?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.abstract
This study examined the ecological properties, 16S rRNA definition and in vitro antagonistic effect of Bacillus spp. against Alternaria spp., Aspergillus flavus, Pseudocercospora pistacina and Septoria sp., which are destructive pathogens of pistachio nut. Bacillus spp. were isolated from rhizosphere soil samples of mined borderlines (Area I), naturally growing pistachio areas (Area II), and pistachio orchards applying standard/conventional agricultural practices (Area III). A total of 106 Bacillus spp. were isolated and 11 taxa were defined through 16S rRNA sequence analyses. Canonical correspondence analysis (CCA) was used to examine the relationship between Bacillus species and environmental variables including macro- and micro-elements, physical and chemical properties of the soil samples. The highest antagonistic activity was exhibited by B. amyloliquefaciens, B. atrophaeus, B. subtilis, B. licheniformis, and B. pumilus strains isolated from area I. Furthermore, antagonistic B. pumilus, B. mycoides, and B. amyloliquefaciens strains showed tolerance to Zn and Mn indicated by CCA. To the best of our knowledge, this is the first report on determination of alternative biological control agents and their possible use in integrated pest management strategies in pistachio orchards.
Introduction
Mediterranean regions of Europe, Northern Africa, Middle Eastern countries, the eastern part of Zagros Mountains (Iran), and Caucasus regions from Crimea to the Caspian Sea have been reported as the origin of Pistacia spp. [Citation1]. With regard to Pistacia taxa, P. vera, P. palaestina, P. khinjuk, P. atlantica, and P. terebinthus, exhibit natural distribution in the Southeastern Anatolia region of Turkey where they are used as rootstocks for P. vera cultivation [Citation2].
Turkey is one of the main countries producing pistachio nut (Pistacia vera L.), together with the USA and Iran, reaching 107,650 tons of production annually [Citation3]. The cultivation is generally conducted under dry conditions in Turkey, unlike in Iran and the USA [Citation2]. Hence, Alternaria spp., Aspergillus flavus, Pseudocercospora pistacina, and Septoria sp. are the main disease-causing fungal agents in the pistachio orchards of Turkey [Citation4,Citation5]. P. pistacina and Septoria sp. are foliar phytopathogens, while Alternaria spp. and A. flavus are the main cause of nut lesions. Alternaria strains are cosmopolitan species that can easily adapt to environmental conditions and are polyphagous fungi that are capable of infecting many other crop species. These strains infect the leaves and nuts of pistachio and result in Alternaria late blight that severely reduces the yield and quality of pistachio nuts [Citation6]. In addition to the yield losses, A. flavus, A. parasiticus, and Alternaria spp. produce mycotoxins in pistachio nuts that are very harmful to human health [Citation7,Citation8].
The Southeastern Anatolia region produces 90% of the total pistachio nuts in Turkey, providing considerable economic income. Extensive fungicide application during each pistachio nut growth season results in heavy metal deposition in the soil, which causes environmental pollution [Citation9]. Integrated pest management of crop plants considers the use of biological control agents, and Bacillus spp. are reported to be effective antagonistic microorganisms in addition to their use in biotechnological and industrial applications [Citation10,Citation11].
