Abstract
A novel laccase from a newly isolated white-rot fungus Trametes sp. MA-X01 was characterized. The sodium dodecyl sulphate polyacrylamide gel electrophoresis (SDS-PAGE) showed that the molecular weight of the laccase produced by Trametes sp. MA-X01 in liquid medium was approximately 62 kDa. The optimal temperature was 60 °C. The enzyme was quite stable at high temperature and maintained about 85% of its highest activity after incubation at 60 °C for 2 h. The laccase could work efficiently over a wide pH range (3.0–5.0) with no significant difference in enzyme activity. Among the metals tested, Mg2+, Mn2+, Zn2+ and Cu2+ could promote the laccase activity, whereas the laccase activity was strongly inhibited by β-mercaptoethanol, l-cysteine and dithiothreitol. Furthermore, the laccase from Trametes sp. MA-X01 exhibited strong ability to decolorize different structural dyes, including azo, heterocyclic and triphenylmethane dyes, in the absence of a redox mediator.
Introduction
Laccases (EC 1.10.3.2) are a group of enzymes with a long history of research. They were first discovered by Yoshida in Rhus vernicifera, and later, laccases were widely found in nature. Laccases belong to the superfamily of multi-copper oxidases, and have been found in bacteria, fungi, plants and insects. Due to their highly diverse origin, the properties and functions of various laccases differ significantly, and execute different physiological functions in different species [Citation1–5]. White rot fungi are filamentous fungi that can cause the decay of wood. Most of them are basidiomycetes which can degrade lignin, causing a white discoloration of the wood and playing an important role in the material circulation in ecosystems. White rot fungi can synthesize and secrete a variety of oxidases including laccase. They have a strong ability to degrade various natural and synthetic chemicals with different structures, such as benzene, aniline, cyanide, polycyclic aromatic hydrocarbons and azo dyes [Citation6–8]. Thus the practical uses of laccases in industry and biotechnology have attracted much research attention.
Laccases are generally glycoproteins containing four copper atoms, and the identified fungal laccases show a similar structure [Citation9,Citation10]. The active site contains four copper atoms of three different types, which play an important role in the catalytic reaction of laccase. One type-1 (T1) copper with a maximum absorption at 600 nm makes the laccase blue. T1 copper, associated with the oxidation of substrate, accepts electrons from the substrate. One type-2 (T2) and two type-3 (T3) copper atoms arrange in a cluster. The electron from T1 is transferred to T2/3 where molecular oxygen is reduced to generate water. During the reaction, O2 in the air is consumed, and the only byproduct is water. Therefore, laccase is considered a ‘green’ enzyme.
Laccase can catalyze a wide range of substrates, such as benzene, aniline and a variety of aromatic amine compounds. In addition, some organic and inorganic metal ions can be the substrates of laccase. Laccases are used in the production of a variety of industrial products. For example, laccase can remove the phenolic compounds that inhibit fermentation in ethanol production [Citation11]. Laccase can replace the chemical reagents in hair dyes to catalyze the dye precursor to produce the colored substances. [Citation12] In the food industry, the excessive phenolic substances in fruit juice (such as apple juice and grape juice) may cause turbidity of the fruit juice, which can be removed by laccase to clarify the juice and enhance its stability [Citation13–15]. Laccase can also be used to eliminate the phenolic substances in the wine, enhance the stability and improve the taste [Citation16,Citation17].
What is more attractive is that laccases can degrade various environmental pollutants such as different structural dyes, antibiotics and polycyclic aromatic hydrocarbons (PAH) [Citation18–20]. However, the practical application of laccase in large-scale industrial production not only requires reduction of the cost of the enzyme, but also needs a variety of candidate enzymes from different species and strains, with specific characteristics and potential applications.
