in . The patients on ‘old’ therapy were 72% of all recruited in the study and the major part of them was with moderate and very severe COPD (GOLD B and D). With the increase in the severity of COPD, the number of exacerbations and the average number of hospitalizations per patient also increased, most notably for males.
Table 3. Patients’ characteristics.
The patients assigned to ‘new’ therapy (12%) with the new INNs and new inhaler devices experienced fewer exacerbations requiring hospitalizations than the patients treated with the medicines available on the Bulgarian market before 2013. The progression of the disease stage in this subgroup was also associated with an increased number of exacerbations requiring hospitalization.
The remaining 16% of the patients who received ‘combined’ INNs as part of their therapy experienced the highest rate of exacerbations and hospitalizations ().
Statistical analysis
Macro analysis level
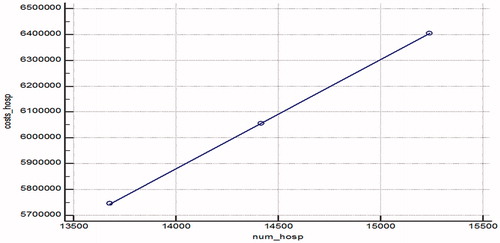
Least square regression analysis was performed to evaluate the relation between the cost of hospitalizations and the number of hospitalizations and between the number of hospitalizations and the DDD utilisation of the different therapeutic groups. The statistical analysis revealed a positive correlation (r2 = 1; p = .0018) between the number of hospitalizations and the increase in their costs ().
Figure 4. Regression model of the correlation between the number of hospitalizations and their cost (in BGN) for the observed period.
A statistically significant decrease in the number of hospitalizations was observed with the increase of DDD utilisation of combined LABA/LAMA treatment (p = .0162), while the utilisation of LABA DDD was positively correlated with an increase in the number of hospitalizations ( and ). We can suppose that the combination therapy and the increase in DDDs utilisation led to a decrease in hospitalizations.
Figure 5. Regression model of the correlation between DDD of LABA/LAMA and LABA utilized and the number of hospitalizations for the observed period.
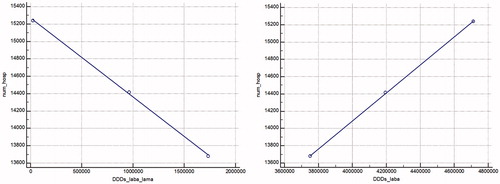
Table 4. Regression equation of the correlation between the number of hospitalizations and the DDD utilization of the different therapeutic groups.
Micro analysis level
The following correlations were investigated: whether age or sex played a role in the number of exacerbations; if there was difference between the different drug classes (new, old and combined) in lowering the number of exacerbations (both with and without hospitalization) and the effect on the number of exacerbations for all drug classes in each individual stage of COPD. The null hypothesis was that there was no significant difference.
Preliminary analysis showed that neither age, nor sex influenced the number of exacerbations; therefore, we focused on estimating the differences between different therapies and the number of exacerbations. The acute events requiring hospitalization and those without hospitalization were recorded and summarized jointly. The analysis showed that the patients on the ‘new’ therapy experienced the least number of exacerbations per year (both with and without hospitalization). With a p value of .000029 for exacerbations without hospitalization and p = .00420 for exacerbations with hospitalization, there was a statistically significant reason to reject the null hypothesis and consider that the choice of therapy affects the number of acute events. The post hoc analysis revealed significant differences between the therapies, with the ‘new’ therapy being better than the other two ones with an average rank of 144.82 vs. 219.55 and 238.53 (for exacerbations without hospitalization), and 167.74 vs. 219.5 and 223.75 (for exacerbations with hospitalization).
To further investigate the magnitude of impact onto the number of events, we broke down the analysis to disease stages. Investigating the effect of all three therapies on each disease stage individually gave some interesting results, which are summarized in .
Table 5. Statistical analysis and Kruskal–Wallis results for exacerbation, broken down by disease stage.
The main statistically significant difference was observed in GOLD 1 (n = 30) patients (p = .01097). The patients on the ‘new therapy’ (n = 7) experienced no exacerbations as opposed to 0.571 for the 21 patients on the ‘old’ therapy and 1.00 for the patients (n= 2) on ‘combination’ therapy. The results might be influenced by the low number of people in the sample, but the post hoc analysis revealed that the average rank of the ‘new’ therapy was the lowest among the three, with the same trend being observed that the combination therapy had the highest average rank and had the most exacerbations.
