abstract
Today’s global problems and challenges have given rise to a new field of interest – bioeconomics. It is strongly related to phycoprospecting, or searching for specific algal strains of commercial importance. There has been growing interest in the small algal class Eustigmatophyceae in recent years. These microscopic stramenopilic algae, which have all the advantages of microalgal cultivation, have proved to be promising commercial sources of valuable compounds (e.g. carotenoids, unsaturated fatty acids, amino acids) in aquaculture, agriculture, biofuels production, medicine, pharmaceutics, cosmetics, wastewater treatment, environmental control, etc. The present review shows the main genera and strains of commercial importance, outlines their main fields of application and some gaps in our knowledge in this aspect. Today, the great promising bioeconomical potential of these algae has generally been recognized, but in the present state of its infancy, it is far from being fully exploited.
Introduction
Many problems and challenges in today’s world, and the shortage of natural resources in particular, have led to the emergence of a new field of interest that seeks to integrate and bridge economics and biology: bioeconomics [Citation1]. Despite that its exact definition is yet disputable, bioeconomics is enlarging its scope owing to the persistent transfer of ideas from economics to biology and vice versa. It already includes ‘renewable biological resources and their conversion into food, feed, bio-based products and bio-energy’ [Citation2]. The search for specific algal strains of commercial importance, or phycoprospecting [Citation3] (sometimes referred to by the broader term bioprospecting [Citation4]), has become one of the most modern branches of recent studies related to the increasing interest in the search for sustainable technologies and mitigating the economic costs. Microscopic algae (microalgae), most of which are highly adaptable phototrophs, do not compete with other sources, like plants which are used for food [Citation5, Citation6], and produce high amounts of valuable compounds, thus attracting considerable interest for biotechnological production of a broad range of products from fuel to pharmaceuticals, functional foods, nutraceuticals, pigments, etc. [Citation7, Citation8]. Initially, the focus was on the best-known groups: blue-green algae (Cyanobacteria/Cyanoprokaryota) and green algae (Chlorophyta and Streptophyta) and they were much better explored. Although the bioeconomical interest in the small stramenopilic algal class Eustigmatophyceae has been growing in recent years, data have not been summarized. The present review shows the main genera and strains of commercial importance, outlines their main fields of application and some gaps in our knowledge in this aspect.
Carotenoids of Eustigmatophyceae
The bioeconomical interest in the microscopic algae of the small stramenopilic class Eustigmatophyceae raised with the discovery of their potential for high extraplastidal carotenoid production. It could be traced back to the paper by Antia and Cheng [Citation9], who studied the marine Nannochloropsis oculata (Droop) Hibberd and provided ‘the first documented evidence of eustigmatophycean production of astaxanthin (free and monoesterified) and astacene in significant amounts, indicating the capacity of this algal type to synthesize the highest oxidation level of four keto-carotenoids known from the algal kingdom, and hitherto found only in the Chlorophyceae, Euglenophyceae and Dinophyceae’ [Citation9, p.47]. This finding was extremely important because all mentioned algal classes contained generally different esterified forms of astaxanthin (AsX) [e.g. Citation10].
Further studies showed that the interest in Nannochloropsis (and in the derived genus Microchloropsis) lay in the availability of a range of valuable pigments (e.g. chlorophyll a, canthaxanthin, zeaxanthin and AsX) in contrast with the green genus Haematococcus, commercially cultivated for its capacity to accumulate AsX [Citation11]. Afterwards, AsX was found in Vischeria (Syn. Eustigmatos) ([Citation12] and references therein). In two Vischeria strains AsX reached 9 and 13% of total carotenoid content showing their commercial potential [Citation12]. Algae are the primary source of this red-coloured pigment (long popular as ‘haematochrom’) in the aquatic food chains and its invaluable role as a dietary supplement and food or feed additive intended for human, animal and aquaculture consumption is known worldwide. Its primary use today is as an animal feed additive to impart coloration, including farm-raised salmon, shrimps, crabs and chicken egg yolks [e.g. Citation13–15]. In the European Union (EU), AsX is considered a food dye with the E number E161j [Citation16]. In the United States, as a food colouring (or colour additive) it has been approved only for specific uses in animal and fish foods [Citation17, Citation18] despite that the plant-derived AsX in particular achieved a status of generally recognized as safe (GRAS), meaning that it can be sold as a dietary supplement [Citation19]. Its main benefits explored in medicine are due to its strong antioxidant activity, which gives it its anti-inflammatory and anti-cancer properties together with skin and eye care potential and possibility to enhance the immune response [Citation14, Citation20, Citation21].
The rich palette of carotenoid pigments in different genera of Eustigmatophyta [Citation12] logically led to the recent studies of their commercial biotechnological potential. Six species of genus Vischeria were reported as possible novel sources of natural β-carotene due to their considerable production in bubble column and flat panel photobioreactors (100 and 470.2 mg L−1, respectively) [Citation22, Citation23]. β-Carotene is best known for its provitamin A activity function and strong stimulatory effect on the immune system. It is now of increasing demand and has extensive applications [Citation22, Citation23]. As AsX, it is broadly used as a colorant and feed additive (E160a [Citation24]), antioxidant, heart-preventive and anticancer agent in the food and aquaculture, pharmaceutical and cosmetics industries [Citation25]. Presently, the primary algal source of β-carotene is the green microalga Dunaliella salina. However, researchers have yet to achieve high β-carotene concentration in the total low biomass yield (1.5–2 g L−1) under harsh conditions of high salinity, high light intensity (>500 µmol photons m−2 s−1) and low temperature [Citation22]. To date, the β-carotene content in the studied Vischeria strains is lower than that in Dunaliella (up to 7% of the dry weight vs. 10%, respectively), although Li et al. [Citation22] believed that it could be increased with improvement of the culture conditions. Further, some eustigmatophyceans (and particularly Vischeria stellata) accumulate higher biomass than Dunaliella and therefore, considering their high intracellular β-carotene content, may be more promising as future natural sources of this pigment. Similar results for V. stellata were obtained by Gao et al. [Citation26].
Apart from the above-mentioned carotenoids, eustigmatophyceans contain violaxanthin (ViX) as one of their primary photosynthetic pigments (for details see [Citation12]). Up to now this red-coloured pigment is sometimes used as a food colourant under the general E number E161e but it is not yet approved for use as a food additive in the EU or the USA [Citation24, Citation27]. ViX has demonstrated strong radical scavenging activity, valid inhibition of lipid peroxidation and red blood cell hemolysis, anti-proliferative, anti-inflammatory and proapoptotic activity against human cancer cell lines in vitro, which indicated that this pigment has great potential to be widely applied in healthcare and medical products [Citation28, Citation29].
Lutein (Lut) is another carotenoid pigment which was relatively recently found in Eustigmatophyta [Citation12]. It accounts for 13–25% of the total carotenoid content in five strains of Vischeria. Therefore, the authors suggested their possible role as its commercial sources considering that this pigment is a high-value product with extensive applications in feed, food, nutraceutical and pharmaceutical industries [Citation12]. Due to its yellow-red colour, it has primarily been used as a colourant in food and supplement manufacturing. As a food additive under the E number E161b, Lut is approved for use in the EU [Citation24], where in animal regulations it is specifically allowed for cats and dogs [Citation16]. In the USA, it was firstly restricted to animal feed, especially for chicken, where it shows up in the colour of the skin and egg yolks, but more recently, crystalline Lut achieved GRAS-status and was allowed for use as an ingredient in milk-based meal replacements [Citation30]. Humans and animals cannot synthesize Lut and can naturally obtain it by ingesting plants. Despite controversial opinions on its exact role and even underestimation and underappreciation by clinicians and vision researchers, recent studies show that dietary supplements containing Lut reduce progression of age-related macular degeneration and cataract formation, enhance curation of other ocular diseases and support the functions of normal eyes [Citation31–37]. Moreover, it was extensively reported that consumption of food rich in Lut is associated with lower incidence of cancer and cardiovascular diseases [Citation37].