Bacillus spp. are prolific soil inhabitants that can be easily isolated from the rhizosphere, and they also produce heat stable endospores, rendering them an ideal organism to use for biological control measures. Bacillus spp. produce lipopeptides, enzymes, and antibiotics to hinder fungal growth as antagonistic mechanisms besides promoting plant growth by triggering the biosynthesis of plant hormones (gibberellic acid and indole-3-acetic acid). In addition, Bacillus spp are reported to induce systemic resistance in plants against phytopathogenic microorganisms. Likewise, B. amyloliquefaciens, B. subtilis, B. pasteurii, B. cereus, B. mycoides, B. sphaericus, B. polymyxa and B. thuringiensis are reported to restrict plant pathogenic fungi from many crop species [Citation12–14]. The use of B. subtilis and B. amyloliquefaciens strains against aflatoxin degradation of pistachio produced by A. flavus and A. parasiticus has been reported from Iran [Citation7,Citation15,Citation16]. A plethora of research has attempted to define the effect of agricultural practices (fertilizer and pesticide applications) on ecosystems and the ubiquitous component microorganisms to understand their quantitative, physiological, biodiversity and biological activity changes [Citation17,Citation18]. Additionally, native strains of rhizobacteria exhibit higher performance when compared with those of exotic strains due to differences in edaphic or climatic conditions. Hence, screening native strains of antagonistic bacteria against plant pathogenic microorganisms is emphasized [Citation11,Citation19,Citation20]. This study aimed: (i) to isolate Bacillus spp. from soil samples of agricultural and natural habitats and characterize their ecological properties; (ii) to define antagonistic Bacillus spp. against Alternaria sp., A. flavus, P. pistacina, and Septoria sp. and (iii) to designate the antagonistic Bacillus spp. with 16S rRNA sequences. To our knowledge, the data obtained through this study contain the first reports on biological activity of Bacillus spp. on P. pistacina and Septoria sp. and may lead to the development of an effective biocontrol system to minimize pre- and post-harvest diseases of pistachio nut orchards.
Materials and methods
Bacillus spp. isolation
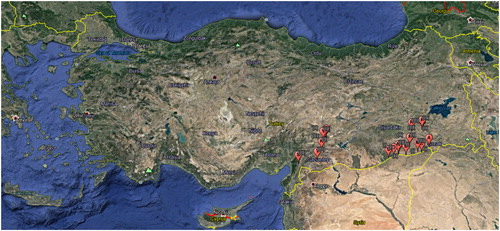
Rhizosphere soil samples were collected from mined borderlines (Area I), naturally growing pistachio sites (Area II) and pistachio orchards applying standard/conventional agricultural practices (Area III) covering five provinces (Mardin, Gaziantep, Adiyaman, Sirnak, and Siirt) of the Southeastern Anatolia region (). The samples of 5–6 different locations for each spot were collected in sterile glass jars and maintained at 4 °C [Citation21]. One gram of soil sample was diluted in 9 mL of physiological serum, shaken well, and maintained for 60 min at 65 °C to kill vegetative bacteria forms. Serial dilutions of 10−1, 10−2, 10−3, 10−4, and 10−5 were prepared, and 0.1 mL of 10−3, 10−4, and 10−5 dilutions were inoculated on nutrient agar (NA) medium. The Petri dishes were incubated at 30 °C for 48 h, and pure colonies were sub-cultured and Gram stained [Citation22]. Gram positive presumptive Bacillus spp. were selected and used in the subsequent analyses.
In vitro screening of antagonistic bacteria
Aspergillus flavus and Alternaria sp. isolates were obtained from the University of Gaziantep, Biology Department culture collections. P. pistacina and Septoria sp. isolates were provided by Pistachio Research Institute (Gaziantep-Turkey). Alternaria sp., P. pistacina and Septoria sp. isolates were pathogenic to pistachio and the A. flavus isolate was aflatoxigenic [Citation23]. The cultivation times of dual cultures were evaluated by considering the fast growing rates of fungal isolates (A. flavus and Alternaria sp.) and secondary metabolite production of Bacillus spp. The antagonistic activity of Bacillus spp. against Alternaria spp., A. flavus, P. pistacina, and Septoria sp. was defined by the dual culture method [Citation24]. Briefly, the bacterial isolates were cultured on Potato dextrose agar (PDA) at 30 °C for 3 days and then the 0.5 mm diameter of fungal disks was placed equidistantly on the other side of the Petri dish (9 cm diameter). The when the control plates were fully covered by fungal mycelia, which was in 5–7 days for Alternaria spp. and A. flavus, 21 days for P. pistacina and Septoria sp. The antagonistic effect of the bacterial isolates on the mycelium growth of Alternaria spp., A. flavus, P. pistacina, and Septoria sp. was then calculated as inhibition of mycelium growth. Fungal radial growth diameters and inhibition zones were determined in the dual cultures and inhibition percentages [Citation24] were calculated using the following formula:
where R is the radial growth (mm) of the fungus in the control dish and r is the radial growth (mm) of the fungus in the plate with different bacteria. The experiments were conducted with three replicates, and mean values were used in the evaluations. The antagonistic activity of Bacillus spp. isolates was classified as medium (inhibition rate % ≤ 30–50) and high (inhibition rate % ≥ 51), according to the method of Gogusgeren [Citation25].
