Abstract
A strain of Microbacterium kitamiense capable of decomposing cellulose was obtained from the soil of Shigatse region in Tibet. Low temperature and cellulase gene MkCel5 was cloned and sequenced. The size of the open-reading frame was 1449 bp; it was predicted to encode a polypeptide with 482 residues with a molecular weight of 51.38 kDa. Sequence analysis indicated that it belongs to the glycoside hydrolase family 5 (GH5), which has high homology with reported endoglucanases. The predicted protein structure had a typical carbohydrate-binding domain and a catalytic domain of cellulase. Site mutagenesis experiments showed that the two glutamates at positions 312 and 404 were crucial in the catalytic process. MkCel5 was identified as a cold-adaptive and neutral enzyme with a maximum activity at 25 °C and pH 7.0. These properties suggested that MkCel5 has potential for application in the cellulose processing industry requiring low temperature.
Introduction
In nature, a lot of natural and renewable biopolymers contain some complex structures which lead to difficulties in the direct utilization [Citation1]. Cellulose is one of the most abundant carbohydrate resources on earth, accounting for 30–50% of the dry weight of plant cell walls and an important material that needs to be processed during food processing [Citation2]. The chemical formula of cellulose is (C6H10O5)n and thousands of small glucose molecules linked by β-1,4-glycosidic bonds compose the cellulose macromolecule [Citation3]. Cellulose macromolecules can be degraded into small molecule, glucoses by cellulase. Actually, cellulase is a composite class of enzyme which is divided into three types: endoglucanase (endo-1, 4-D-glueanase, EC3. 2. 1. 4), exoglucanase (exo-1, 4-D-glucanase, EC3. 2. 1. 91) and glucosidase (1,4-D-glueosidase, EC3.2.1.21) and these three kinds of enzymes break down cellulose by synergistic action [Citation4].
Some of the cellulases previously reported and applied on the market were mesophilic and thermophilic enzymes [Citation5–12], while a few cold-adapted cellulases have been studied, such as CaCel from Cryptopygus antarctica [Citation13], CelG from Fibrobacter succinogenes S85 [Citation14], CelM of Pseudomonas sp. MM15 [Citation15], etc. Compared with medium-temperature cellulase, cold-active enzymes have a unique role in industrial production and food processing [Citation16]. For example, cold-active enzymes below 30 °C have high enzyme activity and catalytic efficiency and also exhibit some advantages: (1) they shorten the processing time, (2) save the cost of heating and cooling, (3) can be inactivated by heat treatment and (4) do not affect the quality of the products. Therefore, such enzymes have an important application value in the low temperature bioprocessing, textile, papermaking, environmental protection and medicine and other fields.
Microbacterium kitamiense, with a growth temperature of 15–37 °C and a growth pH of 6.0–9.0 [Citation17] was found in the soil of the Shigatse region of Tibet. A cellulase gene (named as MkCel5) was successfully cloned, the recombinant plasmid was expressed in Escherichia coli Rosetta (DE3) and the enzyme was purified and studied. The cold-active character of MkCel5 would make it a candidate for the processing industry needing low temperature. The 3D-model structure of the protein and site-directed mutagenesis showed that the two conserved catalytic residues Glu312 and Glu404 were critical in the catalytic process [Citation18].
Materials and methods
Strains and plasmids
The Microbacterium kitamiense strain used in this study was isolated from the soil of Shigatse region in Tibet. It was routinely grown in Luria-Bertani (LB) broth in a constant temperature shaker (Sukun, China) at 37 °C, 225 rpm. The bacterial genomic DNA was extracted using TIANamp Bacterial DNA Kit (TIANGEN, China). The pET24a(+) (Novagen, Germany) plasmid was used as a carrier of the target gene, and Escherichia coli DH5α and E. coli Rosetta (DE3) served as hosts for expression of the recombinant plasmid.