Trametes, a genus of basidiomycetes, includes white-rot fungi with a high laccase production capacity. The laccase activity and the degree of induction differ among different strains isolated from different sources, as the features of the protein are also quite different [Citation21]. In a preliminarily experiment, we isolated a strain of fungus from withered plant materials in Anhui province in China. The ITS sequence analysis showed that this strain belonged to genus Trametes, and was named Trametes sp. MA-X01. In this study, the laccase properties and the ability to degrade different structural dyes were investigated. The results showed that the laccase from Trametes sp. MA-X01 could degrade different dyes in the absence of a redox mediator, which may imply a potential application in the decolorization of textile wastewater containing various dyes.
Materials and methods
Strain and culture conditions
Trametes sp. MA-X01 was isolated in Anhui province in China. In a preliminary study, the ITS sequence was amplified and sequenced. BLAST results showed that this strain belonged to genus Trametes. Thus the strain was named and preserved in our laboratory.
The mycelia were grown on potato dextrose agar (PDA) medium containing 200 g/L potato extract, 20 g/L d-glucose, 3 g/L KH2PO4, 1.5 g/L MgSO4 and 20 g/L agar, at 25 °C. After 7 days of growth, the mycelia were stored at 4 °C, and the stocks were transferred to fresh PDA every three months.
Liquid PDB medium (PDA medium without agar) was used in shake-flask cultures. The pre-cultures were prepared by homogenization of an agar plug sized about 4 cm × 4 cm containing mycelia in 100 mL of PDB medium in a 250-mL flask, followed by culturing at 25 °C with shaking at 160 rpm for 7 days. The pre-cultures were homogenized again and subsequently, 5 mL of homogenized pre-cultures were transferred to 100 mL fresh liquid medium in 250-mL flasks, followed by incubation at 25 °C, with shaking (160 r/min).
Enzyme assay
Laccase activity was determined at room temperature using ABTS [2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid)] as a substrate. The enzyme activity assay was performed in a reaction system with a total volume of 3 mL containing 2.3 mL of 0.2 mol/L sodium acetate buffer (pH = 4.5), 0.5 mL of diluted crude enzyme, and 0.2 mL of 1 mmol/L ABTS. Laccase activity was determined as the increase in the absorbance at 420 nm [ε = 36000 L/(mol·cm)]. One unit of laccase activity was defined as the amount of enzyme required to oxidize 1 μmol of ABTS per minute. All the assays were performed in triplicate, and the results are presented as mean values with standard deviation.
Characterization of laccase
Molecular weight
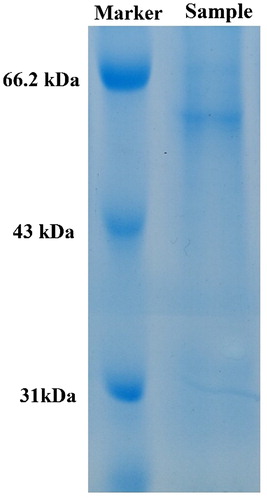
The proteins in the crude enzyme solution were precipitated by ammonium sulphate, and the precipitate was re-dissolved in 0.2 mol/L sodium acetate buffer at pH 4.5. Sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) was performed using 30 μL of the concentrated protein with a 5% stacking gel and a 12% separating gel. After electrophoresis at 160 V for 4 h, the gel was stained with Coomassie brilliant blue R250.