Lut is isomeric with zeaxanthin (ZeX), which is commonly synthesized in higher plants, but has been documented also in algae and in Eustigmatophyta in particular [Citation12]. It amounted to 8–11% of the total carotenoid content of five Vischeria strains [Citation12]. Since the concentration of ZeX in the macula, together with its functions and effects, are quite similar to those of Lut [Citation32, Citation37], it could be supposed that eustigmatophyte algae have commercial potential in future dietary, health-care and medical products. At present, ZeX is being obtained from other plant sources and from the aquatic blue-green algal genus Spirulina [Citation38]. Therefore, it sounds very promising to seek for novel ZeX sources, especially among aeroterrestrial stramenopilic algae. The great potential of eustigmatophyceans as commercial sources of a wide range of carotenoids has been already outlined [Citation12].
The valuable eustigmatophycean carotenoids include also canthaxanthin (CaX) [Citation12], which was firstly isolated from the edible mushroom Cantharellus cibarius. This pigment is known mainly as a food additive for farmed salmon in environments where AsX sources are not available, or is used in combination with AsX [Citation39]. CaX gives farmed salmon a colour similar to pink/red species of wild salmon and could be consequently transferred to humans. Ingested in the human body, despite its subsequent low concentrations, CaX can serve as a carotenoid source alternative to the use of synthetic dietary supplements [Citation40]. In the USA, it is approved as food additive for solid, semisolid and liquid food, and for feed for salmonids and broiler chicken as well [Citation18]. In the EU, under E number E161g [Citation16, Citation24], it is allowed as additive to trout, salmon and poultry feed and is used in broiler chicken feed to enhance the yellow colour of chicken skin. CaX affected positively the diet of representatives of 12 trout families and the commercial diet of the shrimp Penaeus monodon, enhanced by adding cholesterol [Citation41–43]. CaX serves as a potent lipid-soluble antioxidant in animal tissues, including broiler meat and the chick embryo [Citation44–46]. In the egg, the pigment is allocated from the yolk to the developing embryo probably to serve as protection against oxidative damage, especially during the sensitive periods of hatching and early posthatch life [Citation45, Citation46]. The supplementation of broiler breeder diets with CaX improved the hatchability rate, fertility, and reduced the thiobarbituric reactive substances in eggs and embryo mortality [Citation47]. Despite some controversial data on its role in human chronic diseases, CaX has received more attention and has been extensively studied as a component in tanning pills and creams, for its anti-cancer, anti-tumor and anti-dermatosis properties and its very strong modifying effect with respect to the dynamic and structural properties of lipid membranes [Citation48–50]. CaX and other carotenoids could be utilized as chemosensitisers, especially as adjuvants in chemotherapy [Citation51].
Chlorophylls and chlorophyllins in Eustigmatophyceae
Chlorophyll a is the main photosynthetic pigment of Eustigmatophyceae. Although its high amounts and combinations with different chlorophyllins are well-documented [Citation12, Citation52], its application in human activities is still poorly investigated. The health benefits of chlorophyll like detoxication, antioxidant effects, boosting of the immune system, wound healing, weight loss, skin healing and anti-cancer activity are well-known [Citation53, Citation54], and many products of blue-green and green algae (e.g. Aphanizomenon, Spirulina and Chlorella) are broadly used due to its high content and have already achieved high public awareness. Since chlorophyll is registered as a food additive (colourant) under E number E140 [Citation24], its usage from fast-growing microscopic eustigmatophycean algae could be strongly proposed. Chlorophyll is used as food and beverage colouration [Citation55, Citation56].
Vitamins and use of Eustigmatophyceae as food for humans and in aquacultures
Tocopherols (vitamin E) and especially their most abundant form - α-tocopherol - are among the most valuable healthcare products due to their strong antioxidant activity in vivo and their capability to prevent light-induced pathologies of skin and eyes, or degenerative disorders like the socially significant atherosclerosis, cardiovascular diseases and cancer [Citation57]. All tocopherols are synthesized only by photosynthetic organisms. Therefore, seeking for low-cost plant sources of tocopherols is of primary importance. Originally, tocopherols were considered as dietary factor in animal breeding with a positive effect on the reproduction and survival due to improved resistance to stress and diseases [Citation57]. Later, owing to its positive effects, α-tocopherol, under E number E307, was approved as a dietary supplement for humans [Citation24]. Research has demonstrated the significant potential of the marine eustigmatophyte Nannochloropsis oculata (fondly named ‘marine chlorella’) as an α-tocopherol rich source of lower production costs for mariculture [Citation57]. In this species, the bioaccessibility of tocopherols was higher than those of β-carotene and lycopene; it was also higher in comparison with their availability from the diatom Chaetoceros [Citation58]. The experiments under different culture conditions depending on nitrogen source, concentration and growth phase showed the potential to increase the tocopherol content in the studied microalgae, thus proving their importance for large-scale production [Citation57].
In addition to vitamin E, eustigmatophyceans are rich in vitamins B, C, D, and K. This, in combination with their small dimensions and absence of tough cell wall makes eustigmatophyceans easily assimilated by larval animals [Citation59]. The same authors proposed a strain of Monodus subterraneus, grown at relatively high temperature (25–30 °C) as a commercial planktonic feed for tropical aquacultures. Microalgae are commonly required for larval nutrition during a brief period, either for direct consumption in the case of molluscs and peneid shrimp or indirectly as food for the live prey fed to small fish larvae. Despite the advantages of live microalgae in aquaculture, there was a trend to avoid using them due to their high cost and the difficulty in producing, concentrating and storing them [Citation60]. However, the marine Nannochloropsis has been one of the commonly used microalgae in aquacultures [Citation61–64]. Microchloropsis gaditana (Syn. Nannochloropsis gaditana) was considered to be a ‘premium’ food for rotifers [Citation65]. Similar results on the growth rate and composition of 12 marine species from different taxonomic groups as food for Brachionus plicatilis, subsequently used as food for marine fish (mainly Pagrus major), indicated Nannochloropsis sp. (KMMCC-33) as ‘the best microalgal species for the mass culture of the rotifer’ [Citation66]. An alternative method of culturing N. oculata by using green water from red tilapia (Oreochromis sp.) culture system as a fertilizer instead of conventional mediums and fertilizer has been proposed [Citation67].
An effective approach for transferring cDNA of the fish growth hormone (GH) into N. oculata was developed by Chen et al. [Citation68]. The transgenic microalgae were given as food to artemia, which was subsequently used as food for red-tilapia larvae. The results on significantly greater growth of larvae fed by artemia incubated with transgenic microalgae (316% in weight gain and 217% in body length increase versus 104% and 146% respectively in larvae fed by artemia incubated with nontransgenic microalgae) proved the species as a potential good bioreactor material for producing foreign protein, which could be of benefit for both pharmacological and agricultural industries [Citation68]. The authors strongly suggested that, for humans, the consumption of nontransgenic fish fed temporarily on GH-transgenic algae is much safer than direct consumption of GH-transgenic fish, especially when transgenic algae are applied under controlled conditions outdoors.
Lipids (fatty acids and sterols) of Eustigmatophyceae
Considering the broad use of N. oculata as basic food in aquacultures, Patterson et al. [Citation69] studied the sterols and fatty composition of five other marine eustigmatophyceans with the idea to find better algal diet for improved productivity of oyster fisheries. This study was conducted 20 years after the first report of lipids (mainly sterols) in the eustigmatophyte Monodus subterraneus by Mercer et al. [Citation70]. The authors proved the presence of free sterols (mainly cholesterol, and 24-ethylcholesterol and isofucosterol in smaller amounts) in all studied strains, the palmitic acid (16:0) as a major fatty acid in four of the strains and omega-3 eicosapentaenoic acid (EPA; 20:5n-3) as the major fatty acid in two of the strains. The strain Sticho-0-18, rich in EPA as a major fatty acid and in sterols (25.77% of the dry weight, or 0.86 pg/cell), was proposed as the best eustigmatophyte strain for oyster food [Citation69]. A recent study of the sterol content of N. oculata indicated that it might be useful as a potential source of natural anti-inflammatory and anti-cancer compounds [Citation71].