16S rRNA sequence analyses of Bacillus spp
Bacillus spp. were cultured on NA medium for 24 h at 30 °C and genomic DNA was isolated using Invitrogen genomic DNA isolation kits (Thermo Fisher, USA) according to the manufacturer’s instructions. 16S rRNA regions were amplified through polymerase chain reaction (PCR) by 27 forward (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492 reverse (5′-GGTTACCTTGTTACGACTT-3′) primers [Citation26]. The PCR reactions were conducted in 30 µL reaction volume that contained 10 × buffer, 50 mmol/L MgCl2, 10 mmol/L deoxynucleotide triphosphate (dNTP), 10 pmol of each primer and 0.2 µL Taq DNA polymerase. The PCR conditions were 3 min at 94 °C for initial denaturation, followed by 35 cycles of 30 s at 94 °C, 30 s at 55 °C, 1.5 min at 72 °C and a final extension for 10 min at 72 °C. The PCR products with a length of ∼ 1500 bp were sequenced in both directions by using Applied BioS model 3730XL automated DNA sequencing system (Medsantek-Turkey). Both strands of DNA sequence were FASTA formatted and homology was determined using BLASTn within the NCBI (National Center of Biotechnology Information) database. The NCBI accession numbers of Bacillus spp. are presented in . The evolutionary history was inferred using the Neighbour-joining method [Citation27]. The bootstrap consensus tree inferred from 1000 replicates was taken to represent the evolutionary history of the analyzed taxa [Citation28]. The evolutionary distances were computed using the p-distance method and were in the units of the number of base differences per site [Citation29]. Evolutionary analyses were conducted in MEGA v.7 [Citation30].
Table 1. Antagonistic Bacillus spp. isolates against Alternaria sp., Aspergillus flavus, Pseudocercospora pistacina and Septoria sp.
Statistical analyses
Canonical correspondence analysis (CCA) was used to examine the relationship between Bacillus species and environmental variables including macro- and micro-elements (N, P, Ca, Fe, Mn, Mg, Zn, Cu, Pb, Cd, and Cr), physical and chemical properties [Electrical Conductivity (dSm-1), soil pH, lime % (CaCO3) and organic matter] of soil samples obtained from a previous study [Citation9]. Environmental variables were transformed (ln(x + 1), except for pH to decrease skewness [Citation31]. The significance of environmental variables to clarify the variance of data in CCA was tested using the forward selection of Monte Carlo simulations with 499 unrestricted permutations. Bacillus species having more than one occurrence were taken for the multivariate statistical analyses. CCA was performed using the CANOCO program [Citation31]. Weighted average regression was applied to estimate the optima and tolerance values of Bacillus species for environmental variables by using the Calibrate program [Citation32].
Results and discussion
In vitro antagonistic activity of Bacillus spp.
Pistachio is one of the most economically important crop plants in Turkey and it is an exported agricultural product. Alternaria sp., A. flavus, P. pistacina, and Septoria sp. are serious phytopathogenic fungal agents in pistachio in Turkey as well as other producer countries like Iran and the USA where extensive fungicide applications are used in each growing season to control these diseases [Citation33–35]. This study was attempted to investigate the ecological properties and antagonistic activity of Bacillus spp. that were isolated from pistachio growth areas and mined borderlines, and their effect was tested by in vitro studies.
