Abstract
Conventional periodontal therapies have been widely discussed in the literature. The results of these therapies, surgically and non-surgically, have demonstrated high predictability and stable maintenance over time. With some clinical indications, such as inflamed sites with consistent bleeding on probing (BOP), it can be challenging for the root planning procedure to achieve predictable outcomes. Therefore, the aim and objectives of the present study were to evaluate whether the dual-wavelength (980 and 635 nm) laser therapy at lower power settings can be beneficial as a combined non-invasive modality in the management of periodontally diseased root surface in terms of improving the periodontal parameters. Thirty-five healthy patients were enrolled in this study. All patients had at least one pocket in the anterior and posterior teeth with initial periodontal pocket depth (PPD) > 6 mm associated with evidence of clinical inflammation, i.e. bleeding on probing (BOP++ or BOP+++) with no gingival recession. The treatment protocol utilized combined therapy of conventional debridement immediately followed by application of dual-wavelength laser therapy (photo-ablation and photodynamic therapies) at lower power settings. The results showed that all the pockets of involved teeth had an average gain of 3 mm of the clinical attachment level with no sign of BOP and reduction in the mobility of the teeth 35 days post-operatively. Thus, the utilization of the dual-wavelength approach of laser-assisted therapy at lower power settings appears to provide a promising and predictable non-invasive clinical approach in the management of compromised periodontally involved teeth.
Introduction
Following recent documented research; utilization of laser therapy widely serves as an alternative or as an adjunctive therapy to the conventional periodontal treatment modalities. Many studies support the concept of laser therapy in the management of periodontal diseases. Previous studies have suggested that there are numerous benefits to its use in treatment and have found that there is a better homeostatic effect, there can be selective calculus ablation as a result of its use, and there is a reduction of pathogens which contributes to enhancing the optimal treatment outcomes [Citation1–4]. Furthermore, the photonic energy of diode laser wavelength affecting the diseased epithelial lining has great affinity in reducing the dark-pigmented and virulent periodontal disease pathogens such as Porphyromonas gingivalis, which can invade the epithelial cells and interstitial space. Subsequently, this would compromise the periodontal pocket’s heamostatic status [Citation5]. Ochsner [Citation6] defined photodynamic therapy (PDT) as ‘an oxygen-dependent photochemical reaction that occurs upon light-mediated activation of a photosensitising compound leading to the generation of cytotoxic reactive oxygen species, predominantly singlet oxygen’. Anti-microbial photodynamic therapy (aPDT) can be introduced topically into the periodontal pocket avoiding overdose and side effects correlated with the systemic antimicrobial agent application. It may also reduce the incidence of bacterial resistance [Citation7,Citation8]. The most common laser wavelengths utilised in periodontal therapies are the semiconductor diode lasers, Nd:YAG laser (neodymium-doped: yttrium, aluminium and garnet), Er:YAG laser (erbium-doped: yttrium, aluminium and garnet) and CO2 laser (carbon dioxide). These wavelengths range from 635 to 10,600 nm. As a surgical tool, the carbon dioxide (CO2, 10,600 nm) laser can be utilized not only for ablation, but also as an adjunctive modality in mucoperiosteal flap de-epithelialization during traditional flap surgery [Citation9]. Laser-assisted sub-gingival curettage and periodontal pocket decontamination using diodes and Nd:YAG wavelengths demonstrate varying success rates [Citation10–12]. In contrast, some thermal effects (melting, cracking or carbonization) may occur when the root surfaces are directly treated with CO2 laser (10,600 nm) or Nd:YAG (1064 nm) [Citation13–15]. The concept of photodynamic therapy is based on utilization of a suitable light source (Laser or light-emitting diode), which can activate a specific photosensitiser by binding to the target cell [Citation16]. The most common photosensitisers that have been employed for antimicrobial photodynamic therapy (aPDT) are methylene blue and toluidine blue O [Citation16]. Many studies have shown that these photosensitisers are very effective in deactivation of both Gram-positive and Gram-negative periodontal bacteria [Citation17,Citation18]. Noro Filho et al. [Citation19] in a split-mouth randomized clinical trial study showed that the use of methylene blue activated by 660 nm laser wavelength combined with mechanical debridement (scaling and root planning, SRP), is an effective modality in the non-surgical management of chronic periodontitis. Improvement in the clinical attachment levels (CALs) and probing pocket depth (PPD) after 6 months follow-up was noted. Furthermore, Rosa et al. [Citation20] have shown the effectiveness of aPDT with methylene blue dye, activated by 600 nm laser wavelength, in deactivating Staphylococcus aureus biofilms. There is still a gap in the literature to support the effects of diode laser irradiation on the properties of the root surfaces. Some studies [Citation21,Citation22] have shown that this wavelength can cause collateral thermal damage to periodontal hard tissues if the irradiated parameters are too high or if the laser beam is directly aimed towards the root surface. Therefore, the main objective of utilizing a diode laser as an adjunct to the mechanical periodontal therapy is to remove the calcified deposit from the root surface [Citation23]. The aims of the present study were to assess and evaluate whether the effects of dual-wavelength laser therapy, using diode laser (980 nm) and aPDT with 635 nm wavelength, at a very low power setting could be beneficial as a minimally invasive combined modality in the management of periodontally diseased root surface; specifically in relation to inflamed sites where achieving an optimal outcome by conventional treatment can be a challenge. Furthermore, the present study assessed whether the effects of this approach could enhance the healing of the diseased pockets and improve the CAL.
Subjects and methods
Study design
Thirty-five healthy patients with no previous medical history of any medically compromised conditions, immunosuppressing or anticoagulant medications were enrolled in this interventional clinical study. The enrolled female patients were not currently pregnant or during their lactation period. The patients were only non-smokers aged 28–77 years with a diagnosis of chronic periodontitis (Supplemental Figures S1 and S2) according to the International Classification for Periodontal Diseases (1999) [Citation24].
All selected patients had at least one pocket in the anterior and posterior teeth with initial PPD >6 mm associated with evidence of clinical inflammation (BOP ++ or BOP+++) with no gingival recession.
Written informed consent was obtained from all patients. The study was performed in line with the international ethics principles.
Treatment protocol
Upon obtaining written informed consent and prior to commencement of the treatment protocol, the following parameters were recorded at the screening visit and 35 days post-operatively ().
Table 1. FMPS and FMBS at the screening visit prior to treatment.
Full-mouth-bleeding score (FMBS) and the full-mouth plaque score (FMPS) were calculated using the following formula: (number of sites with plaque or bleeding/number of total number of sites) ×100.
CAL was calculated from probing pocket depth and recession, using Hu Friedy UNC 15 periodontal probe, as shown in Supplemental Figure S2.
Tooth mobility was measured based on Miller scoring system (0–3) and bleeding on probing (BOP) was measured as shown in Supplemental Figure S2 (periodontal probe).
All the patients had full-mouth peri-apical radiographs prior to commencing the treatment protocol.
The treatment protocol was based on combined modalities, which were conventional debridement followed immediately by the applications of the photonic energy of the dual-wavelengths; diode 980 nm (photo-ablation therapy) and diode 635 nm (photodynamic therapy). The chosen laser parameters for both wavelengths were based on standardization.
Local anaesthesia (articaine 40 mg/mL in 10 μg/mL epinephrine) was administered as buccal and palatal infiltrations around the sites that were affected by pocketing.
First step: root surface instrumentation
This treatment was carried out by utilizing the diode 980 nm (Lasotronix SMART device) at a power setting of 0.7 W in continuous emission mode with an initiated fibre (Supplemental Figure S3). This was done in order to open the sulcus to facilitate the supra and sub-gingival debridement, which was carried out using Gracey Minifive Curette (Hu Friedy). This curette was inserted in the pocket (Supplemental Figure S4) up to a maximum of 7 mm in order to achieve an optimal outcome. Supplemental Figure S5 shows the site of treatment immediately after root planning.