Before the work by Patterson et al. [Citation69], half of the strains studied by them had been proved as rich in long-chain polyunsaturated fatty acids (PUFAs) [Citation72, Citation73]. PUFAs contain essential fatty acids (EFAs) and participate in many metabolic processes, playing an important role in the life and death of cardiac cells. PUFAs also reduce the blood cholesterol, thus reducing the morbidity risks of coronary heart-diseases and helping in the prevention of hypertension, diabetes Type II, many ocular diseases, arthritis and cystic fibrosis [Citation74–79]. Low EFAs levels, or wrong balance of types among them, could be a factor in a number of illnesses, including osteoporosis [Citation80]. Globally, the primary source for PUFAs and EFAs are fish. However, fish cannot synthesize them but obtain them via the aquatic food chains with algae at the basis. Yet, the application of fish oil as food additive is limited due to problems associated with its typical fishy smell, unpleasant taste and poor oxidative stability, together with inapplicability for certain purposes because of the presence of mixed fatty acids [Citation60]. In addition, it has to be stressed that many fish also accumulate pollutants which change the odour and composition of the extracted oils and that fish are considered declining resources [Citation81]. Therefore, considering the increased interest in these fatty acids for human consumption, in order to meet the demands of the expanding market, it is essential to search for novel algal sources. Studies have shown a high content of EPA in marine Nannochloropsis (up to 44% of the total fatty acid content - TFA) and its potential application for human diet [Citation66, Citation82–86]. Research has vastly concentrated on testing the optimal growth conditions, of environmental conditions and on the effects of nutrients and nutrient starvation for obtaining the maximum EPA yield in different types of reactors [Citation59, Citation84, Citation85, Citation87–105]. Most reports show that higher EPA yield could be achieved via the so-called ‘physiological forcing’, which is based on the development of a set of conditions to maximize the production of a desired biochemical [Citation106]. Successful manipulation of the PUFA and EPA content in the same alga is possible using other techniques (random mutagenesis, ultraviolet mutagenesis, etc.) [Citation8, Citation107–111].
Apart from the marine species, high content of EPA has been demonstrated in the freshwater and aeroterrestrial eustigmatophyceans of genera Monodus (up to 49.3% TFA), Vischeria, Ellipsoidion (18.8% TFA) and Trachydiscus [Citation26, Citation59, Citation90, Citation93, Citation112–121]. Trachydiscus minutus was considered as nearly a top-producer of EPA among microalgae with its 10–36% d.w. content and high productivity of 88 mg L−1 per day (for details see [Citation81]). Similar to the studies of EPA in marine algae, most of the authors applied different culture designs and conditions for obtaining a maximum EPA yield from the freshwater and aeroterrestrial species (e.g. [Citation26, Citation112]).
In this review, the fatty acids found in smaller amounts are not discussed except the mentioning of docosahexaenoic acid (DHA, 22:6n-3) as recorded in the genera Nannochloropsis and Vischeria, and the unusually high content of myristic (14:0) acid (20–54%) in Trachydiscus [Citation66, Citation81, Citation94, Citation114, Citation115, Citation119–125]. The general similarity of PUFA composition in both marine and freshwater eustigmatophyceans has led to the suggestion that freshwater species show potential for use in aquacultures [Citation115]. The freshwater Nannochloropsis limnetica had significantly higher long-chain PUFA content in comparison with green freshwater planktonic algae [Citation122]. Like in other studies, the content of PUFA was highly variable depending on culture conditions with highest concentrations found in non-aerated suspension cultures rich in phosphates. Therefore, N. limnetica was recommended as a high-quality food resource in aquacultures, where it could be used as direct food for rotifers or as an alternative supplement to replace the traditional fish-based food in aquacultures [Citation122]. The same authors stressed on the possibility to use N. limnetica also as an alternative to fish oil produced from fatty fish, which is widely used in human medicine to reduce the risk of myocardial illnesses.
Eustigmatophyceae as potential source for biofuels
The fatty acids and other lipids of Eustigmatophyceae have become a focus of studies due to increased interest in biofuel production from next generation alternative sources [Citation126]. In this respect, Pilatova [Citation81] provided the first review on fatty acid distribution in Eustigmatophyta alongside with the fatty acid profiles and the above-mentioned PUFAs. The studies were oriented towards the finding of prospective oleaginous species, rich mainly in neutral lipids (and triacylglycerol, or TAG, in particular) and the evaluation of the effects of culture growth conditions on the lipid productivity and fatty acid composition [Citation81, Citation126, Citation127]. The study of the neutral lipids in marine Nannochloropsis [Citation73, Citation128, Citation129] led to the discovery of unusual C30 – C32 1, 15 alkyl-diols and monosaturated C32 1, 15 diol. These long-chain alcohols are significant constituents of the lipids of marine Eustigmatophyceae [Citation129]. Therefore, their identification in three freshwater species of the genus Vischeria is also of interest [Citation129]. In addition to these unusual diols and n-alcohols in marine Nannochloropsis, Gelin et al. [Citation130] detected small amounts of C28 – C34 monohydroxy fatty acids both in free and bounded form, which suggests that these microalgae can be potential sources of such fatty acids. Besides the already mentioned EPAs, palmitic and palmitoleic acid, eustigmatophyceans also contain other fatty acids in lesser amounts, for example, arachidonic acid (20:4n-6) and various C18 acids, amongst which oleic acid (18:1) – for example [Citation73, Citation126, Citation131]. Apart from the most studied aquatic halotolerant genus Nannochloropsis [Citation132–134], Zhang et al. [Citation135] found a significantly high lipid content (60.59% d.w.) in the soil eustigmatophyte Vischeria polyphem (Syn. Eustigmatos polyphem) and proposed it as a novel oleaginous alga for biodiesel production due to its high growth and biomass rate. Another novel oleaginous alga, also promising for biodiesel production is Vischeria stellata with total lipid content of about 56% with 52% neutral lipids [Citation26]. Despite all widely known 'theoretical' advantages, generally, based on the maturity of current technology, the true potential of microalgae biofuel towards energy security and its feasibility for commercialization are still questionable. A lot of research is required to bring current microalgae biofuel research to a new dimension and consequently, to revolutionize the entire microalgae biofuel industry towards long-term sustainability [Citation136].
A promising line of research into microalgae biofuel could be marine microalgae. For example, screening of 96 strains of marine microalgae from different groups, outlined the eustigmatous genus Nannochloropsis as the most promising for biodiesel feedstock [Citation137]. Similar results have been obtained after screening of 175 microalgal strains from 21 classes of 7 phyla [Citation7]. Some studies have suggested Nannochloropsis for large-scale biodiesel production [Citation138, Citation139]. Nannochloropsis has become recognized as a model organism to obtain lipids owing to the combination of easy growth at a large scale, high lipid content, new genomic information and innovative genetic transformation techniques [Citation127, Citation140]. As summarized by [Citation8], to exploit the ability of Nannochloropsis to generate special lipids, a great deal of effort has been put into omics studies like genome sequencing, transcriptomic and lipidomic analyses [Citation141–146]. The development of molecular biology tools that enable the generation of Nannochloropsis transformants is increasing [Citation147–149]. The coordination of omics data and functional analyses of key genes related to lipid biosynthesis would yield important information on algal lipid metabolism that is expected to form the basis for future metabolic engineering techniques (for details see [Citation8]).
Eustigmatophyceae for cosmetics, medicine, pharmaceutics and healthcare products
In some cases, whole biomass and residual ‘cake’ after oil extraction may be further utilized as sources of other end products of interest, for example protein, essential amino acids and other nutrients for animal feed. Microalgae have long been recognized as a potential protein source for nutrition applications, but only a few microalgae (e.g. Spirulina, Chlorella and Dunaliella) have been commercially exploited. The interest in the total nutrient content of microalgae is rising due to their richness in macronutrients as a potential future food source preserving natural resources [Citation86, Citation150]. This applies to Eustigmatophyceae as well, whose total nutrient biomass profiles, nutrient bioavailability and safety are subject of investigations [e.g. Citation84, Citation86, Citation150]. Thus algae-rich diets (including those based on Nannochloropsis) are well accepted, well tolerated and suitable for the maintenance of body weight and normal organ function at least in experimental animals (for details see [Citation86]). Another study [Citation150] showed that N. granulata has high content of essential amino acids and of leucine, lysine and tryptophan in particular, non-essential amino acids and crude protein (ca 33% in the whole alga and ca. 40% in lipid extracted algae) together with rich elemental composition of different minerals and trace elements (with highest content of Zn). Studies of total nutrients have also become oriented towards the effects of enriched growth media and cultivation time on nutritional composition [e.g. Citation85].