Gene cloning and site-directed mutagenesis
The predicted cellulase gene MkCel5 was amplified using PrimeSTAR DNA polymerase (TaKaRa, Japan). The primers were MkCel5-F 5′-GGGGAATTCCATATGAGGAAAGCCATGATGAGGAC-3′ and MkCel5-R 5′-CCCCAAGCTTGAGCCCGCTCCGCCCGCGCGGGGGGTG-3′ (Nde I and Hind ш sites are underlined, respectively) according to the nucleotide sequence (GenBank number: PGGU00000). The product was purified by AxyPrep DNA Purification Kit (Axygen, USA) and was connected to the expression vector pET-24a(+), and then the recombinant plasmid was transformed into E. coli Rosetta (DE3) strain after the gene sequence was confirmed. Single site mutated genes were prepared by site-directed mutagenesis using an improved overlapping extended PCR method [Citation19]. Two groups of mutation primers were designed for the first step of PCR to create overlapping gene fragments (E312-F 5′-CGAGATCGCCAACGCACCGAGCGGCGTCTCC-3′; E312-R 5′-GGAGACGC CGCTCGGTGCGTTGGCGATCTCG-3′; E404-F 5′-CCACGTTCGTCACGGCATGGGGAGCGGAGGAG-3′; E404-R 5′-CTCCTCCGCTCCCCATGCCGTGACGAACGTGG-3′; mutation sites are underlined), which then served as template DNA for the second step of PCR to produce a complete gene. Two single-point mutant genes were recombined with plasmid pET-24a(+) and introduced into E. coli Rosetta (DE3) for recombinant protein expression.
Protein expression and purification
E. coli Rosetta (DE3) containing wild-type and mutant genes were cultivated to an OD600 of 0.5 in LB medium containing 30 μg mL−1 kanamycin and chloramphenicol at 37 °C, respectively. Then, isopropyl-β-d-thiogalactoside (IPTG) was added to the cultures at a final concentration of 0.1 mmol L−1 and the protein was induced to expression for 20 h at 20 °C, the bacteria were collected and resuspended in buffer (0.1 M NaH2PO4, 0.01 mol L−1 Tris-HCl, pH 7.0) and ultrasonicated (Shunma, China) on ice. Then the supernatant and precipitation were collected by centrifugation (12,857 g, 20 min, 4 °C) (Eppendorf, Germany) for SDS-PAGE analysis. All three proteins are inclusion bodies and the active proteins were obtained by the inclusion body refolding method to study the enzymatic properties.
Renaturation method was used to collect the precipitate after ultrasonication and centrifugation, then the precipitate was resuspended in lysis buffer (0.1 mol L−1 NaH2PO4, 0.01 mol L−1 Tris-HCl, 5% Triton X-100, pH 7.0) and centrifuged at low temperature for 20 min. The precipitate was washed three times with buffer (0.1 mol L−1 NaH2PO4, 0.01 mol L−1 Tris-HCl, pH 7.0), finally dissolved in a lysis buffer containing high concentration of urea (0.1 mol L−1 NaH2PO4, 0.01 mol L−1 Tris-HCl, 8 mol L−1 urea, pH 7.0) on a vertical rotator for 2 h and centrifuged at low temperature for 20 min to acquire the supernatant. According to the WorkBeads™ 40Ni (TIANGEN, China) protein purification method with some modification, the supernatant was added to a Ni2+-NTA agarose gel column for purification. The bound heteroprotein was eluted with 10 mL elution buffer (0.1 mol L−1 NaH2PO4, 0.01 mol L−1 Tris-HCl, 15 mmol L−1 imidazole, pH 7.0) and the protein of interest was eluted with 2 mL elution buffer (0.1 molL−1 NaH2PO4, 0.01 molL−1 Tris-HCl, 250 mmol L−1 imidazole, pH 7.0), then the elution liquid was dialyzed overnight. After dialysis, the protein was concentrated at low temperature (3214 g, 20 min, 4 °C), and analyzed by SDS-PAGE. Protein concentration was determined by the Bradford method [Citation20].
Characterization of enzyme activity
The standard enzyme activities of MkCel5 and its mutant E312, E404 were determined by the 3,5-dinitrosalicylic acid (DNS) method. The method was as follows: 50 μL of the crude enzyme solution was mixed thoroughly with 50 μL of 1.0% CMC dissolved in 0.2 mol L−1 Na2HPO4-citric acid buffer solution (pH 7.0). After incubation at 25 °C for 60 min, 100 μL DNS reagent was added and the reaction mixture was heated uniformly in boiling water for 5 min to terminate the reaction. After that, the mixture was mixed thoroughly with 800 μL H2O and the absorbance of the mixture was measured at 540 nm. With glucose solution as the standard solution, an enzyme unit is defined as the amount of enzyme required to produce 1 μmol glucose per minute at 25 °C [Citation4].