Consequently, no statistically significant differences were observed for the other three disease stages: p = .959 for GOLD 2 patients (n = 149); p = .776 for GOLD 3 patients (n = 65); and p = .253 for GOLD 4 patients (n = 182). For GOLD 2 and GOLD 3, the average ranks were similar, suggesting that the choice of therapy did not affect the number of exacerbations.
Interestingly, for GOLD 4, despite there being insignificant differences, the average rank of the ‘new’ therapy was lower vs. the other two ones (67.61 vs. 91.20 and 99.27). The patients on the ‘new’ treatment also experienced a lower number of mean exacerbations (2.556 vs. 3.336 and 3.545), which suggests that the new inhaler devices might be more effective as a management therapy than the old or combination therapies. The number of patients on ‘new’ medicines was relatively low (n = 9), so future focus should be on conclusively confirming this with a larger sample size.
At the macro level, the adoption of new therapies is faster and it seems that the regulators are trying to improve the patients’ access to new COPD medicines. The increase in the utilisation in DDDs supports such a conclusion. In contrast to other European countries, the variability in the number of monitored COPD patients and tariff cost of hospitalization could explain the increase in the cost of hospitalizations but their relative share remained almost equal during the observed period. This fact pointed out that the introduction of new medicines did not affect the hospitalization rate at a national level.
In contrast to the regulatory policy, the ‘new’ therapies seem to enter the real-life therapeutic practice more slowly as evident from the micro analysis. Many patients are still on ‘old’ therapy. The analysis also showed that, for GOLD 1 patients, the new therapies could be considered very effective because of the decreased number of hospitalizations. For GOLD 2 and 3, no differences were observed. However, there is a reason to believe that the ‘new’ therapies could be effective for GOLD 4 patients, despite the lack of statistically significant difference, because of their lower average rate between the groups (one hospitalization less for the ‘new’ therapies in comparison to the ‘old’ and the ‘combined’ products). The new therapies are mainly LAMA products, while the old ones are LABAs. Combined medicines are ICS/LABA and LAMA/LABA, but they are prescribed for a limited number of patients. Further studies need to be done to support these results, as well as a follow-up on the results in the longer term. We can expect that the new medicines would be more effective in terms of decreasing the number of hospitalizations due to exacerbations, but these products are slow to enter the real-life practice.
To the best of our knowledge, this is the first study exploring the hospitalization rate at the macro and the micro level. This is also the first national study that explores the entrance of ‘new’ COPD medicines on the national market and attempts to reveal whether there is a consistency in governmental and prescribing policy.
Like other studies [Citation24], we did not find a difference in the hospitalization rate between males and females. Multivariate analysis compared the risk of hospitalization with different therapeutic alternatives and found that the risk of rehospitalization decreases with 16% when patients are treated with ICS (p < .05) and with 41% if patients are treated with ICS and LABA (p < .05) [Citation25]. In another study that followed 437 patients for one year, the frequency of hospitalizations decreased when the treatment included ICS and LABA, in comparison to that with ICS andshort-acting beta-agonists (SABA) (12.1 vs. 18.1%; p < .05) [Citation26]. These studies demonstrated the decrease in the risk of hospitalizations for the group of patients treated with ICS alone or in combinations with LABA, despite poor patient prognosis.
The 52-week FLAME study comparing glycopyrronium/indacaterol with fluticasone propionate/salmeterol in 3360 patients with moderate-to-severe COPD showed that the annual rate of all COPD exacerbations was 3.59 (95% CI: 3.28,3.94) in the indacaterol/glycopyrronium group and 4.03 (95% CI: 3.68, 4.41) in the salmeterol/fluticasone propionate group, representing an 11% lower rate, p = .003 [Citation27]. A systematic review and meta-analysis published in 2017 demonstrates that LABA/LAMA significantly reduced the moderate/severe exacerbation rate compared to LABA/ICS (RR 0.82, 95% CI: [0.75, 0.91]). The adverse event incidence rate was also lower with LABA/LAMA vs. LABA/ICS (RR 0.94, 95% CI: [0.89, 0.99]), including lower pneumonia risk (RR 0.59, 95% CI: [0.43, 0.81]) [Citation28].
Our results showed a statistically significant decrease in the number of hospitalizations in the patients treated with LAMA/LABA compared to those treated with LABA/ICS, where an increase in the number of hospitalizations was observed, albeit statistically insignificant.
Frequent hospitalizations increase the total cost of COPD therapy [Citation29, Citation30]. Therefore, any therapy that might reduce the hospitalization rate should be considered cost saving [Citation31]. We observed an increase in the total hospitalization costs mostly due to an increase in the number of hospitalizations and their insurance tariff during the period. Based on our findings, we can conclude that the LABA/LAMA combination is related to fewer exacerbations, requiring hospitalization. The choice of therapeutic regime should be in line with the GOLD recommendations and personalized to the individual characteristics of the patients.