A total of 21 soil samples (5 from mined borderlines as Area I, 6 from naturally growing pistachio orchards as area II and 10 from pistachio orchards applying standard/conventional agricultural practices as Area III) were analyzed () and 106 Gram-positive Bacillus spp. were isolated (, ). All of the Bacillus spp. isolates were screened in vitro against A. flavus, Alternaria sp., P. pistacina and Septoria sp. The highest number of antagonistic Bacillus spp. was determined from Area I, followed by Area III and Area II (). B. amyloliquefaciens MH323438 (B4), B. atrophaeus MH323450 (D8), B. licheniformis MH324397 (E4), B. pumilus MH324746 (C9), MH324747 (D7), and MH324748 (E7) strains had the highest antagonistic activity and were isolated from Area I. Furthermore, B. subtilis MH321930 (Z2) strain effectively restricted Alternaria sp., P. pistacina, and Septoria sp. that were isolated from Area III ().
Figure 2. Phylogenetic tree of antagonistic Bacillus spp. based on 16S rRNA sequences constructed using Neighbour-joining and MEGA v.7.
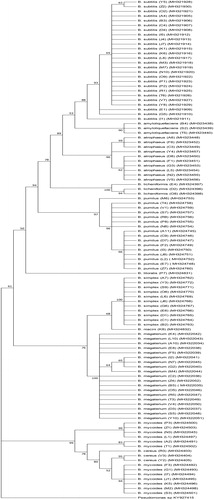
Table 2. Inhibition percentages of Bacillus spp. on Alternaria sp., Aspergillus flavus, Pseudocercospora pistacina and Septoria sp.
The agricultural practices used to increase the quality and quantity of crop yield may result in a rise in the pests and diseases in the ecosystem. Hence, phytopathogenic fungi cause serious losses before and after harvest, and consecutive fungicide applications are conducted to suppress the pathogen populations [Citation36]. Integrated pest management strategies propose the use of biocontrol agents, and Bacillus spp. is considered to be one of the best microorganisms to restrict plant pathogenic fungi [Citation12,Citation14]. B. amyloliquefaciens, B. subtilis, B. pasteurii, B. cereus, B. pumilus, B. mycoides, B. sphaericus, B. polymyxa, and B. thuringiensisare reportedly reduce the disease incidence of phytopathogenic fungi [Citation13,Citation37].
16S rRNA sequence analyses of Bacillus spp. isolates
A total of 106 Bacillus spp. strains were determined to have an effective antagonistic activity against A. flavus, Alternaria sp., P. pistacina, and Septoria sp. by use of 16S rRNA sequence analysis. A PCR product of ∼1500 bp was obtained for all the tested isolates. The sequences exhibited 100% homology with Bacillus cereus, B. mycoides, B. megaterium, B. pumilus, B. simplex, B. atrophaeus, B. litoralis, B. niacini, B. licheniformis, B. amyloliquefaciens, and B. subtilis (). The inhibition percentages of these Bacillus spp. isolates are given in . B. amyloliquefaciens, B. subtilis and B. atrophaeus exhibited the highest level and B. licheniformis had medium level of antagonistic activity against A. flavus. B. amyloliquefaciens, and B. subtilis had the highest activity against Alternaria sp., whereas B. atrophaeus, B. licheniformis, and B. pumilus were determined to have a medium level of antagonistic activity. B. amyloliquefaciens, B. subtilis, B. atrophaeus, and B. licheniformis were highly antagonistic to P. pistacina and Septoria sp. isolates ().
