Abstract
Current treatment options for diffuse glioma patients include maximum safe resection followed by a combination of radiation therapy and chemotherapy with alkylating agents. The DNA-repair enzyme O6-methylguanine-DNA methyltransferase (MGMT) counteracts the cytotoxic effect of alkylating agents and mediates chemoresistance. Disruption of the DNA methylation mechanism in diffuse glioma cells results in epigenetic silencing of MGMT through methylation of cytidine-phosphate-guanosine dinucleotides (CpG) in the promoter region. The methylation status of MGMT is widely accepted to be a strong prognostic factor in diffuse glioma patients. This study was designed to screen Serbian diffuse glioma patients for hypermethylation of the MGMT promoter and to estimate its impact on overall survival. The results obtained in our study on 33 samples of diffuse glioma detected a positive methylation status in 17 patients (51.5%) by methylation-specific polymerase chain reaction. The positive methylation status of the MGMT promoter did not correlate with overall survival. In this study group, the patients older than 50 years had significantly lower overall survival in comparison with younger patients (7 months–19 months median survival). Extent of tumour resection also had influence on overall survival of patients. The relevance of the MGMT promoter methylation status should be further evaluated in a larger study and in association with other markers.
Introduction
Diffuse gliomas are a heterogeneous group of diffusely infiltrating primary brain tumours characterized by their resemblance to glia, although their cellular origin remains controversial [Citation1]. They are considered to be fundamentally incurable, as their complete resection is nearly impossible [Citation2]. Despite their histological heterogeneity, different types of diffuse gliomas share a similar growth pattern and genetic driver mutations, as well as prognostic markers [Citation1, Citation3]. According to the recent World Health Organization (WHO) Classification of Tumors of the Central Nervous System, diffuse gliomas are divided into the WHO grade II and grade III astrocytic tumours, grade II and III oligodendrogliomas, grade IV glioblastomas and the related diffuse gliomas of childhood [Citation4].
Currently the treatment options for newly diagnosed malignant glioma patients include maximum safe resection followed by a combination of radiation therapy and alkylating agents, although significant advances in exploring alternative therapeutic strategies have been made during the past ten years (e.g. immunotherapy including cell-free and cell based vaccines) [Citation5–8]. Besides tumour heterogeneity, one of the main reasons for treatment failure and poor prognosis in malignant glioma patients is intrinsic or acquired chemoresistance to cytotoxic alkylating agents – carmustine (BCNU), lomustine (CCNU) and temozolomide (TMZ) [Citation5, Citation9].
In malignant gliomas, the major mechanism of resistance to alkylating agents relies on the DNA-repair activity of the enzyme O6-methylguanine methyltransferase (MGMT) [Citation9, Citation10]. Alkylating agents are highly reactive molecules that bind to DNA and alkylate the O6 position of guanine. This type of DNA lesion leads to a lethal interstrand crosslink between the N3 position of guanine and the N1 position of cytosine. MGMT enzyme removes the alkyl adduct from O6-methylguanine DNA lesions through a suicide mechanism. Thus, increased MGMT activity in tumour cells counteracts the cytotoxic damage induced by antitumour agents [Citation10]. Since the initial research by Esteller et al. [Citation11], many studies have provided convincing evidence showing that glioma patients with decreased level of MGMT activity have longer overall survival (OS) and progression-free survival (reviewed in [Citation12]). The decrease of MGMT activity in glioma cells is caused by epigenetic silencing of MGMT through hypermethylation of cytidine-phosphate-guanosine dinucleotides (CpG islands) in the promoter region, rather than mutations or deletions. These findings led to the suggestion that the MGMT promoter methylation status could serve as a highly promising prognostic factor for malignant glioma patients [Citation12, Citation13]. However, several studies dealing with the MGMT methylation status in malignant glioma gave controversial results suggesting that the prognostic value of MGMT promoter methylation on GBM remains unclear [Citation12, Citation14–17].
The aim of the present study was to assess the correlation between the methylation status of the MGMT promoter and clinical data from Serbian patients with diffuse glioma.
Materials and methods
Diffuse glioma patients
This study included samples from 33 diffuse glioma patients that were operated at the Neurosurgery Clinic (The Clinical Centre of Niš, Serbia) in June 2013–April 2017. All patients had a Karnofsky score of ≥80% prior tumour removal. The extent of tumour removal varied from total resection (TR, complete removal of visible tumour mass) to partial resection (PR, 10–50% residual tumour mass) and biopsy (B, <10% resection) ().
Figure 1. MRI imaging of a patient who underwent total resection and treatment with TMZ before (A) and after (B) the operation.