Second step: Photo-ablation therapy protocol (diode 980 nm) to remove granulated tissue and reduce the pathogen
This treatment was carried out using an un-initiated fibre of the diode 980 nm at a power setting of 2 W in a gated mode (50% duty cycle) to allow thermal relaxation and reduce collateral thermal damage (Supplemental Figure S6). The tip was inserted into the pocket and kept parallel to the long axis of the root structure and the epithelial lining. The direction of the fiber was maintained in vertical motion and mesio-distally depending on the site of the pocket in slow sweeping motions. This procedure was continued until no further granulated tissue and debris were noted issuing from the pocket.
Third step: aPDT (diode 635 nm) protocol
The steps of application of the aPDT in the diseased sites and its activation with 635 nm intensive light were done as follows:
The photosensitiser (toluidine blue) was inserted via syringe into the pockets that contained residual bacteria (Supplemental Figure S7). Photo-activation of the toluidine blue was performed using an intensive light of 635 nm, at a power setting of 200 mW in continuous emission mode for 30 s, once at the treatment site (Supplemental Figure S8). It was anticipated, based on literature reviews, that singlet oxygen (O2·−) and other various reactive agents would be effective in reducing the pathogens introduced as a result of treatment. The expected results were to achieve photochemical disinfection of the periodontal pocket and subsequently an improvement in the wound healing of the treated sites.
All the patients were given verbal and written post-operative instructions in terms of brushing their teeth on the day after treatment and advise to use interdental brushes. No analgesics were prescribed prior to or after treatment. All the patients had periodontal re-assessment in terms of BOP, pocket depth, mobility of teeth and CAL 35 days post-treatment completion.
Statistical analysis
Statistical analysis of the pre- and post-treatment values of the studied variables, PPD, BOP, tooth mobility and CAL, was done using a formal test, the ‘sign test’. The hypotheses of the test were:
Null hypothesis (H0): the pre-treatment value of a variable was greater than its post-treatment value at a probability of 0.5;
Alternative hypothesis (H1): the pre-treatment value of a variable was greater than its post-treatment value at a probability greater than 0.5.
Intuitively, the test is designed to detect if the pre-treatment value of the variable tends to be greater than its post-treatment value. In the case of the variable ‘tooth mobility’, which is of an ordinal type (recorded on a scale of 1, 2, 3), the following table of frequencies was used:
The variable ‘CAL’ was measured in millimeters and was classified according to the American Association of Periodontology (AAP) scoring scale (0 mm = No CAL, 1–2 mm = Mild, 3–4mm = Moderate and 5–6mm = Severe).
All values are presented as means with standard deviation (±SD).
Results and discussion
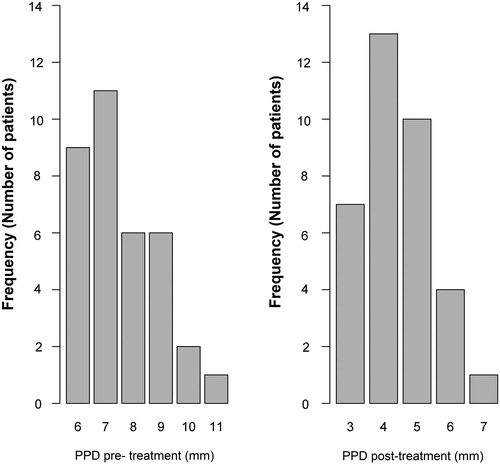
All treatment sites showed improvement in all clinical parameters. Pre-operatively, PPD was 7.54 mm (SD = 1.36) across the age groups, whereas the post-operative measurement showed mean PPD of 4.40 mm (SD = 1.36mm) for the patients included in this study (). The decrease in PPD was statistically significant (p = 5.8 ×·10−11), i.e. the null hypothesis was rejected.
Table 2. Probing pocket depth PPD, POB and CAL prior to treatment and 35 days after treatment.
