Abstract
Microalgae are subject of extensive scientific and commercial interest in terms of both fundamental ecology and biotechnological potential. Here, we describe five Bulgarian aeroterrestrial strains of the Eustigmatos/Vischeria group, which have shown potential as valuable carotenoid producers. The strains are stored in the algal collection of Sofia University (ACUS). The main cytological and morphological diagnostic features of the studied eustigmatophycean stramenopile algae remained stable during long-term cultivation, showing that immediate light microscopy (LM) identification in cultures is reliable. We used a polyphasic approach for taxonomical identification, combining conventional microscopy of culture material with molecular-genetic methods (based on rbcL gene sequencing). This approach led to almost similar taxonomic results, supporting the need for unification of the genera Eustigmatos and Vischeria (which are presently separated on the basis of morphological features). Based on the obtained results, we speculate that there could be new eustigmatophyceaen aeroterrestrial taxa. This, however, needs to be confirmed in more refined molecular-genetic studies with a view to exploiting the full biotechnological potential of the aeroterrestrial Eustigmatos/Vischeria strains described here.
Introduction
There is growing scientific and commercial interest in microalgae owing to accumulating evidence of their significance, in terms of both fundamental ecology and in relation to human use of natural resources. Microalgae act as primary producers, participants in biogeochemical cycles, important couplers in waste-water treatment, environmental remediation and monitoring. In addition, they can be a globally valuable natural source of unsaturated fatty acids, carotenoids, proteins and vitamins as health-promoting compounds, food, dietary supplements, biofuels, fertilisers and animal feed [Citation1–5]. Their sustainable production by exploitation of local strains constitutes one of the major challenges faced by researchers worldwide [Citation6,Citation7]. Such distinct isolates (strains) can have unique properties and multiple applications [Citation8]. Another advantage is that they are adapted to local climatic conditions and pose no risk of becoming noxious invasives [Citation9]. Methods to screen for indigenous species of algae for their inherent phycological resource potential have improved, yet the first essential step in such phycoprospecting studies [Citation8,Citation9] requires well-grounded species selection based on reliable identification, necessary for a feasible microalgal cultivation process [Citation10].
Traditionally, the standard determination procedures included cytological and morphological observations by light microscopy (LM) with intensive use of ultrastructural features for diagnostic purposes provided by electron and confocal laser scanning microscopy [Citation11–15]. However, even these techniques cannot always discriminate between morphologically similar species, which calls for extensive taxonomic expertise, becoming not only more and more time-consuming and expensive, but considerably and increasingly rare [Citation16,Citation17]. In this respect, Sanger DNA sequencing [Citation18,Citation19] and from 2005 onwards next-generation sequencing (NGS) have revolutionized strongly the modern algal systematics [Citation17,Citation20]. Since the 1990s, phylogenetic analyses based on specific molecular markers have become an important tool in the taxonomy of algae. Thus, beyond the traditional light and electron microscopy, many molecular techniques have been developed as alternative methods for species identification in different habitats [Citation21].
However, the algae of aeroterrestrial habitats have always remained underestimated in comparison with their aquatic counterparts mainly due to the need of investigation of living culture material [Citation22–25]. Aeroterrestrial algae were also much less studied by modern molecular genetic methods until recently. This is also valid for the class Eustigmatophyceae (Stramenopiles), which includes the aquatic oil-producing Nannochloropsis/Microchloropsis [Citation26–32] and some other aquatic species [Citation33–37]. The same class contains peculiar, but less studied aeroterrestrial species [Citation38–40]. It appeared to be far more diverse than generally recognized with many new taxa awaiting taxonomic treatment [Citation36,Citation41–43] and recent mitogenomic sequencing on different taxa becoming available [Citation44] despite that the knowledge on species diversity and environmental distribution was evaluated as still being in its infancy [Citation45] and fairly limited [Citation46]. According to Norton et al. [Citation47], only 0.2–2% of the eustigmatophycean species have been discovered so far.
Recently, Eustigmatophyceae, fondly named eustigs [Citation48], are receiving increased attention due to their great biotechnological potential for production of valuable healthy products and biofuel (e.g. [Citation43,Citation49]). This requires phyco-prospective work for discovery and correct identification of promising local strains. The relatively simple morphology of aeroterrestrial eustigmatophyceans calls for application of the polyphasic approach, known also as polyphasic strategy [Citation50]. This approach exploits simultaneously conventional and molecular identification techniques [Citation51] and is known mainly in the taxonomy of prokaryotes [Citation52]. Among algae, the term was commonly used in the studies of cyanoprokaryotes/cyanobacteria [Citation53–58] and rarer was applied in the taxonomy of eukaryotic algae [Citation59–62].
The aim of the present study was to determine by application of the polyphasic approach five aeroterrestrial strains of the Eustigmatos/Vischeria group, collected from soils in different protected areas in Bulgaria, which have shown potential as valuable carotenoid producers [Citation49]. In addition, we made a comparison of main diagnostic cytological and morphological data obtained after short- and long-term cultivation, in order to check its relevance for taxonomic considerations.