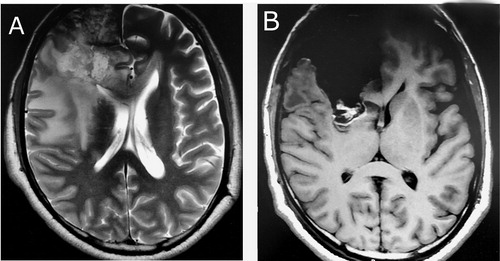
Tumour specimens obtained after tumour resection were snap frozen and stored at −80 °C. These samples were confirmed as glioblastoma (International Classification of Diseases for Oncology (ICD-O)) code 9440/3, anaplastic astrocytoma (ICD-O 9401/3), oligoastrocytoma (ICD-O 9382/3) and oligodendroglioma (ICD-O 9450/3) by an expert neuropathologist (). The mean age of the study group (21 male and 12 female) was 59.64 ± 12.3 years.
Table 1. Distribution of glioma subtypes in the study group.
The patients were divided into two groups based on the postoperative treatment protocol. The patients in group I (n = 11) were treated with temozolomide (TMZ, 6 cycles at a dosage of 150-200 mg/m2) and radiotherapy (60 Gy in 30 fractions). The patients in group II (n = 22) were treated with nitrosoureas (BCNU/CCNU, 6 cycles) and radiotherapy.
Ethics statement
The study protocol was approved by the Ethics Committee of the Faculty of Medicine, Niš, Serbia. All patients included in our study signed written informed consent forms.
DNA isolation and bisulphite conversion
QIAamp® DNA Mini Kit (Qiagen) was used for extraction of genomic DNA [Citation18]. The quantity and quality of isolated DNA was measured by BioSpec-nano UV-Vis Spectrophotometer (SHIMADZU). EpiTect® Bisulfite Kit (Qiagen) was used for modification of 2 μg of genomic DNA by sodium bisulphite.
Analysis of methylation
The MGMT methylation status in glioma samples was determined using methylation-specific polymerase chain reaction (MSP). The MSP was conducted in a total volume of 20 μL containing 1x PCR buffer with 1.5 mmol/L MgCl2 (Qiagen), 0.2 μmol/L deoxyribonucleoside triphosphate (dNTP) mix, 1 U HotStar Taq polymerase (Qiagen), 100 ng of bisulphite converted template DNA and 10 pmol/L of the following forward (F) and reverse (R) primers:
Unmethylated (U) MGMT:
^ F: TTTGTGTTTTGATGTTTGTAGGTTTTTGT
^ R: AACTCCACACTCTTCCAAAAACAAAACA
Methylated (U) MGMT:
^ F: TTTCGACGTTCGTAGGTTTTCGC
^ R: GCACTCTTCCGAAAACGAAACG
The amplification reaction (Mastercycler Gradient, Eppendorf) was performed using the following programme: 95 °C for 15 min, then 35 cycles of 95 °C for 50 s, 59 °C for 50 s and 72 °C for 50 s and final extension at 72 °C for 10 min. A non-template control PCR reaction was also included and all PCR reactions were performed in duplicate.
The amplified PCR products were detected by ultraviolet (UV) light in a 2% agarose gel stained with ethidium bromide. Visible M primer band of MGMT indicated a positive MGMT methylation status, while absence of an M primer PCR product was considered as a negative methylation status of MGMT [Citation11].
Statistical analysis
Statistical analyses were performed in StatSoft, Inc. (2004) STATISTICA (data analysis software system), version 7 (www.statsoft.com). Student’s t-test was used to compare continuous variables.
Overall survival (OS) was measured from the date of surgery to date of death or last follow-up. The Kaplan–Meier method was used to estimate the OS curves and their comparison was performed with the use of an univariate log-rank test.
Results and discussion
Methylation status of the MGMT promoter and clinical parameters
DNA isolated from snap-frozen diffuse glioma samples was subjected to MSP using specific primers for methylated (M) and unmethylated (U) template detection. Methylation data were successfully acquired for all samples obtained from 33 patients ().
Figure 2. MSP amplification of the MGMT promoter CpG region using a methylated (M) and an unmethylated (U) set of primers; lanes 8–27, methylated and bisulphite converted DNA isolated from tumour samples.
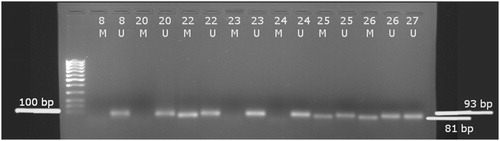
A positive methylation status of the MGMT promoter was detected in 17 patients (51.5%) in this study group (21 male, 12 female; age 59.64 ± 12.3, preoperative and postoperative Karnofsky score ≥80). Their clinical characteristics are shown in .