Optimal pH and temperature
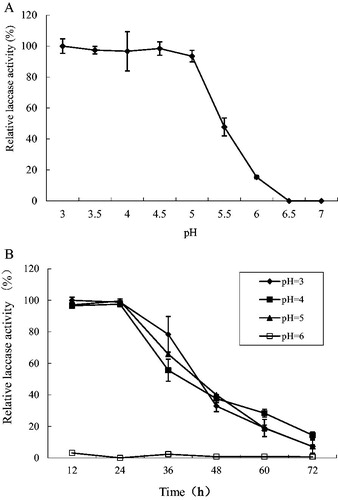
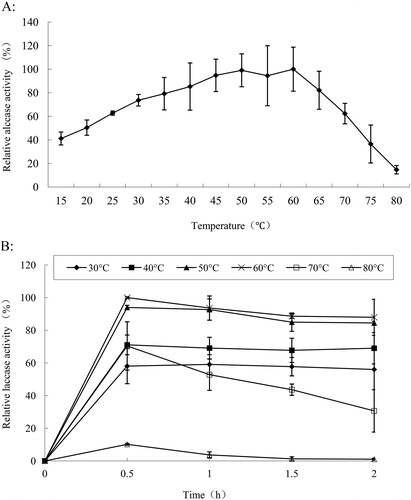
Laccase activity was assayed in buffers of different pH values using ABTS as substrate. Buffers were prepared as follows: 0.2 mol/L sodium citrate buffer (pH 3.0–3.5), sodium acetate buffer (pH 4.0–5.5) and sodium phosphate buffer (pH 6.0–7.0). After determining the optimal pH, laccase activities at 15, 20, 25, 30, 35, 40, 45, 50, 55, 60, 65, 70, 75 and 80 °C were measured. The relative laccase activity was calculated, and the highest activity represents 100% relative activity.
pH and thermal stability
pH stability was examined by measuring the laccase activity after incubation at different pH buffers (pH 3.0–7.0) at room temperature, and the enzyme activity was measured at 12, 24, 36, 48, 60 and 72 h. The effect of temperature on the stability of the enzyme was studied under the optimal pH value. The crude enzyme solution was incubated at 30, 40, 50, 60, 70 or 80 °C, and the laccase activity was determined after 0.5, 1, 1.5 and 2 h. The relative laccase activity was calculated, and the highest activity represents 100% relative activity.
Effects of metal ions and inhibitors on the laccase activity
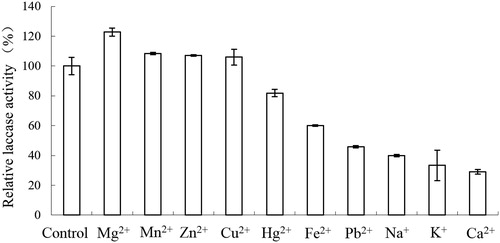
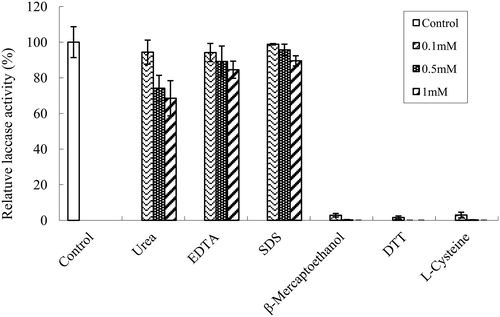
The ions Mn2+ (MnSO4), Ca2+ (CaCl2), Mg2+ (MgCl2), Na+ (NaCl), K+ (KCl), Fe2+ (FeCl2), Zn2+ (ZnSO4), Pb2+ (Pb(NO3)2), Hg2+ (HgCl2) and Cu2+ (CuSO4) were added to the enzymatic reaction mixture, each at a final concentration of 50 mmol/L, and the enzyme activities were detected. Inhibitors, including β-mercaptoethanol, urea, l-cysteine, ethylenediaminetetraacetic acid (EDTA), dithiothreitol (DTT) and sodium dodecyl sulfate (SDS) were added to the enzymatic reaction solution at the final concentrations of 0.1, 0.5 and 1 mmol/L, respectively, and the enzyme activities were determined. The activity of the untreated enzyme was used to represent 100% relative activity.
Dye decolorization
Thirty dyes belonging to different structural types, including azo, heterocyclic and triphenylmethane dyes, were used in the decolorization assay (). The dye decolorization reaction was carried out in 0.2 mol/L pH 4.5 sodium acetate buffer at 50 °C in darkness. The total volume of the reaction system was 10 mL, containing 0.1 mg of the dye and 1 U of the crude enzyme. The reaction system without crude enzyme solution was set up as the negative control. The decolorization rate (%) = (Ai–Af)/Ai × 100%, where Ai is the initial absorbance, and Af is the final absorbance.