To determine the optimal reaction temperature of MkCel5, its activity was measured from 0 °C to 60 °C. The thermostability of MkCel5 was determined by evaluating the residual activity following 1-h incubation at a range of temperatures from 0 °C to 60 °C. The optimum reaction pH of MkCel5 was determined through evaluating the maximal activity in several buffer conditions ranging from pH 3.0 to 11.0 (0.2 mol L−1 Na2HPO4-citric acid (pH 3.0–8.0) and 0.05 mol L−1 glycine-NaOH (pH 9.0–11.0)) after determining the optimum temperature. The determination of pH stability is the maximum residual activity after the enzyme was incubated in different pH buffer (pH 3.0–11.0) for 1 h.
To determine the Km and Vmax values, the activity of the enzymatic reaction was measured at six different concentrations of the substrate CMC (ranging from 1 to 20 mg mL−1) around the dissociation constant (Km) values. The data were in accordance with the Michaelis–Menten equation and the values of Km and Vmax values were calculated using GraphPad Prism software (v.5.1, GraphPad Software, Inc.).
Molecular models of proteins
Swiss-model Workspace [Citation21, Citation22] was used to build the protein structure model based on the reported crystal structure from Thermobifida fusca (PDB code 2CKR, cellulose enzyme protein module) and Paenibacillus fukuinensis (PDB code 4ZY9, chitosan-binding module) as the templates [Citation23]. PyMoL v 1.2.1 was used to predict and explain the simulated images.
Results and discussion
Cloning and sequence analysis of MkCel5
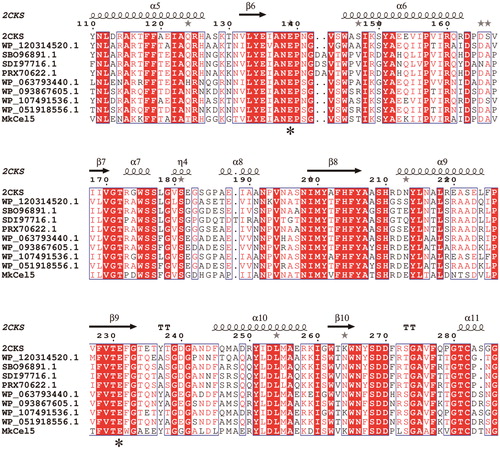
The MkCel5 gene was amplified from genomic DNA of Microbacterium kitamiense. Sequence analysis revealed that the open-reading frame of MkCel5 contained 1449 bp, coding a polypeptide with a size of 482 amino acids. The predicted molecular weight of MkCel5 was 51.38 kDa and pI was 4.94. Conserved domain analysis showed that MkCel5 had two entirely different domains, belonging to the glycoside hydrolase family 5 (GH5, 192–443 residues) and the F5/8 type C domain (46–160 residues), respectively. There is a connection between the two domains, which is rich in Gly and Thr [Citation24, Citation25].
Using BioEdit and ESPript 3.0 for multiple sequence alignment (), we found that the GH5 module of Mkcel5 is similar to Endoglucanase Cel5a (63%, PDB: 2CKS, Thermobifida Fusca), Endo-β-1,4-glucanase (55%, NCBI reference sequence: SBO96891.1, Nonomuraea gerenzanensis), Endoglucanase (55%, NCBI reference sequence: SDI97716.1, Nonomuraea jiangxiensis), GH5 protein (62%, NCBI reference sequence: WP_063793440.1, Streptomyces hirsutus), GH5 protein (63%, NCBI reference sequence: WP_093867605.1, Streptomyces sp. KS_5), GH5 protein (63%, NCBI reference sequence: WP_107491536.1, Thermobifida fusca) and GH5 protein (62%, NCBI reference sequence: WP_051918556.1, Streptomyces sp. PAN_FS17). The F5/8 type C domain of Mkcel5 resembles carbohydrate-binding protein (56%, NCBI reference sequence: WP_120314520.1, Catellatospora citrea) and F5/8 type C domain-containing protein (53%, NCBI reference sequence: PRX70622.1, Nonomuraea fuscirosea). These sequences show very high similarity at some amino acid loci, and are strictly conserved at the two catalytic residue sites Glu312 and Glu404. The two residues serve as catalytic proton donors and active site nucleophiles, respectively, which are capable of maintaining the structure of the substrate isocarbon for the enzyme [Citation18].