The severity of the disease, comorbidities, patient noncompliance and age are risk factors for exacerbations and related hospitalizations [Citation32]. We explore only the severity of the disease and confirm that the patients in severe stage are frequently hospitalized.
This study has some limitations. The period is observed quite short (only four years) and some of the new medicines were included in the PDL at the end of the period. Further observations should be performed in order to examine the utilisation of the new medicines such as umeclidinium/vilanterol, olodaterol/tiotropium and umeclidinium and their correlation with the hospitalization rate in Bulgaria. The reasons behind the decrease in the number of health insured patients with COPD should also be further investigated because this variable might have an impact on the number of hospitalizations as well. We did not explore the patients’ quality of life and its relation to hospitalizations, although there is some evidence that they might be correlated.
Conclusions
The new therapies enter the market relatively quickly but are slow to be adapted to real-life prescribing practices as a choice of therapy. They show a marked decrease in the number of hospitalizations for GOLD 1 and GOLD 4 patients, but a less pronounced difference for GOLD 2 and GOLD 3, suggesting that they might be more effective than old and combined therapies as a maintenance therapy. Further observations should be conducted, as there is a statistically significant decrease in the number of hospitalizations when the patients are treated with LAMA/LABA compared to those treated with LABA/ICS. Frequent hospitalizations increase the total cost of COPD therapy; therefore, LAMA/LABA should be considered as viable alternatives to reduce costs. All of these factors provide important information for decision makers and doctors, allowing for better education or resource allocation. Future cost-effectiveness studies could prove that new therapies have a more favourable cost-effectiveness profile and should be investigated. This study focused on a disease with a high socio-economic impact, in a developing region where information is scarce. It confirmed the global trends, while simultaneously addressing issues in the country. Future studies should also consider assessing other risk factors, such as smoking, which is a major problem in Bulgaria with a high prevalence.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- WHO [Internet]. Burden and mortality of COPD. Geneva (Switzerland): World Health Organization; c2018 [cited 2018 May 04]. Available from: http://www.who.int/respiratory/copd/en/.
- Lozano R, Naghavi M, Foreman K, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128.
- Lopez AD, Shibuya K, Rao C, et al. Chronic obstructive pulmonary disease: current burden and future projections. Eur Respir J. 2006;27(2):397–412.
- Global Strategy for the Diagnosis, Management and Prevention of COPD [Internet]. Global Initiative for Chronic Obstructive Lung Disease (GOLD) 2017; c2018 [cited 2018 May 04]. Available from: http://www.goldcopd.org.
- GOLD (Global Initiative for Chronic Obstructive Lung Disease) [Internet]. Global strategy for the diagnosis, management, and prevention of COPD. 2017 [cited 2018 May 29]. Available from: http://goldcopd.org/download/326/.
- Hurst JR, Vestbo J, Anzueto A, et al. Susceptibility to exacerbation in chronic obstructive pulmonary disease. N Engl J Med. 2010;363(12):1128–1138.
- Chong J, Poole P, Leung B, et al. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease (review). Cochrane Database Syst Rev. 2011 [cited 2018 May 04];5:CD002309. DOI:10.1002/14651858.CD002309.pub3
- Chong J, Leung B, Poole P. Phosphodiesterase 4 inhibitors for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2017 [cited 2018 May 29];9:CD002309. DOI:10.1002/14651858.CD002309.pub5
- Cupurdija V. Economic impact of leading prosperity diseases: COPD in South East Europe. Front Public Health. 2015 [cited 2018 May 04];3:50. DOI: 10.3389/fpubh.2015.00050
- Kamusheva M, Dimitrova M, Van Boven JFM, et al. Clinical characteristics, treatment patterns and socio-economic burden of COPD in Bulgaria. J Med Econ. 2017;20(5):503–509.
- Pavlov P, Ivanov Y, Glogovska P, et al. New epidemiology data on COPD in the Pleven region. Thoracic Med. 2012;2(IV):44–50.