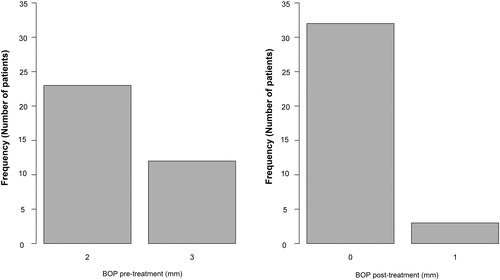
The mean CAL post-operatively was 1.60 mm (SD = 0.88 mm) across the age groups (). This indicates that there were no patients presenting values corresponding to the ‘severe’ class. The vast majority of the patients (74.3%) were in the ‘mild’ class (). The tooth mobility measured pre-operatively across the age groups showed a mean value of 1.91 mm (SD = 0.56 mm), whereas post-operatively the mean mobility was 1.43 mm (SD = 0.50) across the age groups of the study (). The statistical analysis showed that 19 patients out of 35 had maintained the same degree of mobility after treatment, while the other 16 patients showed a lower value. BOP was scored using the periodontal probe, and pre-operatively its mean value was 2.34 mm (SD = 0.48 mm) across the age groups. In contrast, post-operatively the mean result was 0.09 mm (SD = 0.28 mm). This showed a 2.25 mm reduction in the BOP 35 days after the procedure applied in this study (). This reduction was statistically significant (p = 2.9·10−11), i.e. the null hypothesis was rejected.
Table 3. Frequency (number of patients) of CAL according to the AAP CAL scoring guidance.
Table 4. Degree of teeth mobility (Miller scoring) across age groups prior to treatment and 35 days after treatment.
and show a significant reduction in the PPD and BOP values measured post-treatment in comparison to the pre-treatment values and their relationship to the number of patients (frequency). Supplemental Figure S5 illustrates the improvement in the clinical periodontal parameters. No complications were reported following the treatment in terms of post-operative pain (No analgesics were needed) and infection (No antibiotic was required). However, one patient initially reported a persistent hypersensitivity of the treated tooth, which spontaneously ceased 3 weeks after the treatment. The aPDT application using toluidine blue activated by 635 nm wavelength for 30 s, in conjunction with utilization of 980 nm wavelength with low power setting to remove the granulated tissue, was effective in reducing the incidence of periodontal microbes and enhancing the clinical periodontal parameters (Supplemental Figure S9).
According to a recent systematic review by Akram et al. [Citation25], the bactericidal efficacy of aPDT as an adjunct to scaling and root planning (SRP) against periodontal pathogens in periodontal disease has remained debatable. There are reports [Citation26,Citation27] that the bacterial counts decrease significantly following aPDT as an adjunct to SRP compared to SRP alone.
However, other studies have shown that comparable reduction in the counts of periodontal bacteria, were achieved when aPDT alone or as an adjunct to SRP, was compared to SRP alone [Citation28–30]. The present study evaluated the effectiveness of utilizing dual wavelength, and could contribute to this debate. Previous studies have shown [Citation31,Citation32] that using different regenerative therapies would improve the periodontal parameters. Furthermore, the results seen in our study compare favourably with other surgical regenerative therapies [Citation32] and would propose that this minimally invasive therapy with adjunctive use of dual laser wavelengths is an effective modality for the management of periodontally diseased root surface. The photonic energy of each laser wavelength has its own unique potential laser–tissue interaction. So, the ultimate benefits of this approach would help to improve patients’ clinical periodontal parameters to a greater extent than using a mono wavelength modality as a non-invasive therapy in the management of periodontally diseased root surface. The results of our study illustrated that this approach is effective in reducing the pocket depth and the inflammatory clinical signs at the selected pockets. In a randomized controlled study of in vitro animal models (the pig model) by Romanos et al. [Citation33], instrumentation of the soft periodontal tissues (no flap surgery) with a diode laser (980 nm) at a high power setting of 2–4 W, in continuous emission mode, led to a complete epithelial removal in comparison to the conventional treatment methods with the hand instruments in the periodontal pockets. The low power setting of 2 W was able to remove the thin pocket epithelium in the same way in all of the tissues scaled by all of the three examiners independent of the experience level [Citation33]. However, utilization of a higher power setting (4 W) caused significant damage to the underlying connective tissues with coagulation similar to tissue necrosis. This was demonstrated as a result of an increase in the thermal effect of the high power laser setting on the target tissue [Citation33]. The present study, however, demonstrated that utilizing the combined modalities of the diode 980 nm with the low power setting of 2 W in a gated emission mode (average power was 1 W) with aPDT (635 nm diode laser) as a dual-wavelength therapy, is an effective management of the root surface in chronic periodontal diseases with minimal collateral thermal effects on the adjacent tissues.
