Abstract
The aim of this study was to detect aquaporin-5 (AQP5) expression and evaluate the role of the p38-MAPK signalling pathway in mechanical ventilation-associated lung injury. Rats were randomly divided into 5 groups. In Group C, only trachea were cut to retain spontaneous breathing; in Group S and Group L, rats were ventilated with tidal volumes at 12 or 40 mL/kg for 4 h; in Group S + I and Group L + I, rats were given the p38-MAPK inhibitor SB203580 before ventilation. The protein expressions were measured using immunochemistry and western blot. The wet/dry weight ratio (W/D) of the lung tissue was calculated. Broncho-alveolar lavage fluid was collected to examine the pulmonary neutrophil (PMN) count and inflammatory cytokines. Compared with Group C, the other four groups had decreased AQP5 level, along with the increased p38 MAPK level, p-p38 MAPK level, W/D ratio, PMN count, levels of TNF-α and IL-6 and lung injury score (p < 0.05). Moreover, in Group L and Group L + I, the changes in these indicators were more significant than in Group S and Group S + I (p < 0.05), respectively, indicating that larger tidal volume induces more severe lung injury. However, after treatment with SB203580, the changes in the indicators in Group S + I and Group L + I were significantly attenuated compared to those in Group S and Group L, respectively (p < 0.05), suggesting that SB203580 could alleviate the lung injury induced by mechanical ventilation. The inhibition of the p38-MAPK pathway may decrease the occurrence of pulmonary edema induced by mechanical ventilation possibly by up-regulating AQP5 expression.
Introduction
Mechanical ventilation is an important adjuvant treatment to general anesthesia and critically ill patients, but it tends to cause mechanical ventilation-related lung injury [Citation1]. Studies have shown that mechanical ventilation-related lung injury often presents with severe pulmonary edema [Citation2–4].
MAPK signalling is involved in mechanical ventilation-induced pulmonary edema. Mitogen activated protein kinases (MAPKs) signalling pathway is the major signal transduction pathways regulating the production of inflammatory cells and has a close relationship in the LPS-induced ARDS [Citation5]. It has been verified in many rat model and clinical researches. Previous experimental and clinical studies have demonstrated that various damaging factors may lead to a rapid overproduction of proinflammatory cytokines, including TNF-α and IL-6, which are characteristic cytokines associated with the inflammatory process of acute lung injury (ALI) [Citation6]. TNF-α, which is predominantly produced by activated monocytes/macrophages, has a close connection with the p38-MAPK signalling pathway, which may have some impact on the damage of vascular endothelial cells [Citation7].
Aquaporins (AQPs) are membrane-specific channel proteins that are mainly distributed in various glandular cells. AQP5 is mainly located on the membrane of type I alveolar epithelial cells and has the function of maintaining the water balance of cells [Citation8–10]. Studies have shown that AQP5 is associated with different diseases [Citation11–13]. For example, it has been reported that AQP5 plays an important role in the elimination of extravascular lung water, and the increase of AQP5 can contribute to lung injury in patients with sepsis [Citation10]. The deletion of AQP5 slightly increases lung edema and lung injury during HAPE development compared to wild type mice [Citation14]. However, whether mechanical ventilation-induced pulmonary edema is associated with AQP5 down-regulation has not yet been determined, and the relationship between AQP5 and MAPK signalling in mechanical ventilation-induced pulmonary edema is also not fully investigated.
In this study, we determined the changes of AQP5 expression in lung tissue of rats with ventilator-associated lung injury, and explored the mechanism of mechanical ventilation-induced pulmonary edema after blocking the p38-MAPK intracellular signal transduction pathway.
Materials and methods
Animals
A total of 50 healthy specific pathogen-free-level adult Sprague-Dawley rats (male, 200–250 g) were provided by the Laboratory Animal Center of Shandong University of Traditional Chinese Medicine (Jinan, China). They were kept in standard conditions.
Ethics of experimentation
The research protocol was approved by the Animal Care and Use Committee at Qianfoshan Hospital Affiliated to Shandong University.