Microalgae are a source of vitamins, pigments, proteins and other substances beneficial for the skin, but only a few microalgae species are consolidated in the skin care market, wherein the principal ones are the green Chlorella and blue-green Spirulina [Citation151]. However, currently Nannochloropsis is enlisted together with them as ‘traditionally used’ in skin-protection care [Citation152]. The application of Nannochloropsis in cosmetics and cosmeceutics rapidly increases due to its lipid (and especially high PUFA) content and due to the tanning effects of canthaxanthin [Citation49, Citation50]. Extracts of N. oculata and Microchloropsis gaditana could act as an optimum protective sheath against oxidative stress and positively influence collagen synthesis [Citation151, Citation153]. An ingredient of N. oculata with excellent skin elasticity properties and skin-tightening effects (short and long-term) was launched by Pentapharm (Basel, Switzerland) [Citation50, Citation60, Citation151, Citation154]. In future, broader use of Eustigmatophyceae as part of the composition of face and skin care products with anti-ageing, refreshing/regenerating, thickening and anti-irritant properties is to be expected.
The comparative study of antioxidant properties and total phenolic content of various extracts of a diatom, Chaetoceros sp., and Nannochloropsis sp. showed a higher antioxidant capacity of the diatom in comparison with the eustigmatophyte extracts [Citation155]. Different solvent extracts contained different antioxidant capacities in terms of reducing and radical-scavenging power and the correlation between the antioxidant properties and the total phenolic content was not significant, indicating that phenolic compounds might not be a major source of the antioxidant properties found in these two microalgae [Citation155].
A recent study reported a new freshwater eustigmatophyte Forest Park Isolate 5 (FP5), which is able to shift the absorption of chlorophyll a to >705 nm and to grow under solely far-red light [Citation156]. A new case of natural engineering with a novel lineage of endosymbiotic bacteria in eustigmatophyceans was discovered [Citation157]. These authors also found a six-gene operon that possibly encodes a pathway which involves metabolites from both organisms resulting in an isoprenoid–cyclitol-derived compound, probably with antimicrobial or other protective activity. Such new findings give reason for new views and future prospects for the commercial applicability of Eustigmatophyceae.
Considering the increasingly recognized role of microalgae in health and disease prevention [Citation158], the advancement of omics technologies in personalized medicine has spanned over to Eustigmatophyceae as well. They have been used in the improvement of protein extraction protocols, allowing even deeper mining of the proteomes [Citation159, Citation160]. This, along with the lack of toxic records from Eustigmatophyceae (e.g. [Citation86, Citation161]), highlights that this field of potential applications deserves more attention, apparently having been underestimated in comparison with the studies on biofuel and carotenoid production.
Eustigmatophyceae in environmental control
In an environmental aspect, Nannochloropsis was enlisted among promising biocontrol agents of mosquitos [Citation162, Citation163] and marine algae tested for CO2 sequestration [Citation152, Citation164, Citation165]. Eustigmatophyceae could also be applicable in water purification and wastewater treatment according to some successful experiments on their cultivation using different types of wastewaters [Citation67, Citation165–167].
Summary of topics and time-span development of the studies related to the bioeconomical potential of eustigmatophyceae
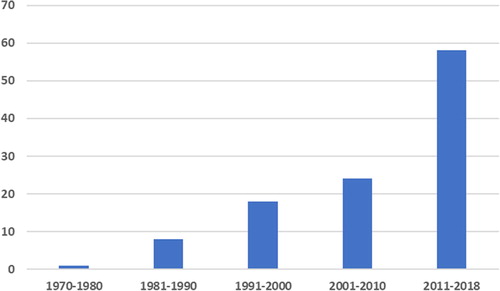
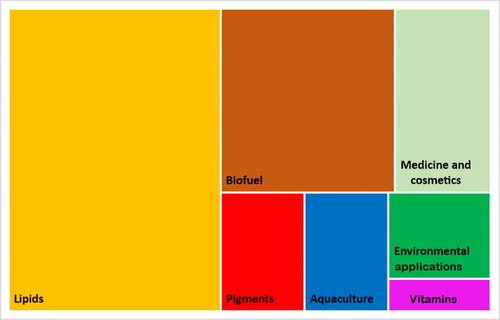
During work on this review, we found 109 scientific papers concerning valuable compounds and different applications of Eustigmatophyceae in human affairs ( and ). There is a general trend towards increasing the number of publications on this topic in the last four decades (). More than 50% of the works were published after 2011, with a twofold increase in the number of papers published in the period 2011–2018 in comparison with the previous decade (59 and 24 papers, respectively). A closer look at the main focus of these papers reveals that most of them are related to studies on lipid content and biofuels (), while other topics have received much less attention so far. The least exploited topic is that of the application of eustigmatophyceaen phytohormones, which had been studied only in Nannochloropsis with finding of abscisic acid and cytokinin as promising for biotechnological purposes [Citation168].
Conclusions
Eustigmatophyceans have all the microalgal advantages of cultivation as a superior feedstock (effective land and CO2 utilization, hundred times higher growth rate in comparison with terrestrial plants and possibility to double their biomass in a day, self-purification if coupled with wastewater treatment) in combination with exceptionally high adaptability. They do not compete with other sources and contain a great palette of valuable bioactive compounds, which could be used by humans separately, or combined in different products. Still, many aspects of the applied research on eustigmatophyceans remain underexplored or yet awaiting to be exploited (e.g. their potential as biofertilizers, as producers of antibiotic metabolites in the pharmaceutical industry, or as compounds in molecular cooking). Therefore, we have to state that the great promising bioeconomical potential of these stramenopilic microalgae has generally been recognized, but in the present state of its infancy is far from being realized to its full potential.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the America for Bulgaria Foundation under Grant AGR.0050.20160121.
References
- Zawojska A, Siudek T. Bioeconomics as interdisciplinary science. Proceedings of the 2016 International Conference “Economic science for rural development” 41; 2016 April 21–22; Jelgava, LLU ESAF; 2016. p. 273–280.
- European Commission Innovating for sustainable growth: A Bioeconomy for Europe. Luxembourg: Publication Office of the European Union. 2012;64.
- Wilkie C, Edmundson SJ, Duncan JG. Indigenous algae for local bioresource production: Phycoprospecting. Energy Sustain Dev. 2011;15:365–371.
- Senhorincho GNA, Laamanen CA, Scott JA. Bioprospecting freshwater microalgae for antibacterial activity from water bodies associated with abandoned mine sites. Phycologia. 2018;57:432–439.
- Patil V, Tran KQ, Giselrød HR. Towards sustainable production of biofuels from microalgae. Int J Mol Sci. 2008;9:1188–1195.
- Khan S, Siddique R, Sajjad W. Biodisel production from algae to overcome the energy crisis. Hayyati J Biosci. 2017;24:163–167.
- Slocombe SP, Zhang Q, Ross M, et al. Unlocking nature's treasure-chest: screening for oleaginous algae. Sci Rep. 2015;5:9844.
- Murakami H, Nobusawa T, Hori K, et al. Betaine lipid is crucial for adapting to low temperature and phosphate deficiency in Nannochloropsis. Plant Physiol. 2018;177:181–193.
- Antia NJ, Cheng JY. The keto-carotenoids of two marine coccoid members of the Eustigmatophyceae. Br Phycol J. 1982;17:39–50.
- Hager A, Stransky H. Das Carotinoidmuster und der Verbreitung des lichtinduzierten Xanthophyllcyclus in verschiedenen Algenklassen. III. Archiv Mikrobiol. 1970;72:68–83.