Kreisler et al. [Citation34] reported on the clinical effects of diode laser (809 nm) irradiation as an adjunct to SRP in the non-surgical management of root surfaces. All patients received conventional SRP using hand instruments that were randomly combined with either GaAlAs diode laser irradiation or rinsing with saline. After 3 months healing, the adjunctive laser therapy (809 nm) showed a significantly higher reduction in the tooth mobility, PPD and CAL, whereas the mean BOP reduction was not significantly different between the groups. The authors did not report if any adverse events were noted related to the laser treatment, knowing that this wavelength has a deeper penetration depth than the 980 nm wavelength.
The results of the present study are in agreement with the report of Kreisler et al. [Citation34] despite the lack of a control group in the present study. Therefore, it seems reasonable to suggest that diode laser (980 nm) irradiation at a lower power setting in a gated emission mode with the effect of the aPDT can provide superior advantages over the conventional non-flap modalities in treating periodontal disease. Advantages can be defined in terms of shorter healing time, reduction of CAL and mobility of the teeth and an improvement of the clinical periodontal parameters. In addition, the present study also showed a reduction in BOP, in contrast to the work of Kreisler et al. [Citation34]. Subsequently, this would minimize the stress on the tissues after the mechanical debridement. It is worth mentioning that the LANAP protocol was firstly described by Gregg et al. [Citation35] and further investigated by Yukna et al. [Citation36] and Nevins et al. [Citation37] in a human prospective clinical study. Despite the evidence showing a reduction in the pocket’s depth and a general gain of the CAL, it seems highly unpredictable that the role of the Nd:YAG laser alone can remove the sub-gingival calculi to achieve periodontal tissue healing after non-surgical and surgical therapies. It is challenging to be convinced that the Nd:YAG laser, which is a soft tissue wavelength of 1064 nm, can remove hard tissue like sub-gingival calculus with no collateral thermal damage taking into consideration its penetration depth of approximately of 7 mm. Furthermore, Sgnolastra et al. [Citation38] undertook a meta-analysis examining Nd:YAG laser wavelength, and identified PD reduction with the use of SPR and Nd:YAG (1064 nm) wavelength compared to SPR alone. The study however, observed no differences in CAL gain, which is in contrast to the findings of the present study. It is important to refer to the benefit of the combined Er,Cr:YSGG (2780 nm) and diode InGaAsP (940 nm) laser therapy, which has added value to the nonsurgical periodontal treatment in bacterial reduction and improvement in the clinical periodontal parameters [Citation39]. The recent systematic review by Betsy et al. [Citation40] reported that although there was a wide range of heterogeneity in the included studies, they all indicated that aPDT has the potential to be an effective adjunct in the treatment of chronic periodontitis. Finally, although the present study has some limitations, it is the first step in utilization of diode 980 nm at a low-power setting combined with aPDT using the 635 nm diode laser, as a dual-wavelength approach, in the management of chronic periodontal diseases of the root surfaces, in order to achieve a better outcome with minimal to no post-operative adverse effects.
5. Conclusions
Utilization of a dual-wavelength approach at a lower power setting, as an adjunct to scaling and root debridement, indicated to provide a promising and predictable clinical approach for the non-surgical management of compromised periodontally involved teeth. The aPDT using toluidine blue as a photosensitiser activated by a 635 nm diode laser appeared to be potentially superior to the traditional modalities of periodontal therapy. Furthermore, it enhanced the clinical periodontal outcome when combined with the 980 nm diode laser therapy as a dual-wavelength approach. However, given the limitations of this study, we recommend further randomized controlled trials/blind studies to be carried out to validate and confirm these results by utilizing the dual-wavelength approach with low laser power settings. This approach could be considered as the first step in improving the patients’ outcomes and perhaps would help to resolve the current debate in the literature.