B. subtilis was reported to have antifungal activity against A. flavus [Citation38–40]. Kumar et al. [Citation40] reported 83% of mycelial growth restriction on PDA medium at 30 °C after the 48 h of incubation; this ratio is higher when compared to that in our study (). This could be due to the cultivation conditions, edaphic or climatic factors or genetic differences of the isolates used. B. pumilus did not exhibit antagonistic activity () against A. flavus in this study. P. pistacina and Septoria sp. mycelial growth were highly inhibited by B. amyloliquefaciens, which was determined as the best antagonistic species among the ones tested in the present study (). Bacillus spp. are the most promising and the most studied genera among other antagonistic bacteria that secrete rigorous secondary metabolites that restrict and inhibit plant pathogenic fungi [Citation41]. B. amyloliquefaciens is reported to synthesize chitinases and lipopeptides, which in turn successfully inhibit destructive phytopathogens, such as Fusarium spp., Rhizoctonia solani, Aspergillus spp., Bipolaris spp., Alternaria spp., Phoma sp., Botrytis cinera, Colletotrichum sp., Penicillium sp., and Sclerotinia sclerotiorum [Citation41–43]. Kildea et al. [Citation37] reported B. megaterium as an antagonistic agent for S. tritici, which causes serious disease in wheat. To our knowledge, the antagonistic effect of B. amyloliquefaciens against P. pistacina and Septoria sp. of pistachio was determined for the first time in this study.
Bacillus spp.–environmental relationship
CCA was applied to evaluate the relationship between predictor variables (environmental factors) and Bacillus species as response variables (, ). The first two axes indicated that physical and chemical variables had important roles in the distribution of the Bacillus taxa and explained 24.7% of the cumulative percentage variance of species data and 60.3% of the Bacillus species–environment correlations.
Figure 3. CCA plot of the species–environmental relationships in the sampling stations. Bacillus spp. are abbreviated as follows: B. cereus (bce), B. mycoides (bmy), B. megaterium (bme), B. pumilus (bpu), B. simplex (bsi), B. atrophaeus (bat), B. licheniformis (bli), B. amyloliquefaciens (bam) and B. subtilis (bsu). Area I (A-E), Area II (F-L), and Area III (M-Z) are indicated with stars, diamonds, and triangles, respectively.
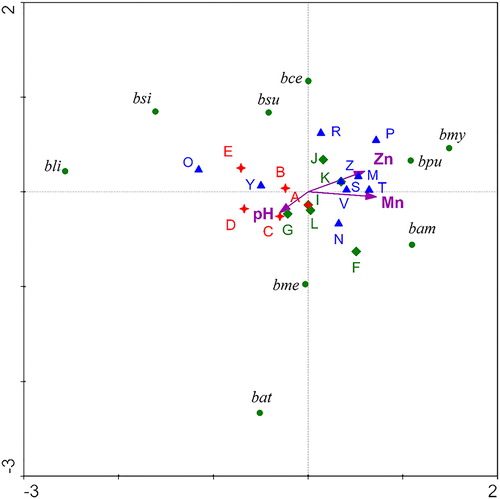
Table 3. Summary of canonical correspondence analysis using Monte Carlo permutation test for Bacillus species-environment variables relationship.
The environmental factors (e.g. Mn, Zn, and pH) exhibited significant effect on the distribution of Bacillus assemblages (). The stations from Area I were located at the negative side of the x-axis, whereas the sites of Area III were mainly found on the positive side of the x-axis, except for O and Y stations. Area III was associated with high Zn and Mn and was characterized by B. pumilus, B. mycoides, and B. amyloliquefaciens. On the other hand, Area I was characterized by B. licheniformis and B. simplex (). The CCA results indicated that B. pumilus, B. mycoides, and B. amyloliquefaciens had tolerance to Zn and Mn. In fact, weighted average (WA) regression analysis showed that the aforementioned Bacillus species had higher optima (e.g., the Zn optima of B. mycoides was 0.89 mg/kg and that of B. pumilus, 0.87 mg/kg). The Mn optima of B. mycoides, B. amyloliquefaciens and B. pumilus were 5.25, 5.33, and 4.94 mg/kg, respectively. Furthermore, B. mycoides, B. amyloliquefaciens, and B. pumilus tolerated relatively high Pb values ranging from 9.96 to 10.94 mg/kg ().