Materials and methods
Strains
The present study was based on five original Bulgarian strains of the Vischeria/Eustigmatos group. Four of them were isolated in 2007 using standard methods of collection from soils in the protected areas of the UNESCO natural heritage Pirin Mt (ACUS 000010, ACUS 00024, ACUS 00025) and in the nearby gorge Kresnensko defile (ACUS 00002) [Citation63]. One strain was isolated later, in 2012, by direct collection [Citation24] from the soils in the region of the Natural landmark Belogradchishki Skali (ACUS 00104) [Citation64,Citation65]. All strains were stored immediately in the algal collection of Sofia University (ACUS) on BBM agar. The strains were identified by LM immediately and their development was observed for one, or one-and-half year (short cultivation period) after their collection in 2007/2012 (for details see [Citation63–65]). In May 2018, after a long cultivation period, they were studied by molecular-genetic methods and checked again by LM. The LM observations from both (short and long-term) cultivation periods were compared.
Light microscopy
LM identification was done using a Motic BA400 microscope [Citation39,Citation40,Citation42,Citation66]. The most recent taxonomic proposals [Citation67] issued afterwards, in September 2018, are considered in the discussion of the results. The microphotographs were done on the same microscope using a Moticam 2 camera and the Image Plus Program.
Polymerase chain reaction
Samples were prepared from standard liquid BBM cultures. Following centrifugation, pellets were stored −80 °C. The frozen samples were disrupted using a Tissue Lyser II (Qiagen) and DNA was isolated using a GeneJT Plant Genomic Purification Kit (Thermo Fischer Scientific). A ∼1-kb region of the rbcL gene was Polymerase chain reaction (PCR) amplified using EustigrbcL-FB (5'-GATCCRATTGAAGCTGC) and EustigrbcL-RB (5′-TTAAGTAATTGGTGCATTTGT) with annealing at 53 °C [Citation36]. Following agarose gel electrophoresis, the amplified DNA fragments were purified using a GeneJT Gel Extraction Kit (Thermo Fischer Scientific) and cloned into a pJET1.2 plasmid vector using a CloneJET PCR Cloning Kit (Thermo Fisher Scientific). Four clones from each strain were sequenced (Macrogen, Inc.) and the DNA sequences were assembled using a Vector NTI v. 10 software package (Thermo Fisher Scientific). The obtained rbcL sequences were compared with the known sequences using BLAST search. Phylogenetic analysis was conducted using MEGA version 4.1, which automatically provides bootstrap values [Citation68].
Results and discussion
Morphological description and identification by means of LM
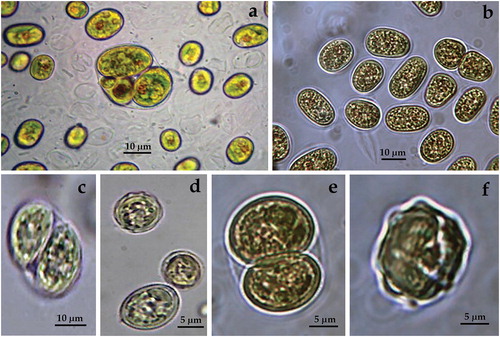
ACUS 00002 (): In 2007–2009, cells were solitary, mainly spherical (11–21 µm in diameter in 2007–2009, ), and less commonly ellipsoidal (6–7 µm wide and 10–11 µm long, ). Very rarely, only in the first period, we observed cells with triangular outline (). In 2018, cells were solitary, spherical, mainly 14–15 µm in diameter and more rarely ellipsoidal, 7–10 µm wide and 15–16 µm long. Cell walls were smooth, but in 2018 in quite a few dead cells the cell wall looked ‘wavy’ due to regular invaginations (). On some vegetative cells and autospores, there were one or a few transparent thickenings (‘warts’) over the cell wall (), the nature of which needs to be clarified. Such ‘large rounded swellings’ which by age turn into ‘conical projections’ were described by Pascher [Citation69] for the genus Vischeria. The plastid was parietal, plate-shaped in adult cells with deep fissures, so that it was strongly lapped and several plastids could be shammed. Each cell contained a large orange-red globule with central or eccentric position (). Reproduction in both periods was by two or four autospores (). Most cells fitted well to the descriptions and figures provided in [Citation39 – Figures 60(a–d)] for Eustigmatos magnus (J. B. Petersen) Hibberd 1981 (14–34 µm in diameter) except the rare presence of ellipsoidal cells and the smaller lowest dimensions (11–21 µm in diameter) in the first period [Citation63]. In 2018, the LM observations tentatively identified the material again as E. magnus due to the dominance of spherical cells with smooth cell walls.
Figure 1. LM microphotographs of strain ACUS 00002 from culture material in 2007–2009 (a, b, e, i) and in May 2018 (c, d, f, g, h).
Note: Scale bar is indicated on each microphotograph.