- Lubián LM, Montero O, Moreno-Garrido I, et al. Nannochloropsis (Eustigmatophyceae) as source of commercially valuable pigments. J Appl Phycol. 2000;12:249–255.
- Stoyneva-Gärtner MP, Stoykova P, Uzunov B, et al. Carotenoids in five aeroterrestrial strains from Vischeria/Eustigmatos group: Updating the pigment pattern of Eustigmatophyceae. Biotechnol Biotechnol Equip. 2019;1. 19p. doi: 10.1080/13102818.2018.1562984.
- Higuera-Ciapara I, Félix-Valenzuela L, Goycoolea FM. Astaxanthin: A review of its chemistry and applications. Crit Rev Food Sci Nutr. 2006;46:185–196.
- Ambati RR, Siew-Moi P, Sarada R, et al. Astaxanthin: Sources, extraction, stability, biological activities and its commercial applications - A review. Mar Drugs. 2014;12:128–152.
- Shah MM, Liang Y, Cheng JJ, et al. Astaxanthin-producing green microalga Haematococcus pluvialis: from single cell to high value commercial products. Front Plant Sci. 2016;7:531.
- European register of food additives pursuant to Regulation (EC) No 1831/2003. Annex II. Luxembourg: Publication office of European Union. 2018;
- Summary of Color Additives for Use in the United States in Foods, Drugs, Cosmetics, and Medical Devices. USA Food and Drug Administration; [cited 2011 Oct 27]. Available from: https://www.fda.gov/ForIndustry/ColorAdditives/ColorAdditiveInventories/ucm115641.htm
- Color Additive Status List. List 4. USA Food and Drug Administration; [cited 2011 Oct 27]. Available from: https://www.fda.gov/ForIndustry/ColorAdditives/ColorAdditiveInventories/ucm106626.htm
- Astaxanthin wins full GRAS status. FDA [cited 2010 Jan 19]. Available from: https://www.nutraingredients-usa.com/Article/2010/01/19/Astaxanthin-wins-full-GRAS-status
- Park JS, Chyun JH, Kim YK, et al. Astaxanthin decreased oxidative stress and inflammation and enhanced immune response in humans. Nutr Metab (Lond). 2010;7:18–28.
- McCall BCK, McPartland R, Moore A, et al. Effects of astaxanthin on the proliferation and migration of breast cancer cells in vitro. Antioxidants. 2018;7:135–143.
- Li Z, Sun M, Li Q, et al. Profiling of carotenoids in six microalgae (Eustigmatophyceae) and assessment of their β-carotene productions in bubble column photobioreactor. Biotechnol Lett. 2012;34:2049–2053.
- Li Z, Ma XQ, Li AF, et al. A novel potential source of β-carotene: Eustigmatos cf. polyphem (Eustigmatophyceae) and pilot β-carotene production in bubble column and flat panel photobioreactors. Bioresour Technol. 2012;117:257–263.
- EU approved additives and E Numbers. UK Food Standards Agency; [2018]. Available from: https://www.food.gov.uk/business-guidance/eu-approved-additives-and-e-numbers
- Prieto A, Pedro Canãvate JP, García-González M. Assessment of carotenoid production by Dunaliella salina in different culture systems and operation regimes. J Biotechnol. 2011;151:180–185.
- Gao B, Yang J, Lei X, et al. Characterization of cell structural change, growth, lipid accumulation, and pigment profile of a novel oleaginous microalga, Vischeria stellata (Eustigmatophyceae), cultured with different initial nitrate supplies. J Appl Phycol. 2016;28:821–830.
- Food additive status list. US Food and Drug Administration; [cited 2018 Apr 01]. Available from: https://www.fda.gov/food/ingredientspackaginglabeling/foodadditivesingredients/ucm091048.htm#ftnV
- Talero E, García-Mauriño S, Ávila-Román A, et al. Bioactive compounds isolated from microalgae in chronic inflammation and cancer. Mar Drugs. 2015;13:6152–6209.
- Wang F, Huang L, Gao B, et al. Optimum production conditions, purification, identification, and antioxidant activity of violaxanthin from microalga Eustigmatos cf. polyphem (Eustigmatophyceae). Mar Drugs. 2018;16:190.
- FDA-3 Agency Additional Correspondence Letter GRAS Notice No. GRN 000140 of 29th July 2016
- Miranda JM, Anton X, Redondo-Valbuena C, et al. Egg and egg-derived foods: Effects on human health and use as functional foods. Nutrients. 2015;7:706–729.
- Li B, Vachali P, Bernstein PS. Human ocular carotenoid-binding proteins. Photochem Photobiol Sci. 2010; 9:1418–1425.
- Nolan JM, Meagher K, Kashani S, et al. What is meso-zeaxanthin, and where does it come from?. Eye (Lond). 2013;27:899–905.
- Koo E, Neuringer M, Sangiovanni JP. Macular xanthophylls, lipoprotein-related genes, and age-related macular degeneration. Amer J Clinic Nutr. 2014;100:336S–346S.
- Pinazo-Durán MD, Gómez-Ulla F, Arias L, et al. Do nutritional supplements have a role in age macular degeneration prevention?. J Ophthalmol. 2014;2014:901686.
- Wang X, Jiang C, Zhang Y, et al. Role of lutein supplementation in the management of age-related macular degeneration: meta-analysis of randomized controlled trials. Ophthalmic Res. 2014;52:198–205.
- Bernstein PS, Li B, Vachali PP, et al. Lutein, zeaxanthin, and meso-zeaxanthin: The basic and clinical science underlying carotenoid-based nutritional interventions against ocular disease. Prog Retin Eye Res. 2016;50:34–66.
- Yu B, Wang J, Suter PM, et al. Spirulina is an effective dietary source of zeaxanthin to humans. Br J Nutr. 2012;108:611–619.
- EFSA. Opinion of the Scientific Panel on additives and products or substances used in animal feed on the request from the European Commission on the safety of use of colouring agents in animal nutrition. EFSA J. 2005;291:1–40.
- Brizio P, Benedetto A, Righetti M, et al. Astaxanthin and canthaxanthin (xanthophyll) as supplements in rainbow trout diet: in vivo assessment of residual levels and contributions to human health. J Agric Food Chem. 2013;61:10954–10959.
- Choubert G, de la Noüe J, Blanc JM. Apparent digestibility of canthaxanthin in rainbow trout: effect of dietary fat level, antibiotics and number of pyloric caeca. Aquaculture. 1991;99:323–329.
- Choubert G, Storebakken T. Digestibility of astaxanthin and canthaxanthin in rainbow trout as affected by dietary concentration, feeding rate and water salinity. Ann Zootech. 1996; 45:445–453.
- Niu J, Li CH, Liu YJ, et al. Dietary values of astaxanthin and canthaxanthin in Penaeus monodon in the presence and absence of cholesterol supplementation: effect on growth, nutrient digestibility and tissue carotenoid composition. Br J Nutr. 2012;108:80–91.
- Surai AP, Surai PF, Steinberg W, et al. Effect of canthaxanthin content of the maternal diet on the antioxidant system of the developing chick. Br Poult Sci. 2003;44:612–619.
- Surai PF. The antioxidant properties of canthaxanthin and its potential effects in the poultry eggs and on embryonic development of the chick. Part 1. Worlds Poult Sci J. 2012;68:465–476.
- Surai PF. The antioxidant properties of canthaxanthin and its potential effects in the poultry eggs and on embryonic development of the chick. Part 2. Worlds Poult Sci J. 2012;68:717–726.
- Rosa AP, Scher A, Sorbara JO, et al. Effects of canthaxanthin on the productive and reproductive performance of broiler breeders. Poult Sci. 2012;91:660–666.
- Sujak A, Gabrielska J, Milanowska J, et al. Studies on canthaxanthin in lipid membranes. Biochim Biophys Acta. 2005;1712:17–28.
- Koller M, Muhr A, Braunegg G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014;6:52–63.
- Mourelle ML, Gómez CP, Legido JL. The potential use of marine microalgae and Cyanobacteria in cosmetics and thalassotherapy. Cosmetics. 2017; 4:46.