The sites of Area I ( and ) were associated with relatively low metal content in the CCA ordination and were characterized by the presence of B. licheniformis and B. simplex. Moreover, the WA results indicated that the aforementioned Bacillus species preferred low metal concentrations. The sites of Area III were associated with high Zn and Mn levels [Citation9] and were characterized by B. amyloliquefaciens, B. mycoides, and B. pumilus assemblages (). The results from the multivariable approach indicated that the aforementioned Bacillus species had high tolerance to Zn and Mn. Accumulation of heavy metals in agricultural soils through fertilization and pesticide applications are detrimental to the ecosystem but microorganisms exhibit great potential to remediate heavy metals by different mechanisms like reduction, alkylation and precipitation [Citation44]. Bioremediation of heavy metals such as Cd, Cu, Pb, and Fe by B. amyloliquefaciens, B. mycoides, B. subtilis, and B. cereus has been reported [Citation45,Citation46]. Therefore, the distribution of B. amyloliquefaciens, B. mycoides, and B. pumilus in pistachio orchards applying standard/conventional agricultural practices may depict that these taxa could be indicators of Zn and Mn accumulated in soils. The results from this study also indicated that heavy metal-tolerant Bacillus spp. (e.g. B. amyloliquefaciens, B. mycoides, and B. pumilus) could alleviate the toxicity of heavy metals to organisms. These findings and the occurrence of the aforementioned species confirmed the different regions’ status with suitable applicability of multivariate approach.
Conclusion
The present study tested the antagonistic effects of 11 Bacillus spp. (B. cereus, B. mycoides, B. megaterium, B. pumilus, B. simplex, B. atrophaeus, B. litoralis, B. niacini, B. licheniformis, B. amyloliquefaciens, and B. subtilis) on Alternaria sp., A. flavus, P. pistacina, and Septoria sp. on pistachio, in which B. amyloliquefaciens was determined to have effective antagonistic potential. A few taxa were prominent in pistachio orchards applying agricultural practices and also exhibited Zn and Mn tolerance indicated by multivariate analyses. Further studies to characterize antifungal substances of B. amyloliquefaciens will improve our understanding and ability to use this species as a biocontrol agent towards A. flavus, Alternaria sp., P. pistacina and Septoria sp. in pistachio.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Zohary M. A monographical study of the genus Pistacia. Palest J Bot Jerusalem Ser. 1952;5:187–228.
- Atli HS, Aydin Y, Arpaci S. Determination of growth, bearing, yield and some quality characteristics of pistachio cultivars grafted on different rootstocks under irrigated conditions. Int Soc Horticult Sci. 2011;912:289–294.
- FAOSTAT, 2017. Crop Production. Food and Agriculture Organization of the United Nations (FAO). Available online: http://faostat.fao.org
- Crous PW, Quaedvlieg W, Sarpkaya K. Septoria-like pathogens causing leaf and fruit spot of pistachio. IMA Fungus. 2013;4:187–199.
- Ozkilinc H, Sarpkaya K, Kurt S, et al. Pathogenicity, morpho-species and mating types of Alternaria spp. causing Alternaria blight in Pistacia spp. inTurkey. Phytoparasitica. 2017;45:719–728.
- Pryor BM, Michailides TJ. Morphological, pathogenic, and molecular characterization of alternaria isolates associated with alternaria late blight of pistachio. Phytopathology. 2002;92:406–416.
- Farzaneh M, Zhi-QiShi ZQ, Ghassempour A, et al. Aflatoxin B1 degradation by Bacillus subtilis UTBSP1 isolated from pistachio nuts of Iran. Food Contr. 2012;23:100–106.