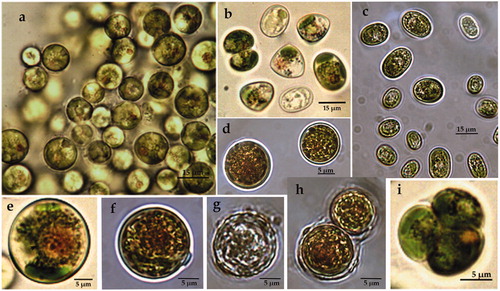
ACUS 00010 (): In 2007–2009, cells were solitary, spherical (9–12 µm, ), or elliptical (12–14 µm wide and 17–20 µm long, ). In adult cells, the walls had many small blunt-conical projections (humps), which were mostly regularly distributed (), while in younger cells only a few projections were visible ). Such projections were visible on elliptic cells (). In 2018, cells were solitary, mainly ellipsoidal, commonly (16)–17–20 µm long and 9–10 µm wide, with smooth cell walls (). There were some ellipsoidal cells (17 × 10 µm) and more rarely spherical cells (12.5 µm in diameter) with regular small humps on cell walls (). The plastid was one, trough-shaped, parietal () or strongly lapped by deep fissures. Each cell contained a big orange-red globule ()). Reproduction was by two or four autospores in each autosporangium, with a sporangium wall which splitted in two equal parts (). In 2007–2009, the cells with spherical shape and with regular projections on their cell walls corresponded well with the description of Vischeria stellata (Chodat ex Poulton) Pascher in [Citation39] and all the cells with smooth walls, as well as the elongated cells were accepted as expressions of morphological variability caused by culture conditions [Citation63]. In 2018, according to the morphology, the material could be also tentatively identified as V. stellata [Citation39,Citation40]. The uncertainty came from the predominance of cells with smooth cell walls, since the sculptured cell walls were pointed out as an important diagnostic feature of the genus. We suppose that the occurrence of smooth cell walls was caused by cultivation as in 2009.
Figure 2. LM microphotographs of strain ACUS 00010 from culture material in 2007–2009 (a, c, g) and in May 2018 (b, d, e, f).
Note: Scale bar is indicated on each microphotograph.
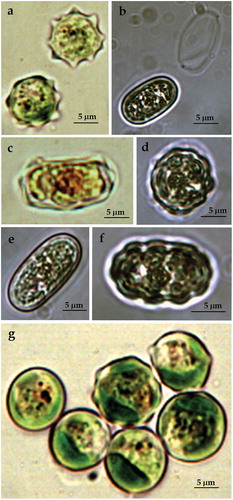
ACUS 00024 (): In 2007–2009, cells were solitary, mainly elliptical (12–14 µm wide and 17–20 µm long). Some cells were sligthly sigmoid (14 µm wide and 50 µm long), symmetrical or asymmetrical (), some were ovoid and rarely spherical (up to 12 µm in diameter – ). Most of the elongated cells were with smooth cell wall, whereas the spherical cells were supplied with small humps (). In 2018, cells were solitary, mainly elongated (commonly 25 µm long and 10–12 µm wide), straight or slightly curved (sigmoid) and more rarely broadly oval (15 µm long and 10 µm wide), . Commonly, the elongated cells were asymmetric – with one broader end (), and much more rarely were ellipsoidal or cylindric (). Almost all cells were with smooth cell wall () but in a few cells tiny thickenings were visible (). The plastid was one, trough-shaped, parietal or strongly lapped by deep fissures. Each cell contained a big orange-red body (). In 2018, almost all cells contained segregated plastids, from (2)5 to 10 (12–14), but only one orange-red globule (). Reproduction was by two or four autospores with a sporangium wall, which mouldered into two equal parts (). Since some sculptured cell walls were seen (), in 2007–2009, the material was referred to atypical Vischeria stellata due to the peculiar cell shape and smooth cell wall of most cells. However, it is possible ‘that the specimens observed belong to a new species of this genus or the changes in their shape and wall sculpture are due to cultivation process’ [Citation63]. In 2018, the material could also be tentatively referred to Vischeria stellata due to the observed cells with sculptured walls, but clearly differed from the typical species.
Figure 3. LM microphotographs of strain ACUS 00024 from culture material in 2007–2009 (a, h) and in May 2018 (b–g, i).
Note: Scale bar is indicated on each microphotograph.
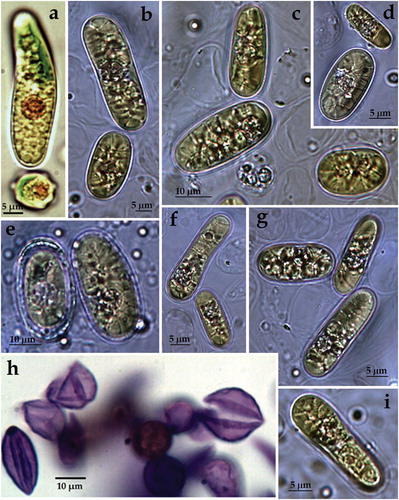
ACUS 00025 (): In 2007–2009, cells were solitary, elliptical (12–14 µm wide and 17–20 µm long; ) or irregularly shaped. Some cells were sligthly sigmoid (14 µm wide and 30–40 (50) µm long) or ovoid and rarely spherical in shape (up to 12 µm in diameter; ). Most of the elongated cells were with smooth cell wall (; exception in ), whereas the spherical cells were supplied with small humps (). In 2018, cells were solitary, mainly ellipsoidal (commonly 25–27 µm long and 10–12 µm wide) and more rarely broadly oval (15 µm long and 10 µm wide) – ), or spherical (10–12 µm in diameter). Extremely rarely some of the ellipsoidal cells were asymmetrical, with one broader end. All cells were with smooth cell wall (). The plastid was one, trough-shaped, parietal () or strongly lapped by deep fissures. In 2018, elongated cells contained four or more segregated plastids (). In both periods each cell contained a big orange-red body (). Reproduction was by two () or four autospores with a sporangium wall, which mouldered in two equal parts. Orange body was visible only in one of the autospores (). According to the cell shape and smooth cell wall, the observed material was accepted as very similar to ACUS 00024 and was referred to ‘atypical Vischeria stellata’, when supposing ‘that the specimens observed belong to a new species of this genus or the changes in their shape and wall sculpture are due to cultivation process’ [Citation63].