Table 2. Clinical characteristics and survival data.
MGMT status and overall survival
Correlation of the methylation status of the MGMT promoter and the overall survival was not found by comparison of survival distributions (log-rank test) ().
Table 3. Comparison of survival distributions (log-rank test).
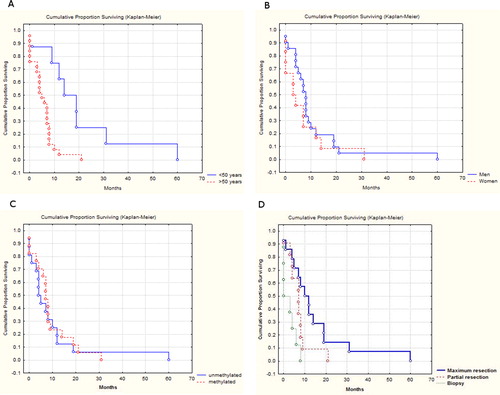
Kaplan–Meier OS curves related to methylation status are shown in .
Figure 3. Kaplan–Meier estimates of overall survival (months) related to age (A), gender (B), MGMT promoter methylation status (C) and type of resection (D).
The MGMT DNA repair enzyme counteracts the cytotoxic effect of DNA alkylating agents such as TMZ and mediates chemoresistance. The introduction of TMZ treatment and Stupp regimen in Serbia significantly improved the survival rates of glioblastoma patients [Citation19–21]. The survival outcome of the diffuse glioma patients from Serbia included in this study was not correlated with MGMT promoter methylation, which corresponds with findings from a recent study dealing with GBM patients in Serbia [Citation19]. Our study, however, has the limitation of a small number of subjects being included.
Since the original research of Esteller et al. [Citation11] in the late 90 s, many studies have delivered results that readily confirm the MGMT methylation status as a significant prognostic factor in malignant glioma. However, several studies have indicated that the prognostic significance of MGMT status in glioma patients may be still controversial [Citation17, Citation22, Citation23]. The heterogeneity in the survival among patients leads to the conclusion that additional genes are likely to be responsible for tumour chemosensitivity. This is supported by the fact that different glioma subtypes have different aetiology [Citation17].
Patients’ age and overall survival
Univariate analyses showed statistically significant improvement in overall survival of patients younger than 50 years (median survival 19 months) in comparison with the group of patients older than 50 years (median survival 7 months) (p < 0.00045) (). This result is in agreement with previous findings that overall survival is significantly higher for younger groups of patients (<50) in comparison with older groups (>50) [Citation19, Citation24–26]. Disruption of the DNA methylation mechanism is associated with aging and is characterized by genome-wide hypomethylation and promoter specific methylation [Citation26–28]. Decline in immune function is associated with elderly people, which also explains the poor prognosis in the group of older patients [Citation28].
Extent of tumour resection and overall survival
Our study confirmed the widely recognized correlation between the extent of the tumour resection and overall survival ( and ). The group of patients with total lesion resection had better overall survival in comparison to those with partial resection and biopsy, thus confirming the survival advantage of complete resection and its cytoreductive effects [Citation19, Citation29].
Conclusions
The analysis of the obtained data showed that the MGMT methylation did not have prognostic value for the diffuse glioma patients included in this study. Due to the small sample size, this result should be further explored in larger-scale, prospective controlled trials. With inclusion of new cases and enlargement of the study group our future investigation will provide more reliable results. Overall, the survival of older patients was significantly lower than that of younger patients, which was most probably a result of disruption of the DNA methylation pattern and the decline in the immune response with age. The extent of tumour resection was positively correlated with overall survival in the diffuse glioma patients from Serbia included in this study.
Acknowledgements
The authors thank their colleague Žaklina Šmelcerović for technical assistance during the laboratory work.
Funding
This research was supported by the Ministry of Education, Science and Technological Development of the Republic Serbia under projects III41018 and grant number 36 by the Faculty of Medicine, University of Niš.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Alcantara Llaguno SR, Parada LF. Cell of origin of glioma: biological and clinical implications. Br J Cancer. 2016;115:1445–1450.
- Lee SC. Diffuse gliomas for nonneuropathologists: The new integrated molecular diagnostics. Arch Pathol Lab Med. 2018;142:804–814.
- Wesseling P, Kros JM, Jeuken J. The pathological diagnosis of diffuse gliomas: towards a smart synthesis of microscopic and molecular information in a multidisciplinary context. Diagn Histopathol. 2011;17:486–494.
- Louis DN, Perry A, Reifenberger G, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820.