- Varga E, Glauner T, Berthiller F, et al. Development andvalidation of a (semi-) quantitative UHPLC-MS/MS method for the determination of 191 mycotoxins and other fungal metabolites in almonds, hazelnuts, peanuts and pistachios. Anal Bioanal Chem. 2013;405:5087–5104.
- Ceyhan DI, Can C, Sarpkaya K, et al. Soil structure of pistachio cultivation areas in Turkey and comparison with borderlines. Indian J Agric Res. 2017;51:360–364.
- Rooney AP, Price NPJ, Ehrhardt C, et al. Phylogeny and molecular taxonomy of the Bacillus subtilis species complex and description of Bacillus subtilis subsp. inaquosorum subsp. nov. Inter J System Evol Microbiol. 2009;59:2429–2436.
- Jin F, Ding Y, Ding W, et al. Genetic diversity and phylogeny of antagonistic bacteria against Phytophthora nicotianae ısolated from tobacco rhizosphere. IJMS. 2011;12:3055–3071.
- Bottone EJ, Peluso RW. Production by Bacillus pumilus (MSH) of an antifungal compound that is active against Mucoraceae and Aspergillus species: preliminary report. J Medical Microbiol. 2003;52:69–74.
- Kloepper JW, Ryu C-M, Zhang S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology. 2004;4:1259–1266.
- Arroyave-Toro JJ, Mosquera S, Villegas-Escobar V. Biocontrolactivity of Bacillussubtilis EA-CB0015 cells and lipopeptides against postharvest fungal pathogens. Biol Contr. 2017;114:195–200.
- Afsharmanesh H, Ahmadzadeh M, Majdabadi A, et al. Enhancement of biosurfactants and biofilm production after gamma irradiation-induced mutagenesis of Bacillus subtilis UTB1, a biocontrolagent of Aspergillus flavus. Arch Phytopathol Plant Protect. 2013;46:1874–1884.
- Siahmoshteh F, Siciliano I, Houda Banani H, et al. Efficacy of Bacillus subtilis and Bacillus amyloliquefaciens in the control of Aspergillus parasiticus growth and aflatoxins production on pistachio. Inter J Food Microbiol. 2017;254:47–53.
- Mikanová O, Šimon T, Kopecký J, et al. Soil biological characteristics and microbial community structure in a fieldexperiment. Open Life Sci. 2015;10:249–259.
- Wang R, Zhang H, Sun L, et al. Microbial community composition is related to soil biological and chemical properties and bacterial wilt outbreak. Sci Reports. 2017;7:343. DOI:10.1038/s41598-017-00472-6.
- Souza A, Cruz JC, Sousa NR, et al. Endophytic bacteria from banana cultivars and their antifungal activity. Genet Mol Res. 2014;13:8661–8670.
- Ahmad Z, Wu J, Chen L, et al. Isolated Bacillus subtilis strain 330-2 and its antagonistic genes identified by theremoving PCR. Sci Reports. 2017;7:1777.
- Jackson ML. Soil chemical analysis. Upper Saddle River, NJ: Prentice Hall Inc. Press; 1962.
- Kim HS, Lee DW, Woo SD, et al. Distribution, serological identification, and PCR analysis of Bacillus thuringiensis isolated from soils of Korea. Curr Microbiol. 1998;37:195–200.
- Konukoğlu F, Kusek M, Sarpkaya K. editors. Discoloration of pistachio nuts grown in the Gaziantep province of Turkey. V International Symposium on Pistachios and Almonds; 2009 Oct 06-10; Şanlıurfa, Turkey.
- Christy JE, Tharmila S, Niranjan K. Antagonistic activity of Trichoderma spp. and Bacillus spp. against Pythium aphanidermatum isolated from tomato damping off. Arch Appl Sci Res. 2012;4:1623–1627.
- Gogusgeren N. Determination strains of Bacillus spp. which produces an effective antibiotic against Trichoderma spp. and investigation of the opportunity to be in situ of these strains at micelle culture of Ganodermalucidum [master’s thesis]. Adana (A): Çukurova University; 2009.