Figure 4. LM microphotographs of strain ACUS 00025 from culture material in 2007–2009 (c, e, d) and in May 2018 (a, b, f, g).
Note: Scale bar is indicated on each microphotograph.
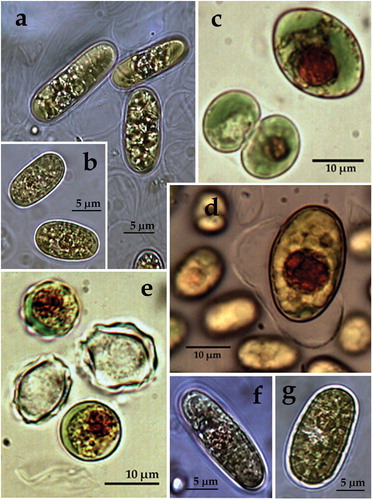
ACUS 00104 (): In 2012–2013, cells were mainly ellipsoidal or broadly oval (13–15 µm long), generally with smooth cell walls () and rarely with humped cell walls (). In 2018, cells were mostly broadly oval, mainly 13–15 (16) µm long and 8–10 µm wide () and some cells were slightly sigmoid bended. There were often elongated cells in a process of cell division in two, 23 × 7 (9) µm (). Almost all cells were with thick but smooth cell walls (). As an exception there were some empty spherical cells (10 µm in diameter) with regularly invaginated or ‘wavy’ cell walls together with some cells with humped cell walls (). The plastid was one per cell, parietal (). Commonly cells contained a single large orange-red globule in an eccentric or central position (). In 2007–2009, reproduction by division in two () or four autospores () was observed, whereas in 2018 only binary division was seen (). In 2012–2013, the cells with projections on the wall resembled the descriptions and figures of the oval and elliptical cells of Vischeria stellata provided in [Citation39, Figures 60i, 61a], whereas all the other material resembled Eustigmatos based on the smooth cell walls. However, the cells shape observed in our culture significantly differed from Eustigmatos, in which the cells should be mainly spherical. According to size, cells were on the upper range of the dimensions for Vischeria stellata (7–14 µm and rarely larger) in [Citation39]. The strain was referred as Vischeria cf. stellata with a note that this is probably a new taxon according to the morphological stability of elongated cells, larger size and general lack of sculptured cell wall [Citation64,Citation65]. In 2018, the LM-based determination led to Eustigmatos according to the cell shape and generally smooth walls, but according to the rare appearance of humps or waves, the material resembled Vischeria. These results supported our former opinion that the isolated material most probably belongs to a new taxon, which combines the features of both genera (the unification of which was done later by Kryvenda et al. [Citation67]) and did not fit to any of their species described so far.
Phylogenetic positioning of the studied strains
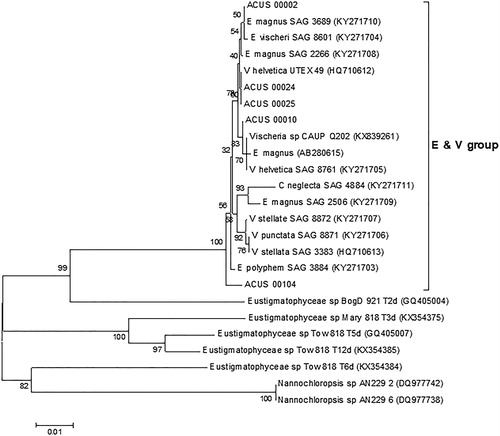
The BLAST search with the rbcL sequences obtained for the studied algae strains revealed high homology to the rbcL sequences of mainly aeroterrestrial algae strains from the Eustigmatos/Vischeria group (), with highest homology (>99% identity) to Eustigmatos magnus SAG36.89, KY271710 aeroterrestrial strain from Nepal (ACUS_00002, ACUS_00010), Eustigmatos polyphem SAG38.84, KY271703 aeroterrestrial strain from Kenya (ACUS_00104) and culture collection Vischeria helvetica UTEX 49, HQ710612 strain (ACUS_00024, ACUS_00025).
Figure 6. Neighbour joining phylogenetic tree of rbcL sequences constructed from studied ACUS strain sequences and their closest homologous sequences retrieved after BLAST search. Bootstrap values greater than 50% confidence are shown at branching points (percentage of 1000 resamplings). Sequence accession numbers are shown in brackets. The Eustigmatos/Vischeria phylogroup is designated with ‘E & V group’.
The inner taxonomy of Eustigmatophyceae is based mainly on structural features of both vegetative and reproductive cells (mainly zoospores): shape of cells, the presence/absence of zoospores, number of flagella per zoospore, number of plastids per cell, etc. [Citation39,Citation40,Citation42,Citation43,Citation66]. Due to the small size and relatively simple morphology, the application of molecular methods for distinguishing of species was proposed [Citation26]. However, 18S rDNA sequence information alone seemed not to be satisfactory and even potentially misleading [Citation70]. That is why more genes, sets of gene and genomic region sequences, which allow more precise affiliation, have been involved in the studies of different photosynthetic stramenopiles and eustigmatophytes in particular [Citation44,Citation67,Citation71]. Therefore, the present study focused on the polyphasic approach, which combines both microscopic and molecular studies in determination of eustigmatophycean strains.