- Eid SY, El-Readi MZ, Wink M. Carotenoids reverse multidrug resistance in cancer cells by interfering with ABC-transporters. Phytomedicine. 2012;19:977–978.
- Braun R, Farré E, Schurr U, et al. Effects of light and circulation clock on growth and chlorophyll accumulation of Nannochloropsis gaditana (Eustigmatophyte). J Phycol. 2014; 50:515–525.
- Mishra VK, Bacheti RK, Husen A. 2011. Medicinal uses of chlorophyll: A critical overview. In: Le H, Salcedo E, editors. Chlorophyll: structure, function and medicinal uses. Hauppauge (NY): Nova Science Publishers; 2011. p. 177–196.
- McGee H. On food and cooking. The science and lore of the kitchen. New York: Scribner; 2004.
- Chlorophyll [cited 2019 Jan 09]. Available from: https://en.wikipedia.org/wiki/Chlorophyll
- Kakhia TI. Dyes, Colors & Pigments. 2015. Available from: http://tarek.kakhia.org.
- Durmaz Y. Vitamin E (α-tocopherol) production by the marine microalgae Nannochloropsis oculata (Eustigmatophyceae) nutrient limitation. Aquaculture. 2007;272:711–722.
- Goh LP, Loh SP, Fatimah MY, et al. Bioaccessibility of carotenoids and tocopherols in marine Microalgae, Nannochloropsis sp. and Chaetoceros sp. Malays J Nutr. 2009;15:77–86.
- Liu C-P, Lin L-P. Morphology and eicosapentaenoic acid production by Monodus subterraneus UTEX 151. Micron. 2005;36:545–550.
- Spolaore P, Joannis-Cassan C, Duran E, et al. Commercial applications of microalgae. J Biosci Bioeng. 2006;101:87–96.
- Fukusho K. Biology and mass production of the rotifer Brachiolnus plicatilis. Int J Aguatic Fish Technol. 1989;1:232–240.
- Yúfera M, Navarro N. Population dynamics on the rotifer Brachionus plicatilis cultured in non-limiting food consumption. Hydrobiologia. 1995;313-314:399–405.
- Kostopolou V, Vadstein O. Growth performance of the rotifer Brachiolnus plicatilis, B. “Nevada” and B. “Cayman” under different food concentrations. Aquaculture. 2007; 273:449–458.
- Campaña-Torres AL, Martínez-Córdova LR, Martínez-Porchas M, et al. Productive response of Nannochloropsis oculata, cultured in different media and their efficiency as food for the rotifer Brachionus rotundiformis. FHYTON. 2012;81:45–50.
- Ferreira M, Coutinho P, Seixas P, et al. Enriching rotifers with “premium” microalgae. Nannochloropsis gaditana. Mar Biotechnol. 2009;11:585–595.
- Bae JB, Hur SB. Selection of suitable species of Chlorella, Nannochloris, and Nannochloropsis in high- and low-temperature seasons for mass culture of the rotifer Brachionus plicatilis. Fish Aquat Sci. 2011;14:323–332.
- Fui CF, Cancerini SY, Shapawi R, et al. Comparison of Nannochloropsis oculata productions cultivated in two different systems: outdoor Red Tilapia (Oreochromis sp.) culture tank and indoor pure culture. Pertanika J Trop Agric Sci. 2018;41:1523–1531.
- Chen HS, Li SS, Huang R, et al. Conditional production of a functional fish growth hormone in the transgenic line of Nannochloropsis oculata (Eustigmatophyceae). J Phycol. 2008;44:768–776.
- Patterson GW, Tsitsa-Tzardis E, Wikfors GH, et al. Sterols of Eustigmatophyceae. Lipids. 1994; 29:661–664.
- Mercer EI, London RA, Kent IS, et al. Sterols, sterol esters and fatty acids of Botrydium granulatum, Tribonema aequale and Monodus subterraneus. Phytochem. 1974;13:845–852.
- Sanjeewa KKA, Fernando IPS, Samarakoon KW, et al. Anti-inflammatory and anti-cancer activities of sterol rich fraction of cultured marine microalga Nannochloropsis oculata. Algae. 2016;31:277–287.
- Suen Y, Hubbard JS, Holzer G, et al. Total lipid production of the green algal genus Nannochloropsis sp. QII under different nitrogen regimes. J Phycol. 1987;23:289–296.
- Volkman JK, Barrett SM, Dunstan GA, et al. C30-C32 alkyl diols and unsaturated alcohols in microalgae of class Eustigmatophyceae. Org Geochem. 1992;18:131–138.
- Ristić-Medić D, Vučić V, Takić M, et al. Polyunsaturated fatty acids in health and disease. J. Serb. Chem. Soc. 2013;78:1269–1289.
- Honoré E, Barhanin J, Attali B, et al. External blockade of the major cardiac delayed-rectifier K + channel (Kv1.5) by polyunsaturated fatty acids. Proc Natl Acad Sci. 1994;91:1937–1941.
- Simopoulos AP. Omega-3 fatty acids in health and disease and in growth and development. Am J Clin Nutr. 1991;54:438–463.
- Herbaut C. Omega-3 and health. Rev Médic Bruxelles. 2006;27:S355–S360.
- Landmark K, Alm CS. Alpha-linolenic acid, cardiovascular disease and sudden death. Tidsskr Nor Laegeforen. 2006;126:2792–2794.
- Reiffel JA, McDonald A. Antiarrhythmic effects of omega-3 fatty acids. Am J Cardiol. 2006;98:50i–60i.
- Kruger MC, Horrobin DF. Calcium metabolism, osteoporosis and essential fatty acids: a review. Prog Lipid Res. 1997;36:131–151.
- Pilatova J. The Potential Use of the Eustigmatophyceae in the Production of Biofuels [master’s thesis]. Praha: Charles University; 2013.
- Seto A, Wang HL, Hesseltine CW. Culture conditions affect eicasapentanoic acid content of Chlorella minutissima. J Amer Oil Soc. 1984;61:891–894.
- Sukenik A, Carmeli Y, Berner T. Regulation of fatty acid composition by irradiance level in the eustigmatophyte Nannochloropsis sp. J Phycol. 1989;25:686–692.
- Rebolloso-Fuentes MM, Navarro-Pérez A, Garcia-Camacho F, et al. Biomass nutrient profiles of the microalga Nannochloropsis. J Agric Food Chem. 2001;49:2966–2972.
- Safafar H, Michael ZH, Møller P, et al. High-EPA biomass from Nannochloropsis salina cultivated in a flat-panel photo-bioreactor on a process water-enriched growth medium. Mar Drugs. 2016;14:144.
- Neumann UF, Derwenskus A, Gille S, et al. Bioavailability and safety of nutrients from the microalgae Chlorella vulgaris, Nannochloropsis oceanica and Phaeodactylum tricornutum in C57BL/6 mice. Nutrients. 2018;10:965.
- Hodgson PA, Henderson RJ, Sargent JR, et al. Patterns of variation in the lipid class and fatty acid composition of Nannochloropsis oculata (Eustigmatophyceae) during batch culture. J Appl Phycol. 1991;3:169–181.
- Sukenik A, Carmeli Y. Lipid synthesis and fatty acid composition in Nannochloropsis sp. (Eustigmatophyceae) grown in a light-dark cycle. J Phycol. 1990;469:463–469.
- Sukenik A. Ecophysiological considerations in the optimization of eicosapentanoic acid production by Nannochloropsis sp. (Eustigmatophyceae). Biores Technol. 1991;35:263–269.
- Cohen Z. Production potential of eicosapentaenoic acid by Monodus subterraneus. J Am Oil Chem Soc. 1994;71:941–945.
- Cohen Z. Monodus subterraneus. In: Cohen Z, editor. Chemicals from microalgae. London: CRC Press; 1999. p. 25–40.
- Zou N, Zang C, Cohen Z, et al. Production of cell mass and eicosapentanoic acid (EPA) inultrahigh cell density cultures of Nannochloropsis sp. (Eustigmatophyceae). Eur J Phycol. 2000;35:122–133.