- Bal EBB, Bal MA. Effects of chemicaladditivesandensiling time on whole plant wheat silage microbial profiles inferred by phenotypic and 16S ribosomal DNA analyses. World J Microbiol Biotechnol. 2012;28:767–776.
- Saitou N, Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;4:406–425.
- Felsenstein J. Confidence limits on phylogenies: an approach using the bootstrap. Evolution. 1985;39:783–791.
- Nei M, Kumar S. Molecular evolution and phylogenetics. New York: Oxford University Press; 2000.
- Kumar S, Stecher G, Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874.
- Ter Braak CJF, Smilauer P. CANOCO Reference Manual and User’s Guide to CANOCO for Windows: Software for Canonical Community Ordination (version 4). Ithaca, NY: Microcomputer Power Press; 1998.
- Juggins S, Ter Braak CJF. CALIBRATE - a program for species-environment calibration by [weighted-averaging] partial least squares regression. London (LDN): University College Press; 1992.
- Moradi M, Ershad J, editors. Determination of density of the molds Aspergillus species in the Kerman pistachio orchards in different months of years. Proceeding of the 14th Iranian Plant Protection Congress; 2000 Sept 5–8; Isfahan, Iran. Isfahan University of Technology p:128; 2000.
- Ma Z, Michailides TJ. Characterization of iprodione-resistant Alternaria isolates from pistachio in California, Pesticide Biochemistry and Physiology. Phytopathology. 2004;80:75–84.
- Sarpkaya K. Studies on biology, epidemiology and control of Pseudocercospora pistacina on pistachio [dissertation]. Adana (A): Çukurova University; 2014.
- Cuervo-Parro j, Ramírez- Lepe M, Cortes TR. Biological control of phytopathogenic fungi. Henderson: OMICS International; 2015.
- Kildea S, Ransbotyn V, Khan MR, et al. Bacillus megaterium shows potential for the biocontrol of Septoria tritici blotch of wheat. Biol Contr. 2008;47:37–45.
- Zhang T, Shi ZQ, Hu LB, et al. Antifungal compounds from Bacillus subtilis B-FS06 inhibiting the growth of Aspergillus flavus. World J Microbiol Biotechnol. 2008;24:783–788.
- Zuo R, Chang J, Yin Q, et al. Inhibiting Aspergillus flavus growth and degrading a flatoxin B1 by combined beneficial microbes. Afr J Biotechnol. 2012;11:12903–12909.
- Kumar R, Singh VP, Sharma A. A study biological control of Aspergillus flavus using Psudomonas fluorescens and Bacillus subtilis. IRJSE. 2014;2:213–218.
- Shafi J, Tian H, Ji M. Bacillus species as versatile weapons for plan tpathogens: a review. Biotechnol Biotechnol Equip. 2017;31:446–459.
- Ji SH, Paul NC, Deng JX, et al. Biocontrolactivity of Bacillus amyloliquefaciens CNU114001 against fungal plant disease. Mycobiology 2013;41:234–242.
- Hosssain MJ, Ran C, Liu K, et al. Deciphering the conserved in plant disease control through comparative genomics of Bacillus amyloliquefaciens subsp. plantarum. Front Plant Sci. 2015;8:631. DOI: 10.3389/fpls.2015.00631
- Dzionek A, Wojcieszyńska D, Guzik U. Natural carriers in bioremediation: a review. Electronic J Biotechnol. 2016;23:28–36.
- Costa ACA, Duta FP. Bioaccumulation of copper, zinc, cadmium and lead by Bacillus sp., Bacillus cereus, Bacillus sphaericus and Bacillus subtilis. Braz J Microbiol. 2001;32:1–5.
- Syed S, Chinthala P. Heavy metal detoxification by different Bacillus species isolated from solar salterns. Scientifica. 2015;2015:1. DOI: 10.1155/2015/319760