Within the polyphasic approach, the presence/absence of zoospores deserves special attention as one of the key structural features in the inner taxonomy of Eustigmatophyceae. The lack of zoospores in the studied material could be attributed to the solid agar medium used for cultivation rather than to their real absence in the studied strains. This is in accordance with the results of Muratova [Citation72], who observed only autospores in cultures and successfully applied a diluted medium to obtain zoospores from Eustigmatos polyphem.
The literature data on the morphological diagnostic features of both genera Eustigmatos and Vischeria, united by their lageniform uniflagellated zoospores, shows that the main difference between them lies in the character of the cell wall – always smooth in Eustigmatos and sculptured with projections (humps) in Vischeria. This allows formation of angular cells in Vischeria, which was accepted as the main characteristic feature of the genus [Citation39,Citation40,Citation70,Citation73]. However, the cytological and morphological data from our observations showed that this feature are not always clearly manifested even in the same culture, and a strain could contain cells with both smooth and humped cell walls. The results were similar after long-term cultivation of the material, with a main trend towards more cells with smooth walls during the cultivation period. This result is in accordance with the note made by Neustupa and Němcová [Citation70, 382] for Vischeria sp. that ‘in culture the presence of angular cells could be quite rare occasion so that the populations are very often indistinguishable from members of genus Eustigmatos’. Similar observations were reported by Safiullina on Eustigmatos magnus, which formed polymorphic cells, sometimes similar to the cells of Vischeria [Citation74]. Thus, the LM observations conducted in our study show the need for re-assessment of the taxonomy of both genera Eustigmatos and Vischeria. This suggestion is in agreement with the paper of Kryvenda et al. [Citation67] unifying both genera under the older name Vischeria.
The cytological and morphological data obtained in our study showed that very few and practically insignificant deviations in the main diagnostic features could be observed after long-term cultivation period (6 and 11 years). This result indicated that such long-term comparison is not really needed for taxonomic purposes and almost immediate identification in cultures is reliable.
According to our LM observations carried out earlier (2007–2009, 2012) and recently (2018), we identified the strains under investigation as belonging to Eustigmatophyceae and to the Eustigmatos/Vischeria group in particular. This was confirmed by molecular-genetic methods based on rbcL sequencing and BLAST analysis. LM identified two of the strains, ACUS 00002 and ACUS 00010, as Eustigmatos magnus and Vischeria stellata with more certainty, which generally coincided with the phylogenetic analysis. The molecular results clearly supported the general similarity observed via LM of both peculiar strains, ACUS 00024 and ACUS 00025, with straight or sigmoid, symmetric or asymmetric elongated cells. They grouped mainly near to Vischeria representatives. Similar, but only symmetric and straight elongated cells were noted as ‘elongated stages’ in ‘aged cultures’ of an unidentified strain of Vischeria [Citation70, 7, 25–27]. The authors outlined the similarity of their material with Vischeria punctata Vischer (originally isolated from soil in Switzerland [Citation75]). However, due to the presence of ‘distinct elongated ellipsoidal stage in the life cycle’ which had ‘not yet been reported in the genus Vischeria’, without careful investigation of cultures, they could not state whether it should be referred to this species or be interpreted as a separate species [Citation70, 383]. Noteworthily, these authors obviously observed also many spherical (globular) cells and autospores with smooth cell walls [Citation70, ]. Because the elongated cells diverse in symmetry, shape and size prevailed in the initial stages of cultivation of both strains ACUS 00024 and ACUS 00025, and still dominated in the cultures maintained for a long time, we believe that this is a stable feature of both strains grouped together in the obtained cladogram. Therefore, we suppose that they should be interpreted as a new species of the genus Vischeria. We also suggest that the peculiar strain ACUS 00104, which contained cells typical of both genera Vischeria and Eustigmatos, but did not fit to any of their species described so far, most probably represented a new taxon. The conducted phylogenetic analysis also showed the separate position of this strain (). Thus, we have to underline the need for further elucidation of the phylogenetic relations and taxonomic position of our original ACUS strains using more genes, genomic regions and genome sequences for more precise affiliation of the studied photosynthetic stramenopiles [e.g. Citation44,Citation67,Citation71]. This is also of practical importance, considering our previous results which proved the accumulation of significant amounts of carotenoids (e.g. lutein, canthaxanthin, astaxanthin) in the studied strains [Citation49], and the recent increase of interest in phycoprospecting based on reliable identification [Citation8–10].
Conclusions
The results of rbcL phylogenetic analysis fully supported the LM identification of the studied original strains of promising commercial importance as carotenoid sources and their proposed affiliation to the Eustigmatos/Vischeria group. On the other hand, the resolution of the present rbcL analysis was not sufficient to affiliate the studied strains to some particular species, which requires their further investigation. Therefore, a multigene phylogenetic study of the aeroterrestrial eustigmatophycean strains of ACUS is currently underway in our laboratory, involving the studied strains and other strains closely related to them based on rbcL analysis. This would pave the way for further research into the strains’ biotechnological potential.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the America for Bulgaria Foundation under Grant AGR.0050.20160121.
References
- Mata TM, Martins AA, Caetano NS. Microalgae for biodiesel production and other applications: a review. Renew Sustain Energy Rev. 2010;14:217–232.
- Yen H-W, Hu I-C, Chen C-Y, et al. Microalgae-based biorefinery-from biofuels to natural products. Bioresour Technol. 2013;135:166–174.