Table 1. Biodegradation of different dyes by laccase produced by Trametes sp. MA-X01.
Results and discussion
Molecular weight of the laccase from Trametes sp. MA-X01
As shown in , the crude enzyme solution was concentrated by ammonium sulphate precipitation and subjected to SDS-PAGE. Only one band with a size of approximately 62 kDa was observed in the sample. Laccase activity was undetectable in the supernatant after the ammonium sulphate precipitation, and the laccase activity of the concentrated protein sample was approximately six times higher than that of the crude enzyme solution (data not shown), indicating that most of the proteins in the fermentation broth had precipitated and concentrated. Since the content of the protein impurities in the fermentation broth was low, the crude enzyme solution was used to determine the enzymatic properties in the following tests.
Optimal pH and pH stability
The optimal pH and pH stability of the laccase produced by Trametes sp. MA-X01 are shown in . The laccase showed better laccase activity under acidic conditions, and there was no significant difference in the enzyme activity in the pH range of 3.0–5.0. However, when the pH was increased to 5.5, the laccase activity decreased rapidly to approximately 47.8% of the highest laccase activity, and the laccase became inactive at pH 6.5 ().
The pH stability of the laccase is shown in . The activity was almost constant within a day in the pH range of 3.0–5.0, and then it gradually decreased. The laccase activity was reduced to less than 10% on the third day of the incubation.
Optimal temperature and thermal stability
The optimal temperatures of laccases from different sources of fungi vary widely [Citation22], ranging from 20 °C to 80 °C. Strains of the same species from different regions may also show significant differences in their biological characteristics due to their adaptation to the environment. The laccase produced from Trametes sp. MA-X01 was a thermophilic enzyme, the laccase activity increased as the reaction temperature increased, up to a maximum activity at 60 °C. After that, the activity decreased rapidly, and at 80 °C, the laccase activity was about 15% of the highest activity ().
Thermal stability assay was conducted at 30–80 °C. As shown in , the enzyme exhibited good thermal stability. The laccase was not only thermophilic, but also a thermotolerant enzyme. With incubation at 30 °C and 40 °C for 2 h, there was almost no change in the enzyme activity: the activities were 55% and 70% of the highest activity, respectively. Consistent with the results about the optimal temperature, the maximum activity was observed with incubation at 60 °C for 0.5 h. With incubation at 50 °C or 60 °C for 1 h, the laccase activity slightly decreased, the residual activity was about 92%. After 2 h of incubation, the activity was reduced to about 85% of the highest activity. If the temperature was increased to 70 °C, the activity decreased rapidly with the prolongation of the incubation time. Under incubation for 1 h, the activity was approximately 50% of the maximum, and with incubation for 2 h, approximately 30% enzyme activity remained. The laccase was inactive after incubation at 80 °C for 1 h.
Effects of metal ions and inhibitors on the laccase activity
The effects of the metal ions at respective final concentrations of 50 mmol/L on the laccase activity are shown in . Among them, Mg2+, Mn2+, Zn2+ and Cu2+ promoted the activity, whereas the enzyme activity increased by 22.8%, 8.3%, 7.2% and 5.9%, respectively. Pb2+, Na+, K+ and Ca2+ showed strong inhibitory effects on the enzyme activity, decreasing it to 45.8%, 40%, 33.3% and 29%, respectively.
The inhibitory effects of several protein denaturants including urea, EDTA and SDS on the enzyme activity were enhanced with increasing concentration, but the overall effects were minor. The relative activities at the concentration of 1 mmol/L were 68.5%, 84.5% and 90%, respectively. The effects of protein reductants (β-mercaptoethanol, l-cysteine, DTT) on the enzyme activity were much greater, with inhibition rates close to 100% ().
These traits are similar to some of the currently reported strains in genus Trametes [Citation23,Citation24]. While it is noteworthy that the thermal stability of this enzyme was relatively good, with 85% of the enzyme activity retained after incubation at 60 °C for 2 h, the enzyme could also work efficiently at a wide pH range (pH 3.0–5.0). Thus, the laccase produced by Trametes sp. MA-X01 has the potential to be applied in many biotechnological processes.