- Xuecheng XNZ. Effect of temperature, light intensity and pH on the growth and fatty acid compositions of Ellipsoidion sp. J Ocean Univ Qingdao. 2001;4:013–023.
- Hu H, Gao K. Optimization of growth and fatty acid composition of a unicellular marine picoplankton, Nannochloropsis sp., with enriched carbon sources. Biotechnol Lett. 2003; 25:421–425.
- Tonon T, Harvey D, Larson TR, et al. Long chain polyunsaturated fatty acid production and partitioning to triacylglycerols in four microalgae. Phytochem. 2002;61:15–24.
- Xu F, Hu H, Cong W, et al. Growth characteristics and eicosapentaenoic acid production by Nannochloropsis sp. in mixotrophic conditions. Biotechnol Lett. 2004;26:51–53.
- Converti A, Casazza AA, Ortiz EY, et al. Effect of temperature and nitrogen concentration on the growth and lipid content of Nannochloropsis oculata and Chlorella vulgaris for biodiesel production. Chem Eng Process: Process Intensific. 2009;48:1146–1151.
- Hoffmann M, Marxen K, Schulz R, et al. TFA and EPA productivities of Nannochloropsis salina influenced by temperature and nitrate stimuli in turbidostatic controlled experiments. Mar Drugs. 2010;8:2526–2545.
- Pal D, Khozin-Goldberg I, Cohen Z, et al. The effect of light, salinity, and nitrogen availability on lipid production by Nannochloropsis sp. Appl Microbiol Biotechnol. 2011;90:1429–1441.
- Lin Q, Gu N, Li G, et al. Effects of inorganic carbon concentration on carbon formation, nitrate utilization, biomass and oil accumulation of Nannochloropsis oculata CS 179. Biores Technol. 2012;111:353–359.
- Recht L, Zarka A, Boussiba S. Patterns of carbohydrate and fatty acid changes under nitrogen starvation in the microalgae Haematococcus pluvialis and Nannochloropsis sp. Appl Microbiol Biotechnol. 2012;94:1495–1503.
- Simionato D, Sforza E, Corteggiani Carpinelli E, et al. Acclimation of Nannochloropsis gaditana to different illumination regimes: effects on lipids accumulation. Biores Technol. 2011;102:6026–6032.
- Arudchelvam Y, Nirmalakhandan N. Energetic optimization of algal lipid production in bubble columns: Part 1: Evaluation of gas sparging. Biomass Bioenergy. 2012;46:757–764.
- Arudchelvam Y, Nirmalakhandan N. Energetic optimization of algal lipid production in bubble columns: Part II: Evaluation of CO2 enrichment. Biomass Bioenergy. 2012;46:765–772.
- Iwai M, Hori K, Sasaki-Sekimoto Y, et al. Manipulation of oil synthesis in Nannochloropsis strain NIES-2145 with a phosphorus starvation-inducible promoter from Chlamydomonas reinhardtii. Front Microbiol. 2015;6:912.
- Falkowski PG. Potential strategies for regulating the flux of carbon into specific chemical classes of algae. In: Ramos, J Jones MC, editors. Polysaccharides from microalgae: A new agroindustry. Proceedings of the International Workshop DUML. Beaufort (NC); 1988. p. 83–91.
- Chatuverdi R, Uppalapati SR, Alamsjash MA, et al. Isolation of quizalofop-resistant mutants of Nannochloropsis oculata (Eustigmatophyceae) with high eicosapentanoic acid following N-methyl-N-nitrosourea-induced random mutagenesis. J Appl Phycol. 2004;16:135–144.
- Zhang J, Liu S, Sun X, et al. Fatty acid composition analyses of DCMU resistant mutants of Nannochloropsis oculata (Eustigmatophyceae). J Ocean Univ Qindao. 2003;2:63–68.
- Cañavate JP, Armada I, Hachero-Cruzado I, et al. Interspecific variability in phosphorus induced lipid remodelling among marine eukaryotic phytoplankton. New Phytol. 2017;213:700–713.
- Dolch LJ, Rak C, Perin G, et al. A palmitic acid elongase affects eicosapentaenoic acid and plastidial monogalactosyldiacylglycerol levels in Nannochloropsis. Plant Physiol. 2017;173:742–759.
- Nobusawa T, Hori K, Mori H, et al. Differently localized lysophosphatidic acid acyltransferases crucial for triacylglycerol biosynthesis in the oleaginous alga Nannochloropsis. Plant J. 2017;90:547–559.
- Iwamoto H, Sato S. Production of EPA by freshwater unicellular algae. J Amer Oil Chem Soc. 1986;63:434–440.
- Hu Q, Hu Z, Zvi C, et al. Enhancement of eicosapentaenoic acid (EPA) and J-linolenic acid (GLA) production by manipulating algal density of outdoor cultures of Monodus subterraneus (Eustigmatophyta) and Spirulina platensis (Cyanobacteria). Eur J Phycol. 1997;32:81–86.
- Vazhappilly R, Chen F. Eicosapentaenoic acid and docosahexaenoic acid production potential of microalgae and their heterotrophic growth. J Amer Oil Chem Soc. 1998;75:393–397.
- Volkman JK, Barrett SM, Blackburn SI. Fatty acids and hydrocy fatty acids in three species of freshwater eustigmatophytes. J Phycol. 1999;35:1005–1012.
- Xu N, Zhang X, Fan X, et al. Effects of nitrogen source and concentration on growth rate and fatty acid composition of Ellipsoidion sp. (Eustigmatophyta). J Appl Phycol. 2001;13:463–469.
- Khozin-Goldberg I, Didi-Cohen S, Shayakhmetova I, et al. Biosynthesis of eicosapentaenoic acid (EPA) in the freshwater eustigmatophyte Monodus subterraneus (Eustigmatophyceae) 1. J Phycol. 2002;38:745–756.
- Khozin-Goldberg I, Cohen Z. The effect of phosphate starvation on the lipid and fatty acid composition of the fresh water eustigmatophyte Monodus subterraneus. Phytochem. 2006;67:696–701.
- Iliev I, Petkov G, Lukavsky J, et al. The alga Trachydiscus minutus (Pseudostaurastrum minutum): growth and composition. Gen Appl Plant Physiol. 2010;36:222–231.
- Rezanka T, Petránková M, Cepák V, et al. Trachydiscus minutus, a new biotechnological source of eicosapentaenoic acid. Folia Microbiol (Praha). 2010;55:265–269.
- Gigova L, Ivanova N, Gacheva G, et al. Response of trachydiscus minutus (xanthophyceae) to temperature and light(1). J Phycol. 2012; 48:85–93.
- Krienitz L, Wirth M. The high content of polyunsaturated fatty acids in Nannochloropsis limnetica (Eustigmatophyceae) and its implication for food web interactions, freshwater aquaculture and biotechnology. Limnol - Ecol Manag Inland Waters. 2006;36:204–210.
- Roncarati A, Meluzzi A, Acciarri S, et al. Fatty acid composition of different microalgae strains (Nannochloropsis sp., Nannochloropsis oculata (Droop) Hibberd, Nannochloropsis atomus Butcher and Isochrysis sp.) according to the culture phase and carbon dioxide concentration. J World Aquaculture Soc. 2004;35:401–411.
- Cepák V, Přibyl P, Kohoutková J, et al. Optimization of cultivation conditions for fatty acid composition and EPA production in the eustigmatophycean microalga Trachydiscus minutus. J Appl Phycol. 2014;26:181–190.
- Jo MJ, Hur SB. Growth and nutritional composition of Eustigmatophyceae Monodus subterraneus and Nannochloropsis oceanica in autotrophic and mixotrophic culture. Ocean Polar Res. 2015;37:61–71.
- Khozin-Goldberg I, Boussiba S. Concerns over the reporting of inconsistent data on fatty acid composition for microalgae of the genus Nannochloropsis (Eustigmatophyceae). J Appl Phycol. 2011;23:933–934.
- Ma XN, Chen TP, Yang B, et al. Lipid production from Nannochloropsis. Mar Drugs. 2016;14:61.
- Volkman JK, Brown MR, Dunstan GA, et al. The biochemical composition of marine microalgae from the class Eustigmatophyceae. J Phycol. 1993;29:69–78.