Animal treatment and grouping
Rats were randomly divided into 5 groups (n = 10/group): control group (Group C), small tidal volume group (Group S), large tidal volume group (Group L), small tidal volume plus inhibitor group (Group S + I), and large tidal volume plus inhibitor group (Group L + I), according to different treatments. The rats were fasted for 12 hours before the experiment. After anesthesia with intraperitoneal injection of 3.5 mL/kg of 10% chloral hydrate, tracheotomy was performed in rats and a tracheal tube was placed. For the rats in Group C, only trachea were cut to retain spontaneous breathing, while the rats of the other four groups were connected to small animal ventilators (model ALC-V8A; Gene&I, Beijing, China), with I:E (Absorbing ratio) = 1:3 and RR (Respiratory Rate) 50 times/min. In Group S, the rats were ventilated with tidal volumes at 12 mL/kg for 4 hours and the rats in Group L were ventilated with tidal volumes at 40 mL/kg for 4 hours. The rats in Group S + I were given 4 hours of small tidal volume ventilation at 12 mL/kg and the specific P38-MAPK inhibitor SB203580 (Sigma-Aldrich Trading Co., Ltd., Shanghai, China). And the rats in Group L + I were given 4 hours of large tidal volume ventilation at 40 mL/kg and specific p38-MAPK inhibitor SB203580. SB203580 was injected intravenously 30 min before mechanical ventilation at a dose of 10 mg/kg [Citation15].
BALF collection and PMN count
The rats in Group C were sacrificed immediately after the tracheotomy, while the rats in the other four groups were sacrificed by bloodletting through the abdominal aorta at the end of the mechanical ventilation. Shortly afterwards, thoracotomy was done. The right main bronchus was clamped and the left main bronchus was lavaged with 1 mL normal saline 3 times. After that, the bronchoalveolar lavage fluid (BALF) was collected after centrifugation (400×g). The precipitate was diluted with 1 mL of normal saline for the polymorphnuclear neutrophil (PMN) counts.
General observation and wet/dry weight ratio of the lung
The left lung was observed for general morphology, colour and exudation on the tissue surface. Then, the wet weight of the left lung was analyzed with an electronic analytical balance. The left lung was then dried in a 37 °C oven until a constant weight was obtained. Then, the lung wet/dry weight ratio (W/D) was calculated.
The lower lobe of the right lung was fixed by perfusion of 4% paraformaldehyde for H&E staining and immunohistochemical detection. The upper lobe of the right lung was placed into a −80 °C refrigerator for the later measurement of protein expression by Western blot.
H&E staining and scoring
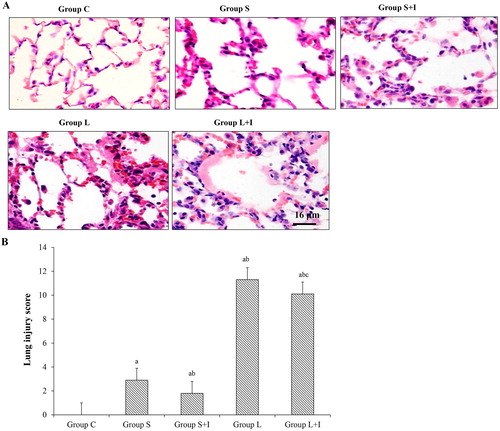
The section of the lower lobe of the right lung was embedded in paraffin after fixation with 4% paraformaldehyde. Routine H&E (hematoxylin and eosin) staining was performed. The pathological changes of the lung tissues were observed under a light microscope (Olympus Research Microscope HB-2, Japan). The lung injury score was calculated. The scoring criteria () ranging from 0 to 12 points included the following four aspects: alveolar wall thickening, inflammatory cell infiltration, pulmonary hyaline membrane formation and atelectasis change as previously described [Citation16].
Table 1. Scoring criteria for lung injury.