- Mudimu O, Rybalka N, Bauersachs T, et al. Biotechnological screening of microalgal and cyanobacterial strains for biogas production and antibacterial and antifungal effects. Metabolites. 2014;4:373–393.
- Matos J, Cardoso C, Bandarra NM, et al. Microalgae as healthy ingredients for functional food: a review. Food Funct. 2017;8:2672–2685.
- Sathasivam RI, Ki J-S. A review of the biological activities of microalgal carotenoids and their potential use in healthcare and cosmetic industries. Marine Drugs. 2018;16:1–26.
- Bongiovani N, Sanchez-Puerta MV, Popovich C, et al. Molecular and phylogenetic identification of an oil-producing strain of Nanochloropsis oceanica (Eustigmatophyceae) isolated from the south-western Atlantic coast (Argentina). Rev Biol Mar Oceanogr. 2014;49:615–623.
- Chikkaswamy BK, Paramanik RC. Molecular distinction of algae using molecular marker. Int J Curr Microbiol Appl Sci. 2016;5:485–489.
- Minhas AK, Hodgson P, Barrow CJ, et al. The isolation and identification of new microalgal strains producing oil and carotenoid simultaneously with biofuel potential. Bioresour Technol. 2016;211:556–565.
- Wilkie C, Edmundson SJ, Duncan JG. Indigenous algae for local bioresource production: Phycoprospecting. Energy Sustain Dev. 2011;15:365–371.
- Suriya GN, Gaurav K, Sivaji S, et al. Isolation, identification and outdoor cultivation of thermophilic freshwater microalgae Coelastrella sp. FI69 in bubble column reactor for the application of biofuel production. Biocatal Agric Biotechnol. 2018;14:357–365.
- Kreimer G, Kawai H, Muller DG, et al. Reflective properties of the stigma in male gametes of Ectocarpus siliculosus (Phaeophyceae) studied by confocal laser scanning microscopy. J Phycol. 1991;27:268–276.
- Zakrys B, Milanowski R, Empel J, et al. Two different species of Euglena. E. geniculata and E. myxocylindracea (Euglenophyceae), are virtually genetically and morphologically identical. J Phycol. 2002;38:1190–1199.
- Ferroni L, Baldisserotto C, Fasulo MP, et al. Adaptive modifications of the photosynthetic apparatus in Euglena gracilis Klebs exposed to manganese excess. Protoplasma. 2004;224:167–177.
- Škaloud P, Radochová B. Confocal microscopy of the green-algal chloroplast. Czech Phycol. 2004;4:183–190.
- Weatherill K, Lambiris I, Pickett-Heaps JD, et al. Plastid division in Mallomonas (Synurophyceae, Heterokonta). J Phycol. 2007;43:535–541.
- Tang CQ, Leasi F, Obertegger U, et al. The widely used small subunit 18S rDNA molecule greatly underestimates true diversity in biodiversity surveys of the meiofauna. Proc Natl Acad Sci USA. 2012;109:1208–16212.
- Groendahl S, Kahlert M, Fink P. The best of both worlds: A combined approach for analyzing microalgal diversity via metabarcoding and morphology-based methods. PLOS One. 2017;12:1–15.
- Sanger F, Coulson AR. A rapid method for determining sequences in DNA by primed synthesis with DNA polymerase. J Mol Biol. 1975;94:441–448.
- Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467.
- Kim KM, Park J-H, Bhattacharya D, et al. Applications of next-generation sequencing to unravelling the evolutionary history of algae. Int J Syst Evol Microbiol. 2014;64:333–345.
- Medlin LK. Molecular techniques for identification and characterization of marine biodiversity. Ann Mar Biol Res. 2016;3:1015–1023.
- Johansen JR, Shubert LE. Algae in soils. Nova Hedwigia. 2001;123:297–306.
- Uzunov B, Stoyneva MP, Gärtner G. Review of the studies on aero-terrestrial cyanoprokaryotes and algae in Bulgaria with a Checklist of the recorded species. I. Phytol Balc. 2007;13:65–73.
- Gärtner G, Stoyneva MP, Mancheva AD, et al. A new method in collection and cultivation of aerophytic and endolithic algae. Ber Nat Med Verreins Innsbruck. 2010;96:27–34.
- Gärtner G, Uzunov BA, Dimitrova PH, et al. Review of the studies of aeroterrestrial algae along the Bulgarian Black Sea coast (1890–2017) with special attention to the newly described and threatened species. Acta Zool Bulg. 2018;11:53–55.
- Andersen RA, Brett RW, Potter D, et al. Phylogeny of the Eustigmatophyceae based upon 18S rDNA, with emphasis on Nanochloropsis. Protist. 1998;149:171–174.
- Krienitz L, Hepperle D, Stich H-B, et al. Nannochloropsis limnetica (Eustigmatophyceae), a new species of picoplankton from freshwater. Phycologia. 2000;35:219–227.
- Radakovits R, Jinkerson RE, Fuerstenberg SI, et al. Draft genome sequence and genetic transformation of the oleaginous alga Nannochloropis gaditana. Nat Commun. 2012; [cited 2018 Nov 14] 3:686. DOI:10.1038/ncomms1688
- Vieler A, Wu G, Tsai C-H, et al. Genome, functional gene annotation, and nuclear transformation of the heterokont oleaginous alga Nannochloropsis oceanica CCMP1779. PLоS Genet. 2012;[cited 2018 Nov 14]8:e1003064. [25 p.]. DOI:10.1371/journal.pgen.1003064
- Corteggiani Carpinelli E, Telatin A, Vitulo N, et al. Chromosome scale genome assembly and transcriptome profiling of Nannochloropsis gaditana in nitrogen depletion. Mol Plant. 2014;7:323–335.