- Puduvalli VK, Chaudhary R, McClugage SG, et al. Beyond alkylating agents for gliomas: quo vadimus? Am Soc Clin Oncol Educ Book. 2017;37:175–186.
- Khan MN, Sharma AM, Pitz M, et al. High-grade glioma management and response assessment-recent advances and current challenges. Curr Oncol. 2016;23:e383–e391.
- Wang H, Xu T, Jiang Y, et al. The challenges and the promise of molecular targeted therapy in malignant gliomas. Neoplasia (New York, NY). 2015;17:239–255.
- Davis ME. Glioblastoma: overview of disease and treatment. Clin J Oncol Nurs. 2016;20:S2–S8.
- Sarkaria JN, Kitange GJ, James CD, et al. Mechanisms of chemoresistance to alkylating agents in malignant glioma. Clin Cancer Res. 2008;14:2900–2908.
- Damia G, D'Incalci M. Mechanisms of resistance to alkylating agents. Cytotechnology. 1998;27:165–173.
- Esteller M, Garcia-Foncillas J, Andion E, et al. Inactivation of the DNA-repair gene MGMT and the clinical response of gliomas to alkylating agents. N Engl J Med. 2000;343:1350–1354.
- Binabaj MM, Bahrami A, ShahidSales S, et al. The prognostic value of MGMT promoter methylation in glioblastoma: a meta-analysis of clinical trials. J Cell Physiol. 2018;233:378–386.
- Li Q, Guo J, Wang W, et al. Relationship between MGMT gene expression and treatment effectiveness and prognosis in glioma. Oncol Lett. 2017;14:229–233.
- Zawlik I, Kita D, Vaccarella S, et al. Common polymorphisms in the MDM2 and TP53 genes and the relationship between TP53 mutations and patient outcomes in glioblastomas. Brain Pathol. 2009;19:188–194.
- Hegi ME, Diserens A-C, Gorlia T, et al. MGMT Gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003.
- Mineura K, Yanagisawa T, Watanabe K, et al. Human brain tumor O6-methylguanine-DNA methyltransferase mRNA and its significance as an indicator of selective chloroethylnitrosourea chemotherapy. Int J Cancer. 1996;69:420–425.
- Brell M, Tortosa A, Verger E, et al. Prognostic significance of O6-methylguanine-DNA methyltransferase determined by promoter hypermethylation and immunohistochemical expression in anaplastic gliomas. Clin Cancer Res. 2005;11:5167–5174.
- Christians A, Hartmann C, Benner A, et al. Prognostic value of three different methods of MGMT promoter methylation analysis in a prospective trial on newly diagnosed glioblastoma. PLoS One. 2012;7:e33449.
- Illic R, Somma T, Savic D, et al. A survival analysis with identification of prognostic factors in a series of 110 patients with newly diagnosed glioblastoma before and after introduction of the stupp regimen: a single-center observational study. World Neurosurg. 2017;104:581–588.
- Nikolov V, Stojanović M, Kostić A, et al. Factors affecting the survival of patients with glioblastoma multiforme. J Buon. 2018; 23:173–178.
- Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–996.
- Jesien-Lewandowicz E, Jesionek-Kupnicka D, Zawlik I, et al. High incidence of MGMT promoter methylation in primary glioblastomas without correlation with TP53 gene mutations. Cancer Genet Cytogenet. 2009;188:77–82.
- Kalkan R, Atli Eİ, Özdemir M, et al. IDH1 mutations is prognostic marker for primary glioblastoma multiforme but MGMT hypermethylation is not prognostic for primary glioblastoma multiforme. Gene. 2015;554:81–86.
- Thumma SR, Fairbanks RK, Lamoreaux WT, et al. Effect of pretreatment clinical factors on overall survival in glioblastoma multiforme: a Surveillance Epidemiology and End Results (SEER) population analysis. World J Surg Oncol. 2012;10:75.
- Montecino-Rodriguez E, Berent-Maoz B, Dorshkind K. Causes, consequences, and reversal of immune system aging. J Clin Invest. 2013;123:958–965.
- Johnson AA, Akman K, Calimport SRG, et al. The role of DNA methylation in aging, rejuvenation, and age-related disease. Rejuvenation Res. 2012;15:483–494.
- Yang J, Yu L, Gaiteri C, et al. Association of DNA methylation in the brain with age in older persons is confounded by common neuropathologies. Int J Biochem Cell Biol. 2015;67:58–64.
- Jung M, Pfeifer GP. Aging and DNA methylation. BMC Biol. 2015;13:7.
- Thakkar JP, Dolecek TA, Horbinski C, et al. Epidemiologic and molecular prognostic review of glioblastoma. Cancer Epidemiol Biomarkers Prev. 2014;23:1985–1996.