Immunohistochemistry
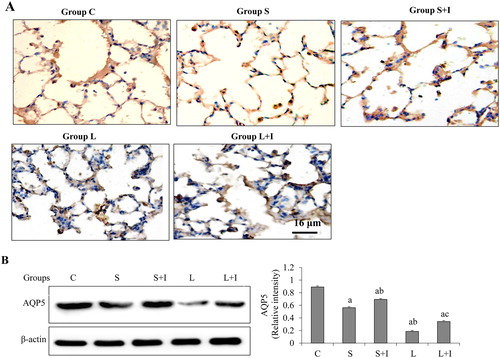
The expression of AQP5 was determined by immunohistochemistry, following the instructions of the immunohistochemistry kit (ZSGB-BIO, Beijing, China). After deparaffinization and antigen retrieval, endogenous peroxidase activity was blocked by incubation with 3% hydrogen peroxide in methanol. Non-specific binding was blocked by incubation in 10% goat serum for 15 minutes. Rabbit anti-rat AQP5 primary antibodies (1:300) were added and incubated at 37 °C for 1.0–1.5 hours. After that, secondary antibodies of goat anti-rabbit (1:1000) were added and incubated at 37 °C for 2 hours. Afterwards, the section was coloured with diaminobenzidine, and counter stained with hematoxylin. PBS was used instead of primary antibody for a negative control. Five randomly selected fields at 400 × magnification in each sample were used. The expression level of AQP5 was quantified based on the brown intensity.
Western blot
Total proteins were extracted from 50 mg of lung tissue after incubation with 50 μL RIPA lysis buffer at 4 °C for 30 min. The proteins (30 μg of protein per lane) were then fractionated by 10% sodium dodecyl sulphate polyacryl amide gel electrophoresis (SDS-PAGE) and electro-transferred onto PVDF membranes (Millipore, MA, USA). The primary antibodies included AQP5 (sc-28628, 1:800, Santa Cruz), p38 MAPK (14511, 1:1000, Cell Signaling Technology, MA, USA), Phospho-p38 MAPK (Thr180/Tyr182) (4511, 1:1000, Cell Signaling Technology, MA, USA) and β-actin (ab6276, 1:5000, Abcam, Cambridge, MA, USA). The secondary antibody was horseradish peroxidase-conjugated secondary antibody (Santa Cruz, USA). The bound antibodies were visualized using an enhanced chemiluminescence reagent (Millipore, MA, USA) and quantified by densitometry using a ChemiDoc XRS + image analyzer (Bio-Rad, USA). Gray density of bands was normalized to that of β-actin (loading control). Triplicate experiments with triplicate samples were performed.
Quantification of cytokines in BALF
The levels of IL-6 and TNF-α in the BALF supernatants were determined using the corresponding ELISA kits (YuanyeBiological Technology Co. LTD, Shanghai, China) according to the manufacturer’s instructions.
Statistical analysis
All data were expressed as mean values with standard deviation (±SD) and were analyzed using SPSS 18.0 software (SPSS, Inc., Chicago, Illinois). The comparison between groups was done by one-way analysis of variance (ANOVA) followed by LSD analysis. The rank data were compared by rank sum test. A p < 0.05 was considered statistically significant.
Results and discussion
Pathological changes in different groups
To determine the pathological changes of the lung, H&E staining was performed and the lung injury score was calculated. As shown in , the lung tissue of the group C animals was smooth and there was no haemorrhage. The alveolar wall was composed of single-layer alveolar epithelial cells. The structure was intact. There was no inflammatory cell infiltration in the alveoli or hyaline membrane formation. The alveolar wall of the S group animals was slightly thickened, and there were a small number of inflammatory cells in the alveolar wall. There was no hemorrhage in the alveoli and no hyaline membrane was formed. The S + I group was similar to the S group and had fewer inflammatory cells than the alveolar wall of the S group. In group L, we observed slight hemorrhages on the surface of the lung. Microscopically, the alveolar wall was thickened. There was a large amount of inflammatory cell infiltration in the alveoli, alveolar hemorrhage, atelectasis and hyaline membrane formation. In addition to a large number of inflammatory cell infiltration in the L + I group, intra-alveolar hemorrhage and inflammatory exudation in the alveolar wall were less than those in the L group. As shown in , compared with Group C, the lung injury score in the other groups increased significantly (p < 0.05). Compared with Group S, the score in Group L and Group L + I significantly increased, while that in Group S + I significantly decreased (p < 0.05). And compared with Group L, the score in Group L + I was significantly reduced (p < 0.05). These results suggest that the greater the tidal volume, the more severe the lung tissue damage, and the inhibitor can reduce the extent of lung tissue damage under the same tidal volume.