Acknowledgments
The authors would like to express their gratitude to all their patients who enrolled in this study. They would also like to extend their appreciation to the senior dental nurses who were of a great support in this study.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Aoki A, Ando Y, Watanabe H, et al. In vitro studies on laser scaling of subgingival calculus with an erbium:YAG laser. J Periodontol. 1994;65:1097–1106.
- Aoki A, Sasaki KM, Watanabe H, et al. Lasers in nonsurgical periodontal therapy. Periodontol 2000. 2004;36:59–97.
- Ando Y, Aoki A, Watanabe H, et al. Bactericidal effect of erbium YAG laser on periodontopathic bacteria. Lasers Surg Med. 1996;19:190–200.
- Folwaczny M, George G, Thiele L, et al. Root surface roughness following Er:YAG laser irradiation at different radiation energies and working tip angulations. J Clin Periodontol. 2002;29:598–603.
- Yilmaz O. The chronicles of Porphyromonas gingivalis: the microbium, the human oral epithelium and their interplay. Microbiology (Reading, Engl). 2008;154:2897–2903.
- Ochsner M. Photophysical and photobiological processes in the photodynamic therapy of tumors. J Photochem Photobiol B. 1997;39:1–18.
- Hamblin MR, Hasan T. Photodynamic therapy: a new antimicrobial approach to infectious diseases?. Photochem Photobiol Sci. 2004;3:436–450.
- Wainwright M. Photodynamic antimicrobial chemotherapy. J Antimicrob Chemother. 2004;48:2173–2178.
- Centty IG, Blank LW, Levy BA, et al. Carbon dioxide laser for de-epithelialization of periodontal flaps. J Periodontol. 1997;68:763–769.
- Cobb CM, McCawley TK, Killoy WJ. A preliminary study on the effects of the Nd:YAG laser on root surfaces and subgingival microflora in vivo. J Periodontol. 1992;63:701–707.
- Moritz A, Schoop U, Goharkhay K, et al. Treatment of periodontal pockets with a diode laser. Lasers Surg Med. 1998;22:302–311.
- Liu CM, Hou LT, Wong MY, et al. Comparison of Nd:YAG laser versus scaling and root planning in periodontal therapy. J Periodontol. 1999;70:1276–1282.
- Wilder-Smith P, Arrastia AM, Schell MJ, et al. Effect of ND:YAG laser irradiation and root planning on the root surface: structural and thermal effects. J Periodontol. 1995;66:1032–1039.
- Tucker D, Cobb CM, Rapley JW, et al. Morphologic changes following in vitro CO2 laser treatment of calculus-ladened root surfaces. Lasers Surg Med. 1996;18:150–156.
- Gümü P, Buduneli N. Photodynamic therapy and periodontal treatment. Clin Anti-Inflam Anti-Allergy Drugs. 2015;2:38–42.
- De Olivereira RR, Schwatrz-Filho HO, Novaes AB, Jr, et al. Antimicrobial photodynamic therapy in the nonsurgical treatment of aggressive periodontitis: a preliminary randomized controlled clinical study. J Periodontal. 2007;78:965–973.
- Chan Y, Lai CH. Batericidal effects of different laser wavelengths on periodontopathic germs in photodynamic therapy. Lasers Med Sci. 2003;8:51–55.
- Maisch T, Szeimies RM, Jori G, et al. Antibacterial photodynamic therapy in dermatology. Photochem Photobiol Sci. 2004; 3:907–917.
- Noro Filho GA, Casarin RC, Casati MZ, et al. PDT in non-surgical treatment of periodontitis in HIV patietns: a split-mouth randamized clincial trail. Lasers Surg Med. 2012;44:296–302.
- Rosa LP, Silva FC, Nader SA, et al. Effectiveness of antimicrobial photodynamic therapy using a 660 nm laser and methylene blue dye for inactivating Staphylococcus aureus biofilms in compact and cancellous bones: An in vitro study. Photodiagnosis Photodyn Ther. 2015;12:276–281.
- Monzavi A, Shahabi S, Fekrazad R, et al. Implant surface temperature changes during Er:YAG laser irradiation with different cooling systems. J Dent (Tehran). 2014;11:210–215.