Dyes decolorization
lists the degradation ability of the laccase produced by Trametes sp. MA-X01 towards 30 dyes of different structure, including azo, heterocyclic and triphenylmethane dyes. With no additional redox mediator (such as ABTS), this enzyme showed a strong ability to degrade a variety of dyes, while the degradation rate differed among different dyes. For example, among the azo dyes, Direct Blue 53 could be effectively decolorized, the achieved decolorization rate being more than 80% after 48 h reaction. Over 50% decolorization rate was observed for Direct Blue 14, Acid Orange 10, Acid Red 18, Acid chrome blue K and Janus green B. However, the degradation rate of Solvent Yellow 2 was only 5%. Among the heterocyclic dyes, the 48-h decolorization rates of Acid Red 94 and azocarmine B were higher than 50%, and the 48-h decolorization rates of Basic Red 2, Fluorescein, litmus and orcein were higher than 40%. Among the triphenylmethane dyes, the degradation rate of Basic green 4 was higher than 85%, and the degradation rates of methyl green and Light Green SF were approximately 75%.
Such differences in the degradation rates are not only related to the enzymatic properties, but also to the nature of the substrates. The preliminary estimates based on the 24-h and 48-h degradation rates showed that the degradation rates of different dyes were also significantly different. Future work should pay more attention to the details of degradation mechanism and laccase catalytic reaction conditions, optimization of the reaction system and selection of the laccase that is optimal for specific degradants, while reducing the costs of the input.
Conclusions
The characteristics of a novel laccase from the strain Trametes sp. MA-X01were studied. The molecular weight of the laccase was approximately 62 kDa on SDS-PAGE. The laccase was a thermophilic enzyme with an optimal reaction temperature of 60 °C, and also a thermotolerant enzyme with 85% of the enzyme activity retained after incubation at 60 °C for 2 h. The enzyme could also work efficiently in the pH range of 3.0–5.0. Mg2+, Mn2+, Zn2+ and Cu2+ can promote the enzymatic reaction. Without redox mediators, the enzyme had a good ability to degrade different kinds of dyes, including azo, heterocyclic and triphenylmethane dyes. In summary, the laccase from Trametes sp. MA-X01 is one of the promising candidates for the decolorization of textile wastewater containing various dyes.
| Abbreviations | ||
| SDS-PAGE | = | sodium dodecyl sulfate–polyacrylamide gel electrophoresis |
| ITS | = | internal ranscribed spacer |
| ABTS | = | 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) |
| PDA | = | potato dextrose agar |
| EDTA | = | ethylenediaminetetraacetic acid |
| DTT | = | dithiothreitol |
| SDS | = | sodium dodecyl sulfate |
| PAH | = | polycyclic aromatic hydrocarbons |
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Chandra R, Chowdhary P. Properties of bacterial laccases and their application in bioremediation of industrial wastes. Environ Sci Process Impacts. 2015;17:326–342.
- Pollegioni L, Tonin F, Rosini E. Lignin-degrading enzymes. FEBS J. 2015;282:1190–1213.
- Bryan AC, Jawdy S, Gunter L, et al. Knockdown of a laccase in Populus deltoides confers altered cell wall chemistry and increased sugar release. Plant Biotechnol J. 2016;14:2010–2020.
- Shi L, Chan S, Li C, Zhang S. Identification and characterization of a laccase from Litopenaeus vannamei involved in anti-bacterial host defense. Fish Shellfish Immunol. 2017;66:1–10.
- Dittmer NT, Kanost MR. Insect multicopper oxidases: diversity, properties, and physiological roles. Insect Biochem Mol Biol. 2010;40:179–188.