- Wang D, Ning K, Li J, et al. Nannochloropsis genomes reveal evolution of microalgal oleaginous traits. Plos Genet..[cited 2014; Jan 9]; 10:e1004094. [10 p.]. DOI:10.1371/journal.pgen.1004094
- Fawley MW, Jameson I, Fawley KP. The phylogeny of the genus Nannochloropsis (Monodopsidaceae, Eustigmatophyceae), with descriptions of N. australis sp. nov. and Microchloropsis gen. nov. Phycologia. 2015;54:545–552.
- Hegewald E, Padisák J, Friedl T. Pseudotetraëdriella kamillae: taxonomy and ecology of a new member of the algal class Eustigmatophyceae (Stramenopiles). Hydrobiologia. 2007;586:107–116.
- Prior SW, Fawley MW, Fawley KP. DNA sequence analysis of freshwater Eustigmatophyceae, a potential source of essential fatty acids. J Ark Acad Sci. 2009;63:139–144.
- Přibyl P, Eliás M, Cepák V, et al. Zoosporogenesis, morphology, ultrastructure, pigment composition, and phylogenetic position of Trachydiscus minutus (Eustigmatophyceae, Heterokontophyta). J Phycol. 2012;48:231–242.
- Fawley K, Eliáš M, Fawley M. The diversity and phylogeny of commercially important algal class Eustigmatophyceae, including the new clade Goniochloridales. J Appl Phycol. 2014;26:1773–1782.
- Fawley M, Fawley K. Rediscovery of Tetraëdriella subglobosa Pascher, a member of the Eustigmatophyceae. Fottea. 2017;17:96–102.
- Hibberd DJ. Notes on taxonomy and nomenclature on the algal classes Eustigmatophyceae and Tribophyceae (synonym Xanthophyceae). Bot J Linn Soc. 1981;82:93–119.
- Ettl H, Gärtner G. Syllabus der Boden-, Luft- und Flechtenalgen. Sttutgart: Gustav Fischer; 1995.
- Ettl H, Gärtner G. Syllabus der Boden-, Luft- und Flechtenalgen. 2. Auflage. Berlin: Springer Spectrum; 2014.
- Hofbauer KW. 2015. Class Eustigmatophyceae. In: Frey W, editor. Syllabus of plant families, 13th ed., A. Engler’s Syllabus der Pflanzenfamilien, 2/1 Photautotrophic eukaryotic algae. Stuttgart: Gebr. Borntraeger Verlagsbuchhandlung; 2015. p. 109–117.
- Ott DW, Oldham-Ott CK, Rybalka N, et al. Xanthophyte, eustigmatophyte and raphidophyte algae. In: Wehr JD, Sheath RG, Kociolek JP, editors. Freshwater algae of North America, 2nd ed. San Diego, CA: Academic Press; 2015. p. 483–534.
- Elias M, Amaral R, Fawley KP, et al. Eustigmatophyceae. In: Archibald JM, Simpson JM, Alastair GB, et al. editors. Handbook of the protists. Cham: Springer International publishing AG; 2016.
- Ševčíková T, Klimeš V, Zbránková V, et al. A comparative analysis of mitochondrial genomes in Eustigmatophyte algae. Genome Biol Evol. 2016;8:705–722.
- Trzcińska M, Pawlik-Skowrońska B, Krokowski D, et al. Genetic and morpholofical characteristics of two ecotypes of Eustigmatos calaminaris sp. n. (Eustigmatophyceae) inhabiting Zn- and Pb-loaded calamine mine spoils. Fottea. 2014;14:1–13.
- Rampen SW, Datema M, Rodrigo-Gámiz M, et al. Sources and proxy potential of long chain alkyl diols in lacustrine environments. Geochim Cosmochim Acta. 2014;144:59–71.
- Norton TA, Melkonian M, Andersen RA. Algal biodiversity. Phycologia. 1996; 35:308–326.
- Margulis L, Chapman A. Kingdoms and domains. 4th ed. An illustrated guide to the Phyla of life on Earth. [place unknown]: Elsevier; 2009.
- Stoyneva-Gärtner MP, Stoykova P, Uzunov B, et al. Carotenoids in six aeroterrestrial strains from Vischeria/Eustigmatos group: updating the pigment pattern of Eustigmatophyceae. Biotechnol Biotechnol Equip. 2019; Forthcoming.
- Ramasamy D, Mishra AK, Lagier J-C, et al. A polyphasic strategy incorporating genomic data for the taxonomic description of novel bacterial species. Int J Syst Evol Microbiol. 2014;64:384–391.
- Das S, Dash HR, Mangwani N, et al. Understanding molecular identification and polyphasic taxonomic approaches for genetic relatedness and phylogenetic relationships of microorganisms. J Microbiol Meth. 2014;103:80–100.
- Vandamme P, Pot B, Gillis M, et al. Polyphasic taxonomy, a consensus approach to bacterial systematics. Microbiol Rev. 1996;60:407–438.