- Han J, Dai L, Zhong N. Indacaterol on dyspnea in chronic obstructive pulmonary disease: a systematic review and meta-analysis of randomized placebo-controlled trials. BMC Pulm Med. 2013 [cited 2018 May 04];13:26. DOI:10.1186/1471-2466-13-26
- Geake JB, Dabscheck EJ, Wood-Baker R, et al. Indacaterol, a once-daily beta2-agonist, versus twice-daily beta2-agonists or placebo for chronic obstructive pulmonary disease. Cochrane Database Syst Rev. 2015 [cited 2018 May 04];1:CD010139. DOI: 10.1002/14651858.CD010139.pub2
- Rootmensen GN, van Keimpema AR, Jansen HM et al. Predictors of incorrect inhalation technique in patients with asthma or COPD: a study using a validated videotaped scoring method. J Aerosol Med Pulm Drug Deliv. 2010;23(5):323–328.
- Sulaiman I, Cushen B, Greene G, et al. Objective assessment of adherence to inhalers by Patients with Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med. 2017;195(10):1333–1343.
- Dahl R, Jadayel D, Alagappan VK, et al. Efficacy and safety of QVA149 compared to the concurrent administration of its monocomponents indacaterol and glycopyrronium: the BEACON study. Int J Chron Obstruct Pulmon Dis. 2013;8:501–508.
- Wedzicha J, Decramer M, Ficker JH, et al. Analysis of chronic obstructive pulmonary disease exacerbations with the dual bronchodilator QVA149 compared with glycopyrronium and tiotropium (SPARK): a randomised, double-blind, parallel-group study. Lancet Respir Med. 2013;1(3):199–209.
- D`Urzo A, Rennard S, Kerwin EM, et al. Efficacy and safety of fixed-dose combinations of aclidinium bromide/formoterol fumarate: the 24-week randomized, placebo-controlled AUGMENT-COPD study. Respir Res. 2014 [cited 2018 May 04];15:123. DOI: 10.1186/s12931-014-0123-0
- National Health Insurance Fund in Bulgaria [Internet]. Official website. Available from: https://www.nhif.bg/
- National Council on Pricing and Reimbursement [Internet]. Positive Drug List. Annex 1. Available from: http://portal.ncpr.bg/registers/pages/register/list-medicament.xhtml
- National Health Insurance Fund [Internet]. National framework contract. Available from: https://www.nhif.bg/get_file?section=document&uuid=504f49c1-397e-49c2-b478-2c7c49504386
- WHO [Internet]. Introduction to drug utilization research. World Health Organization. Geneva Switzerland: WHO; 2003 [cited 2018 May 04]. Available from: https://www.whocc.no/filearchive/publications/drug_utilization_research.pdf
- Tachkov K, Kamusheva M, Pencheva V, et al. Evaluation of the economic and social burden of chronic obstructive pulmonary disease (COPD). Biotechnol Biotechnol Equip. 2017;31(4):855–861.
- Papaioannou A, Bania E, Alexopoulos E, et al. Sex discrepancies in COPD patients and burden of the disease in females: a nationwide study in Greece (Greek Obstructive Lung Disease epidemiology and health economics: GOLDEN study). Int J Chron Obstruct Pulmon Dis. 2014;9:203–213.
- Soriano JB, Kiri VA, Pride NB, et al. Inhaled corticosteroids with/without long-acting beta-agonists reduce the risk of rehospitalization and death in COPD patients. Am J Respir Med. 2003;2:67–74.
- Kiri VA, Bettoncelli G, Testi R, et al. Inhaled corticosteroids are more effective in COPD patients when used with LABA than with SABA. Respir Med. 2005;99:1115–1124.
- Wedzicha JA, Banerji D, Chapman KR, et al. Indacaterol-glycopyrronium versus salmeterol-fluticasone for COPD. N Engl J Med. 2016;374:2222–2234.
- Rodrigo GJ, Price D, Anzueto A, et al. LABA/LAMA combinations versus LAMA monotherapy or LABA/ICS in COPD: a systematic review and meta-analysis. Int J Chron Obstruct Pulmon Dis. 2017;12:907–922.
- Jeetvan GP, Saurabh PN, Dalal AA. Indirect costs in chronic obstructive pulmonary disease: a review of the economic burden on employers and individuals in the United States. Int J Chron Obstruct Pulmon Dis. 2014;9:289–300.
- Geitona M, Hatzikou M, Steiropoulos P, et al. The cost of COPD exacerbations: a university hospital-based study in Greece. Respir Med. 2011;105(3):402–409.
- Simoens S. Cost-effectiveness of pharmacotherapy for COPD in ambulatory care: a review. J Eval Clin Pract. 2013;19:1004–1011.
- Alexopoulos E, Foteini M, Mitsiki E, et al. Frequency and risk factors of COPD exacerbations and hospitalizations: a nationwide study in Greece (Greek Obstructive Lung Disease Epidemiology and health economics: GOLDEN study. Int J Chron Obstruct Pulmon Dis. 2015;10:2665–2674.