- Afreen S, Shamsi TN, Baig MA, et al. A novel multicopper oxidase (laccase) from cyanobacteria: purification, characterization with potential in the decolorization of anthraquinonic dye. PLoS One. 2017;12:e0175144. DOI: 10.1371/journal.pone.0175144.
- Xu H, Guo MY, Gao YH, et al. Expression and characteristics of manganese peroxidase from Ganoderma lucidum in Pichia pastoris and its application in the degradation of four dyes and phenol. BMC Biotechnol. 2017;17:19. DOI: 10.1186/s12896-017-0338-5
- Chang YT, Lee JF, Liu KH, et al. Immobilization of fungal laccase onto a nonionic surfactant-modified clay material: application to PAH degradation. Environ Sci Pollut Res Int. 2016;23:4024–4035.
- Mot AC, Silaghi-Dumitrescu R. Laccases: complex architectures for one-electron oxidations. Biochemistry (Mosc). 2012;77:1395–1407.
- Hoegger PJ, Kilaru S, James TY, et al. Phylogenetic comparison and classification of laccase and related multicopper oxidase protein sequences. FEBS J. 2006;273:2308–2326.
- Li J, Lin J, Zhou P, et al. One-pot simultaneous saccharification and fermentation: a preliminary study of a novel configuration for cellulosic ethanol production. Bioresour Technol. 2014;161:171–178.
- Jeon JR, Kim EJ, Murugesan K, et al. Laccase-catalysed polymeric dye synthesis from plant-derived phenols for potential application in hair dyeing: Enzymatic colourations driven by homo- or hetero-polymer synthesis. Microb Biotechnol. 2010;3:324–335.
- Lettera V, Pezzella C, Cicatiello P, et al. Efficient immobilization of a fungal laccase and its exploitation in fruit juice clarification. Food Chem. 2016;196:1272–1278.
- Gassara-Chatti F, Brar SK, Ajila CM, et al. Encapsulation of ligninolytic enzymes and its application in clarification of juice. Food Chem. 2013;137:18–24.
- Yin L, Ye J, Kuang S, Guan Y, et al. Induction, purification, and characterization of a thermo and pH stable laccase from Abortiporus biennis J2 and its application on the clarification of litchi juice. Biosci Biotechnol Biochem. 2017;81:1033–1040.
- Toushik SH, Lee KT, Lee JS, et al. Functional applications of lignocellulolytic enzymes in the fruit and vegetable processing industries. J Food Sci. 2017;82:585–593.
- García-Guzmán JJ, Hernández-Artiga MP, Palacios-Ponce de León L, et al. Selective methods for polyphenols and sulphur dioxide determination in wines. Food Chem. 2015;182:47–54.
- Shankar S, Shikha. Effect of metal ions and redox mediators on decolorization of synthetic dyes by crude laccase from a novel white rot fungus Peniophora sp. (NFCCI-2131). Appl Biochem Biotechnol. 2015;175:635–647.
- Weng SS, Liu SM, Lai HT. Application parameters of laccase-mediator systems for treatment of sulfonamide antibiotics. Bioresour Technol. 2013;141:152–159.
- Zafra G, Cortés-Espinosa DV. Biodegradation of polycyclic aromatic hydrocarbons by Trichoderma species: a mini review. Environ Sci Pollut Res Int. 2015;22:19426–19433.
- Fonseca MI, Tejerina MR, Sawostjanik-Afanasiuk SS, et al. Preliminary studies of new strains of Trametes sp. from Argentina for laccase production ability. Braz J Microbiol. 2016;47:287–297.
- Morozova OV, Shumakovich GP, Gorbacheva MA, et al. "Blue" laccases. Biochemistry Mosc 2007;72:1136–1150.
- Zheng F, An Q, Meng G, et al. A novel laccase from white rot fungus Trametes orientalis: Purification, characterization, and application. Int J Biol Macromol. 2017;102:758–770.
- Savinova OS, Moiseenko KV, Vavilova EA, et al. Properties of two laccases from the Trametes hirsuta 072 multigene family: twins with different faces. Biochimie. 2017;142:183–190.