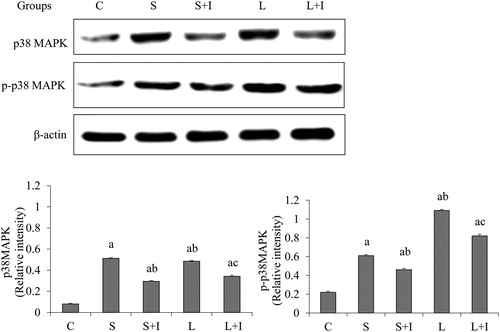
Detection of p38 MAPK and p-p38 MAPK in the lung
The expression of p38 MAPK and p-p38 MAPK was analyzed by western blot (). We found that p38 MAPK and p-p38 MAPK levels were significantly increased in Group S, Group S + I, Group L and Group L + I as compared to those in Group C (p < 0.05). After treatment with SB203580, which specifically blocks the p38-MAPK signalling way, the expression of p38 and p-p38 significantly decreased in the lung tissues (p < 0.05, Group S vs. Group S + I, and Group L vs. Group L + I, respectively). This result indicates that SB203580 can successfully reduce the expression of p38 MAPK and p-p38 MAPK in the lung tissue.
AQP5 expression analysis
To determine AQP5 expression, immunohistochemistry and western blot analysis were performed. In Group C, strong AQP5 expression was observed in the lung tissues, which was obviously higher than that in the other four groups (). Compared with Group S, the expression of AQP5 was down-regulated in Group L. When the animals were treated with SB203580, the expression of AQP5 in Group L + I was upregulated compared with Group L. And the expression of AQP5 in Group S + I was increased when compared with Group S (). The expression changes of the AQP5 protein were further confirmed by western blot (). These results suggest that AQP5 expression on the alveolar epithelial membranes was reduced with increased ventilation, and SB203580 reduced the AQP5 loss and up-regulated its expression.
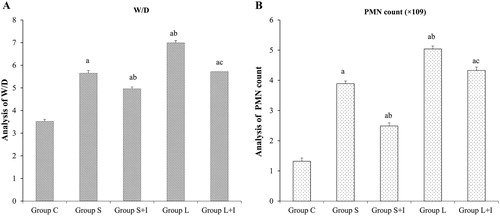
W/D ratio and PMN counts
The W/D ratio and PMN counts were calculated. As shown in , compared with Group C, we observed increased W/D ratio and PMN counts in the other four groups (p < 0.05). The animals ventilated with large tidal volumes showed higher W/D ratio and PMN counts than those ventilated with small tidal volumes (p < 0.05, Group S vs. Group L; p < 0.05, Group S + I vs. Group L + I). Our results then revealed that compared with Group S or Group L, the increased W/D ratio and PMN counts were down-regulated after the SB203580 treatment in Group S + I and Group L + I, respectively (p < 0.05 Group S vs. Group S + I; p < 0.05 Group L vs. Group L + I). These results imply that AQP5 expression was reduced on the alveolar epithelial membranes with increased ventilation, and SB203580 reduced the AQP5 loss and up-regulated its expression.
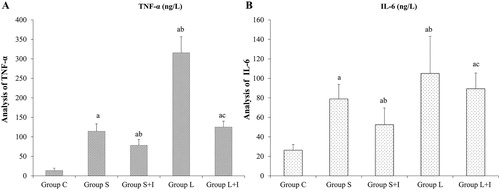
Cytokine levels in BALF
The pulmonary inflammatory cytokine levels analyzed by ELISA are shown in . The levels of pro-inflammatory cytokines (IL-6 and TNF-α) in the BALF of rats in Group S, Group S + I, Group L and Group L + I were significantly higher than those in the control group (p < 0.05); while these levels were decreased significantly after treatment with the p38-MAPK inhibitor SB203580 (p < 0.05, Group S vs. Group S + I; Group L vs. Group L + I, respectively). These data demonstrated that along with the increase in ventilation, the lung tissue damage was aggravated, and the inflammatory factors exuded in the alveoli increased. The addition of SB203580 reduced this effect.