- Zhao Y, Yin Y, Tao L, et al. Er:YAG laser versus scaling and root planning as alternative or adjuvant for chronic periodontitis treatment: a systematic review. J Clin Periodontol. 2014;41:1069–1079.
- O’Leary TJ. The impact of research on scaling and root planning. J Periodontol. 1986;57:69–75.
- Amitage G. Development of a classification system for periodontal diseases and conditions. Ann Periodontol. 1999;4:1–6.
- Akram Z, Al-Shareef SA, Daood U, et al. Bactericidal efficacy of photodynamic therapy against periodontal pathogens in periodontal disease: A systematic review. Photomed Laser Surg. 2016;34:137–149.
- Theodoro LH, Silva SP, Pires JR, et al. Clinical and microbiological effects of photodynamic therapy associated with nonsurgical periodontal treatment. A 6-month follow-up. Lasers Med Sci. 2012;27:687–693.
- Novaes AB, Jr, Schwartz–Filho HO, de Oliveira RR, et al. Antimicrobial photodynamic therapy in the non-surgical treatment of aggressive periodontitis: microbiological profile. Lasers Med Sci. 2012;27:389–395.
- Birang R, Shahaboui M, Kiani S, et al. Effect of nonsurgical periodontal treatment combined with diode laser or photodynamic therapy on chronic periodontitis: a randomized controlled split-mouth clinical trial. J Lasers Med Sci. 2015;6:112–119.
- Cappuyns I, Cionca N, Wick P, et al. Treatment of residual pockets with photodynamic therapy, diode laser, or deep scaling. A randomized, split-mouth controlled clinical trial. Lasers Med Sci. 2012;27:979–986.
- Nastri L, Donnarumma G, Porzio C, et al. Effects of toluidine blue-mediated photodynamic therapy on periopatho- gens and periodontal biofilm: in vitro evaluation. Int J Immunopathol Pharmacol. 2010;23:1125–1132.
- Aichelmann-Reidyand ME, Reynolds MA. Predictability of clinical outcomes following regenerative therapy in infra-bony defects. J Periodontol. 2008;79:378–393.
- Matarasso M, Iorio-Siciliano V, Blasi A, et al. Enamel matrix derivative and bone grafts for periodontal regeneration of infrabony defects. A systematic review and meta-analysis. Clin Oral Invest. 2015;19:1581–1593.
- Romanos GE, Henze M, Banihashemi S, et al. Removal of epithelium in periodontal pockets after diode (980nm) laser application in the animal model. An in Vitro study. Photomd Laser Surg. 2004;22:177–183.
- Kreisler M, Al Haj H, D’Hoedt B, et al. Clinical efficacy of semiconductor laser application as an adjunct to conventional scaling and root planning. Lasers Surg Med. 2005;37:350–355.
- Gregg RH, McCarthy DK. Laser ENAP for periodontal bone regeneration. Dent Today. 1998;17:88–91.
- Yukna RA, Carr RL, Evans GH. Histologic evaluation of an Nd:YAG laser-assisted new attachment procedure in humans. Int J Periodontics Restorative Dent. 2007;27:577–587.
- Nevins M, Kim SW, Camelo M, et al. prospective 9-month human clinical evaluation of laser-assisted new attachment procedure (LANAP) therapy. Int J Periodontics Restorative Dent. 2014;34:21–27.
- Sgnolastra F, Severino M, Petrucci A, et al. Nd:YAG laser as an adjunctive treatment to nonsurgical periodontal therapy: A meta-analysis. Lasers Med Sci. 2014;29:887–895.
- Ciurescu C, Vanweersch L, Franzen R, et al. The antibacterial effect of the combined Er,Cr:YSGG and 940 nm diode laser therapy in treatment of periodontitis: a pilot study. Lasers Dent Sci. 2018; 2:43–51.
- Betsy J, Presanthila J, Subhash N, et al. Is Antimicrobial photodynamic therapy effective as an adjunct to scaling and root planing in patients with chronic periodontitis? A Systematic Review. Biomolecules. 2017;7:1–15.