- Zapomělová E, Jezberová J, Hrouzek P, et al. Polyphasic characterization of three strains of anabaena reniformis and aphanizomenon aphanizomenoides (cyanobacteria) and their reclassification to Sphaerospermum gen. nov. (incl. anabaena kisseleviana)(1). J Phycol. 2009;45:1363–1373.
- Moustaka-Gouni M, Kormas KA, Polykarpou P, et al. Polyphasic evaluation of Aphanizomenon issatschenkoi and Raphidiopsis mediterranea in a Mediterranean lake. J Plankt Res. 2010;32:927–936.
- Zapomělová E, Skácelová Pumann P, et al. Biogeographically interesting planktonic Nostocales (Cyanobacteria) in the Czech Republic and their polyphasic evaluation resulting in taxonomic revisions of Anabaena bergii Ostenfeld 1908 (Chrysosporum gen. nov.) and A. tenericaulis Nygaard 1949 (Dolichospermum tenericaule comb. nova). Hydrobiologia. 2012;698:353–365.
- Komarek J, Kaštovský J, Mareš J, et al. Taxonomic classification of cyanoprokaryotes (cyanobacterial genera) 2014, using a polyphasic approach. Preslia. 2014;86:295–335.
- Rott E, Pentecost A, Mareš J. 2018. Introduction: recent developments in cyanobacterial research with special reference to aquatic habitats, molecular ecology and phylogenetic taxonomy. Hydrobiologia. 2018;811:1–6.
- Aguilera A, Gómez EB, Kaštovský J, et al. The polyphasic analysis of two native Raphidiopsis isolates supports the unification of the genera Raphidiopsis and Cylindrospermopsis (Nostocales, Cyanobacteria). Phycologia. 2018;57:130–146.
- Pröschold T, Leliaert F. Systematics of the green algae: conflict of classic and modern approaches. In: Brodie J, Lewis JM, editors. Unravelling the algae: the past, present, and future of algal systematics. Boca Raton, FL: CRC Press; 2007. p. 123–153.
- Škaloud P. Polyphasic approaches in the taxonomy of green aerophytic algae [dissertation]. Prague: Charles University; 2008.
- Kawasaki Y, Nakada T, Tomita M. Taxonomic revision of oil‐producing green algae, Chlorococcum oleofaciens (Volvocales, Chlorophyceae), and its relatives. J Phycol. 2015;51:1000–1016.
- Saber AA, Fučíková K, McManus HA, et al. Novel green algal isolates from the Egyptian hyper-arid desert oases: a polyphasic approach with a description of Pharao desertorum gen. et sp. nov. (Chlorophyceae, Chlorophyta) ). J Phycol. 2018;54:342–357.
- Uzunov BA. Aeroterrestrial algae from Pirin Mountain (Bulgaria) [dissertation]. Innsbruck: University of Innsbruck; 2009.
- Mancheva AD. [Investigation of aerophytic algae from the natural landmark Belogradchik rocks] [dissertation]. Sofia (Bulgaria): Sofia University “St Kliment Ohridski”; 2013. Bulgarian.
- Stoyneva MP. Contribution to the knowledge on the biodiversity of hydro- and aerobiontic prokaryotic and eukaryotic algae in Bulgaria [D.Sc.thesis]. Sofia: Sofia University “St Kliment Ohridski”; 2014. Bulgarian.
- Ott D, Oldham-Ott CK. Eustigmatophyceae, Raphidophyceae, and Tribophyceae. In: Wehr JD, Sheath RG, editors. Freshwater algae of North America. San Diego (CA): Academic Press; 2003. p. 423–469.
- Kryvenda A, Rybalka N, Wolf M, et al. Species distinctions between closely related strains of Eustigmatophyceae (Stramenopiles) emphasizing ITS2 sequence-structure data: Eustigmatos and Vischeria. Eur J Phycol. 2018; [cited 2018 Sep 06] [22p.]; DOI:10.180/09670262.2018.1475015
- Tamura K, Dudley J, Nei M, et al. MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol. 2007;24:1596–1599.
- Pascher A. Heterokonten. Leipzig: Akad. Verlagsges; 1939.
- Neustupa J, Němcová Y. Morphological and taxonomical study of three terrestrial eustigmatophyceans species. Nova Hedwigia. 2001;123:373–386.
- Yang EC, Boo GH, Kim HJ, et al. Supermatrix data highlight the phylogenetic relationships of photosynthetic stramenopiles. Protist. 2012;163:217–231.
- Muratova KR. [Life cycle and zoosporogenenis of the microscopic alga Eustigmatos polyphem (Pitchmann) Hibberd (Eustigmatophyta)], [dissertation]. UFA: Bashkir State Pedagogik University; 2012.Russian.
- Hibberd D. J., et al. Phylum Eustigmatophyta. In: Margulis L, Corliss JO, Melkonian M, editors. Handbook of protoctista. Boston (MA): Jones and Bartlett Publishers; 1990. p. 326–333.
- Safiullina LM. 2009. [The morphological variability of the soil algae Eustigmatos magnus (B. Petersen) Hibberd (Eustigmatophyta) under the influence of environmental factors] [dissertation]. UFA: Bashkir State Pedagogical University; 2009. Russian.
- Vischer W. Heterokonten aus alpinen Böden, speziell dem schweizerischen Nationalpark. Ergebn. wiss. Unters. Sweiz. Nationalpark, N. F. 1945;1:477–512.