Comparative analysis
Clinical studies generally select large tidal volume ventilation set at 35–45 mL/kg and small tidal volume ventilation set at 6–15mL/kg [Citation17]. Lung injury is mainly manifested as structural destruction of the alveolar wall, along with increased pulmonary inflammatory exudation and different degrees of pulmonary oedema [Citation18,Citation19]. The alveolar surface is covered with about 90% of type I flat alveolar cells and only a small number of type II alveolar cells [Citation20]. Generally, the alignment of these alveolar epithelial cells means that liquids cannot penetrate under normal conditions. Type II alveolar cells can secrete surface-active substances, not only to maintain the expansion of the alveoli but also to prevent the alveolar interstitial fluid into the alveolar space [Citation21].
AQP5 is a transmembrane protein located on the alveolar type I epithelial cell membrane and can regulate the transport of water inside and outside the alveolar epithelial cells [Citation22–24]. When mechanical ventilation-induced lung injury occurs, voluminal injury and barotrauma cause alveolar wall structure damage [Citation25]. Even worse, large tidal volume ventilation causes alveolar wall rupture, pulmonary vascular injury and inflammatory cell exudation [Citation26]. Reduced number of AQP5 or a lack of function may result in the inability to transfer the excessive water out of cells and thus, lung injury is aggravated [Citation27]. Although extensive research has focused on the effects of mechanical ventilation on alveolar epithelial cell function and the mechanisms which impair the ability to efficiently transport fluid across the alveolar epithelium [Citation28–32], the molecular mechanisms that underlie the physiological processes have only recently begun to be understood. Several reports show that mechanical ventilation induces down-regulation of sodium channel expression and activity in rat lungs and in cultured lung epithelial cells. The relationship between lung edema and AQP5 however remains not clear, in spite of many research findings of changes of AQP5 in hyperoxia induced lung injury and LPS induced lung injury [Citation33–35].
Fabregat et al. [Citation36] found that a small tidal volume of 10 mL/kg for 2 h and 4 h induced mild damage to lung tissue and that the changes of pulmonary edema and lung permeability were not statistically significant. Interestingly, the increase in AQP5 gene expression started from 2 h of small tidal volume ventilation, whereas the protein expression of AQP5 was increased after 4 h ventilation. It is speculated that the increase in AQP5 aims to accelerate the water transport on both sides of the cell membrane, thus avoiding interstitial pulmonary oedema. Further, Fabregat et al. [Citation37] reported the relationship between AQPs and ventilation in mechanically ventilated lung injury. They found that a decrease in AQP5 expression in rat lung tissue during mechanical ventilation with high ventilation directly caused a further deterioration of pulmonary oedema. Based on these results, we speculate that upon stimulation (such as inflammation and mechanical stretching), the expression of AQP5 is up-regulated to enhance the water transport on both sides of the lung epithelial membrane. However, when the AQP5 expression is decreased, the functional pulmonary oedema may develop into interstitial oedema or even lung epithelial cell oedema, which is accompanied by destruction of AQP5 and decreased ability of cells to transport water, forming a vicious circle.
We therefore investigated whether AQP5 would respond to VILI through the p38-MAPK signalling pathway. This pathway is a family of serine/threonine protein kinases including p38 MAPK, extracellular signal-regulated kinase and c-Jun N-terminal regulatory kinase. The p38-MAPK signalling pathway plays an important role in the regulation of stress and inflammation [Citation38]. Many factors such as injury, infection and oxidative stress can activate p38MAPK [Citation39]. AQP5 is a seven-transmembrane protein with its intracellular signal transduction pathway yet unclear [Citation40]. To determine the signalling pathways which result in downregulation of AQP5, we tested the p38-MAPK signalling pathway, which is activated by inflammation. To further explore of the effect of p38 MAPK activation on mechanical ventilation-induced lung injury, we adopted SB203580, a specific inhibitor to the p38 MAPK-ATP binding site. We found that the inflammatory response in the groups with inhibitors was significantly reduced. Accompanied by this, after the application of the p38 MAPK inhibitor, the expression of AQP5 was up-regulated, which may further accelerate the removal of water from the lung epithelial cell membrane and thus reduce the occurrence of pulmonary oedema.
Conclusions
The results from this study suggest that application of a p38-MAPK pathway inhibitor might protect against mechanical ventilation induced lung injury and decrease the occurrence of pulmonary edema caused by mechanical ventilation. The potential mechanisms might be related to regulation of AQP5 expression of alveolar epithelial cells.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ngiam N, Kavanagh BP. Ventilator-induced lung injury: the role of gene activation. Curr Opin Crit Care. 2012;18:16–22.