- Volkman JK, Barrett SM, Blackburn SI, et al. Microalgal biomarkers: a review of recent research developments. Org Geochem. 1998;29:1163–1179.
- Gelin F, Volkman JK, de Leeuw JW, et al. Mid-chain hydroxy long-chain fatty-acids in microalgae from genus Nannochloropsis. Phytochemistry. 1997;46:641–646.
- Sukenik A. Production of eicosapentaenoic acid by the marine eustigmatophyte Nannochloropsis. In: Cohen Z, editor. Chemicals from microalgae. London: CRC Press; 1999. p. 41–56.
- Boussiba S, Vonshak A, Cohen Z, et al. Lipid and biomass production by the halotolerant microalga Nannochloropsis salina. Biomass. 1987;12:37–47.
- Chiu SY, Chien YK, Ming TT, et al. Lipid accumulation and CO2 utilization of Nannochloropsis oculata in response to CO2 aeration. Bioresour Technol. 2009;100:833–838.
- Chi Y, Chen F, Takiguchi Y. Effect of nitrogen source on biomass and lipid production of a marine Microalga, Nannochloropsis oceanica IMET1. GSC. 2015;05:101–106.
- Zhang JJian, Wan LLin, Xia S, et al. Morphological and spectrometric analyses of lipids accumulation in a novel oleaginous microalga, Eustigmatos cf. polyphem (Eustigmatophyceae). Bioprocess Biosyst Eng. 2013;36:1125–1130.
- Lam MK, Lee KT. Microalgae biofuels: a critical review of issues, problems and the way forward. Biotechnol Adv. 2012;30:673–690.
- Doan TTY, Sivaloganathan B, Obbard JF. Screening of marine microalgae for biodiesel feedstock. Biomass Bioenergy. 2011;35:2534–2544.
- Moazami N, Ashori A, Ranjbar R, et al. Large-scale biodiesel production using microalgae biomass of Nannochloropsis. Biomass Bioenergy. 2012;39:449–453.
- Dinesh Kumar S, Santhanam P, Grace FLG. The techniques in microalgae bioremediation and algal co-product development. In: Santharaman P, Begum A, Pachiappan P, editors. Basic and applied phytoplankton biology. Singapore: Springer Nature; 2018. p. 191–210.
- Jinkerson RE, Radakovits R, Posewitz MC. Genomic insights from the oleaginous model alga Nannochloropsis gaditana. Bioengineered. 2013;4:37–43.
- Radakovits R, Jinkerson RE, Fuerstenberg SI, et al. Draft genome sequence and genetic transformation of the oleaginous alga Nannochloropis gaditana. Nat Commun. 2012;3:686.
- Vieler A, Wu G, Tsai C-H, et al. Genome, functional gene annotation, and nuclear transformation of the heterokont oleaginous alga Nannochloropsis oceanica CCMP1779. PLоS Genet. 2012;8:1–25.
- Wang D, Ning K, Li J, et al. Nannochloropsis genomes reveal evolution of microalgal oleaginous traits. PLоS Genet. 2014;10:1–13.
- Li N, Xu C, Li-Beisson Y, et al. Fatty acid and lipid transport in plant cells. Trends Plant Sci. 2016;21:145–158.
- Poliner E, Panchy N, Newton L, et al. Transcriptional coordination of physiological responses in Nannochloropsis oceanica CCMP1779 under light/dark cycles. Plant J. 2015;83:1097–1113.
- Alboresi A, Perin G, Vitulo N, et al. Light remodels lipid biosynthesis in Nannochloropsis gaditana by modulating carbon partitioning between organelles. Plant Physiol. 2016;171:2468–2482.
- Kilian O, Benemann CSE, Niyogi KK, et al. High-efficiency homologous recombination in the oil-producing alga Nannochloropsis sp. Proc Natl Acad Sci USA. 2011;108:21265–21269.
- Wang Q, Lu Y, Xin Y, et al. Genome editing of model oleaginous microalgae Nannochloropsis spp. By CRISPR/Cas9. Plant J. 2016;88:1071–1081.
- Wei L, Xin Y, Wang Q, et al. RNAi-based targeted gene-knockdown in the model oleaginous microalgae Nannochloropsis oceanica. Plant J. 2017;89:1236–1250.
- Tibbetts SM, Milley JE, Lall SP. Chemical composition and nutritional properties of freshwater and marine microalgal biomass cultured in photobioreactors. J Appl Phycol. 2015;27:1109–1119.
- Stolz P, Obermayer B. Manufacturing microalgae for skin care. Cosmet Toilet. 2005;120:99–106.
- Pachiappan P, Santhanam P, Begum A, et al. 2018 An introduction to plankton. In: Santharaman P, Begum A, Pachiappan P, editors. Basic and applied phytoplankton biology. Singapore: Springer Nature; 2018. p. 1–24.
- Letsiou S, Kalliampakao K, Gardikis K, et al. Skin protective effects of Nannochloropsis gaditana extract on H2O2-stressed human dermal fibroblasts. Front Mar Sci. 2017;4:221.
- Mourelle L, Gόmes CP, Martin MC, et al. Microalgae and Thermalism: Perspectives. J Jpn Soc Balneol Climatol Phys Med. 2014;5:537–538.
- Goh SH, Yusoff FM, Loh SP. A comparison of the antioxidant properties and total phenolic content in a diatom, Chaetoceros sp. and a green microalga, Nannochloropsis sp. J Agric Sci. 2010;2:123–130.
- Wolf BM, Niedzwiedzki DM, Magdaong NCM, et al. Characterization of a newly isolated freshwater Eustigmatophyte alga capable of utilizing far-red light as its sole light source. Photosyn Res. 2018;135:177–189.
- Yurchenko T, Ševčíková T, Přibyl P, et al. A gene transfer event suggests a long-term partnership between eustigmatophyte algae and a novel lineage of endosymbiotic bacteria. ISME J. 2018;12:2163–2175.
- Levine I, Fleurence J, editors. Microalgae in health and disease prevention. London UK: Elsevier; 2018.
- Karthikaichamy A, Deore P, Rai V, et al. Time for multiple extraction methods in proteomics? A comparison of three protein extraction methods in the eustigmatophyte alga Microchloropsis gaditana CCMP526. OMICS. 2017;21:678–683.
- Nice EC. Challenges for omics technologies in the implementation of personalized medicine. Expert Rev Prec Medic Drug Develop. 2018;3:229–231.
- Kagan ML, Matulka RA. Safety assessment of the microalgae Nannochloropsis oculata. Toxicol Rep. 2015;2:617–623.
- Rai SV, Rajashekhar M. Effect of twelve species of marine phytoplankton on larval survival and development of the mosquito Culex quinquefasciatus. Int J Mar Sci. 2015;57:1–5.
- Balakrishnan S, Santhanam P, Manikam N, et al. A method of bio-efficacy potential of microalgae for the control of vector mosquitoes. In: Santharaman P, Begum A, Pachiappan P, editors. Basic and applied phytoplankton biology. Singapore: Springer Nature; 2018. p. 109–122.
- Matsumoto H, Shioji N, Hamasaki A, et al. Carbon dioxide fixation by microalgae photosynthesis using actual flue gas discharged from a boiler. Appl Biochem Biotechnol. 1995;51:681.
- Matsumoto H, Shioji N, Hamasaki A, et al. Basic study on optimization of raceway-type algal cultivator. J Chem Eng Japan / JCEJ. 1996;29:541–543.
- Biondi N, Bassi N, Chini Zittelli G, et al. Nannochloropsis sp. F&M-M24: oil production, effect of mixing on productivity and growth in an industrial wastewater. Environ Prog Sustainable Energy. 2013; 32:846–854.
- McGinn PJ, Dickinson KE, Bhatti S, et al. Integration of microalgae cultivation with industrial waste remediation for biofuel and bioenergy production: opportunities and limitations. Photosyn Res. 2011;109:231–247.
- Lu Y, Xu J. Phytohormones in microalgae: a new opportunity for microalgal biotechnology? Trends Plant Sci. 2015;20:273–282.