- Matthay MA, Bhattacharya S, Gaver D, et al. Ventilator-induced lung injury: in vivo and in vitro mechanisms. Am J Physiol Lung Cell Mol Physiol. 2002;283:L678–L682.
- Ricard JD, Dreyfuss D, Saumon G. Ventilator-induced lung injury. Curr Opin Crit Care. 2002;8:12–20.
- Uhlig S. Ventilation-induced lung injury and mechanotransduction: stretching it too far? Am J Physiol Lung Cell Mol Physiol. 2002;282:L892–L896.
- Meng Y, Yu CH, Li T, et al. Expression and significance of Toll-like receptor-4 in rats lung established by passive smoking or associated with intratracheal instillation of lipopolysaccharide. Zhonghua Yi Xue Za Zhi. 2013;93:2230–2234.
- Cribbs SK, Matthay MA, Martin GS. Stem cells in sepsis and acute lung injury. Crit Care Med. 2010;38:2379–2385.
- Giebelen IA, van Westerloo DJ, LaRosa GJ, et al. Local stimulation of alpha7 cholinergic receptors inhibits LPS-induced TNF-alpha release in the mouse lung. Shock. 2007; 28:700–703.
- Kreda SM, Gynn MC, Fenstermacher DA, et al. Expression and localization of epithelial aquaporins in the adult human lung. Am J Respir Cell Mol Biol. 2001;24:224–234.
- Pedersen PS, Braunstein TH, Jørgensen A, et al. Stimulation of aquaporin-5 and transepithelial water permeability in human airway epithelium by hyperosmotic stress. Pflugers Arch. 2007;453:777–785.
- Tao B, Liu L, Wang N, et al. Effects of hydrogen-rich saline on aquaporin 1, 5 in septic rat lungs. J Surg Res. 2016; 202:291–298.
- Chang YL, Lin CS, Wang HW, et al. Chlorpheniramine attenuates histamine-mediated aquaporin 5 downregulation in human nasal epithelial cells via suppression of NF-κB activation. Int J Med Sci. 2017; 14:1268–1275.
- Kawedia JD, Yang F, Sartor MA, et al. Hypoxia and hypoxia mimetics decrease aquaporin 5 (AQP5) expression through both hypoxia inducible factor-1α and proteasome-mediated pathways. PLos One. 2013;8:e57541. [cited 2018 Jan 04];
- Rump K, Unterberg M, Bergmann L, et al. AQP5-1364A/C polymorphism and the AQP5 expression influence sepsis survival and immune cell migration: a prospective laboratory and patient study. J Transl Med. 2016;14:321.
- She J, Bi J, Tong L, et al. New insights of aquaporin 5 in the pathogenesis of high altitude pulmonary edema. Diagn Pathol.. 2013;8:193. [cited 2018 Jan 04];
- Liu S, Feng G, Wang GL, et al. p38MAPK inhibition attenuates LPS-induced acute lung injury involvement of NF-kappaB pathway. Eur J Pharmacol. 2008;584:159–165.
- Li KZ, Yao SL, Sun GY, et al. Effects of mechanical ventilation on the expression of endotoxin receptor CD14 in alveolar macrophages in rats. J Shandong Univ. 2006; 44:1142–1145.
- Ricard JD, Dreyfuss D, Saumon G. Production of inflammatory cytokines in ventilator-induced lung injury: a reappraisal. Am J Respir Crit Care Med. 2001;163:1176–1180.
- Casetti AV, Bartlett RH, Hirschl RB. Increasing inspiratory time exacerbates ventilator-induced lung injury during high-pressure/high-volume mechanical ventilation. Crit Care Med. 2002; 30:2295–2299.
- Lee SM, Kim WH, Ahn HJ, et al. The effects of prolonged inspiratory time during one-lung ventilation: a randomised controlled trial. Anaesthesia. 2013; 68:908–916.
- Verbrugge SJ, Lachmann B, Kesecioglu J. Lung protective ventilatory strategies in acute lung injury and acute respiratory distress syndrome: from experimental findings to clinical application. Clin Physiol Funct Imaging. 2007;27:67–90.
- Maier BB, Hladik A, Lakovits K, et al. Type I interferon promotes alveolar epithelial type II cell survival during pulmonary Streptococcus pneumoniae infection and sterile lung injury in mice. Eur J Immunol. 2016;46:2175–2186.
- Krane CM, Melvin JE, Nguyen HV, et al. Salivary acinar cells from aquaporin 5-deficient mice have decreased membrane water permeability and altered cell volume regulation. J Biol Chem. 2001; 276:23413–23420.
- Li J, Xu M, Fan Q, et al. Tanshinone IIA ameliorates seawater exposure-induced lung injury by inhibiting aquaporins (AQP) 1 and AQP5 expression in lung. Respir Physiol Neurobiol. 2011;176:39–49.
- Wang F, Huang H, Lu F, et al. Acute lung injury and change in expression of aquaporins 1 and 5 in a rat model of acute pancreatitis. Hepatogastroenterology. 2010;57:1553–1562.
- Slutsky AS. Lung injury caused by mechanical ventilation. Chest. 1999;116:9S–15S.
- Ma T, Fukuda N, Song Y, et al. Lung fluid transport in aquaporin-5 knockout mice. J Clin Invest. 2000;105:93–100.
- Towne JE, Krane CM, Bachurski CJ, et al. Tumor necrosis factor-alpha inhibits aquaporin 5 expression in mouse lung epithelial cells. J Biol Chem. 2001;276:18657–18664.
- Hardiman KM, Matalon S. Modification of sodium transport and alveolar fluid clearance by hypoxia: mechanisms and physiological implications. Am J Respir Cell Mol Biol. 2001;25:538–541.
- Mairbäurl H, Wodopia R, Eckes S, et al. Impairment of cation transport in A549 cells and rat alveolar epithelial cells by hypoxia. Am J Physiol. 1997;273:797–806.
- Sartori C, Allemann Y, Scherrer U. Pathogenesis of pulmonary edema: learning from high-altitude pulmonary edema. Respir Physiol Neurobiol. 2007;159:338–349.
- Tomlinson LA, Carpenter TC, Baker EH, et al. Hypoxia reduces airway epithelial sodium transport in rats. Am J Physiol. 1999;277:881–886.
- Zemans RL, Matthay MA. Bench-to-bedside review: the role of the alveolar epithelium in the resolution of pulmonary edema in acute lung injury. Crit Care. 2004; 8:469–477.
- Davieds B, Gross J, Berger MM, et al. Inhibition of alveolar Na transport and LPS causes hypoxemia and pulmonary arterial vasoconstriction in ventilated rats. Physiol Rep. 2016;4:e12985. [cited 2018 Jan 04]
- Lin X, Xu Z, Wang P, et al. Role of PiCCO monitoring for the integrated management of neurogenic pulmonary edema following traumatic brain injury: A case report and literature review. Exp Ther Med. 2016;12:2341–2347.
- Rump K, Siffert W, Peters J, et al. The transcription factor NMP4 binds to the AQP5 promoter and is a novel transcriptional regulator of the AQP5 gene. DNA Cell Biol. 2016;35:322–327.
- Fabregat G, García-de-la-Asunción J, Sarriá B, et al. Increased expression of AQP 1 and AQP 5 in rat lungs ventilated with low tidal volume is time dependent. PLoS One. 2014;9:e114247. [cited 2018 Jan 04]
- Fabregat G, García-de-la-Asunción J, Sarriá B, et al. Expression of aquaporins 1 and 5 in a model of ventilator-induced lung injury and its relation to tidal volume. Exp Physiol. 2016;101:1418–1431.
- Zhang LP, Zhao Y, Liu GJ, et al. Glabridin attenuates lipopolysaccharide-induced acute lung injury by inhibiting p38MAPK/ERK signaling pathway. Oncotarget. 2017;8:18935–18942.
- Du J, Zhang L, Yang Y, et al. ATP depletion-induced actin rearrangement reduces cell adhesion via p38 MAPK-HSP27 signaling in renal proximal tubule cells. Cell Physiol Biochem. 2010;25:501–510.
- Jiao G, Li E, Yu R. Decreased expression of AQP1 and AQP5 in acute injured lungs in rats. Chin Med J. 2002;115:963–967.