Congo red staining method to identify enzyme activity
The Congo red dye can turn the carboxymethyl cellulose (CMC) medium red. The cellulase decomposes the CMC to form a small molecule, so there will be seen a hydrolytic halo on the medium. In this study, a transparent hydrolytic halo was formed on the medium by the wild-type and the two mutants, but the transparent halos produced by the two mutant enzymes were not as clear as that of the wild-type enzyme (). It can be seen that the enzyme activities were significantly reduced on account of the replacement of Glu312 or Glu404 by alanine, indicating that these two residues play an important role in the catalytic action of the enzyme.
Expression and characterization of enzyme
The proteins encoded by MkCel5, E312 and E404 genes with 6His-tag at the N-terminal were expressed in E. coli Rosetta, and the resulting inclusion bodies were renatured and purified using an affinity chromatography column. The standard enzyme activity of the wild-type enzyme was 0.6 U mg−1 and the standard enzyme activities of the mutants E312 and E404 were 0.003 and 0.005 U mg−1, respectively. Since the value of the wild-type enzyme activity was higher than the values of the mutants, it can be seen that MkCel5 has strong catalytic ability for cellulose and the data were consistent with the Congo red staining experiment. To calculate the Km and Vmax of MkCel5, we measured the enzyme activity at different substrate concentrations and brought the obtained data to the Michaelis–Menten equation to draw the curve. The Km value of MkCel5 was 42.11 mg mL−1 and the calculated Vmax was 1.51 mmol min−1 mg−1. shows the properties of the reported cold-active cellulase. Compared with other cold-active enzymes, MkCel5 has lower viability, but as a novel cold-active cellulase gene resource, it has reference significance for enzyme function research. Our future research will focus on increasing the expression of soluble enzymes and enzyme activity.
Table 1. Some reported cold active cellulases and their properties.
Effect of temperature, pH, metal ions and some reagents on the activity of MkCel5
MkCel5 reached its maximum enzyme activity at 25 °C, retained 40% of the maximum activity at 0 °C, decreased above 40 °C and completely lost activity at temperatures higher than 50 °C (). Thermal stability experiments showed that the enzyme was very stable at 0–30 °C, and still retained 80% of its maximum activity after incubation for 1 h at 0 °C, but the enzyme was rapidly inactivated above 30 °C (). MkCel5 belongs to the cold-active enzymes. Margesin and Schinner [Citation26] defined cryogenic enzyme or cold-active enzyme as the optimal catalytic temperature at around 30 °C, and the enzyme with certain catalytic efficiency at 0 °C. In fact, some enzymes have an optimum temperature above 30 °C, but have strong enzyme activity at low temperatures or even 0 °C. In , we can see that MkCel5’s properties are superior to those of other cold-active enzymes. Its optimum temperature is low and most of the enzyme activity is retained at 0 °C, this shows that it is very stable and very suitable for low temperature applications
Figure 3. Influence of pH, temperature, metal ions and reagents on the activity and stability of purified recombinant MkCel5. Influence of temperature on protein activity (a) and stability (b); influence of pH on protein activity (c) and stability (d). Effect of metal ions and reagents on the activity of MkCel5 (e).
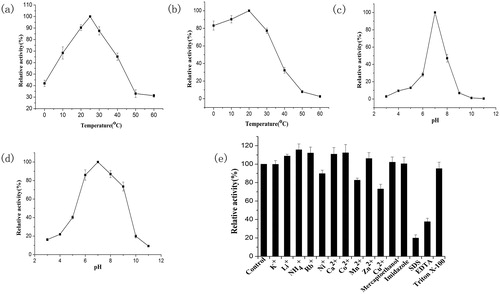
MkCel5 was active within a relatively narrow pH range (pH 5.0–8.0) () and reached the maximum activity at pH 7.0 (). In , we can see that MkCel5 works in acidic, neutral and weakly alkaline environments compared to most acidic cold-active enzymes. Rb+, Ca2+ and Co2+ at a concentration of 1 mmol L−1 could stimulate the enzyme activity; Cu2+ and Mn2+ could reduce the activity of the enzyme but it still retained most of its activity (>80%). The surfactant SDS and the metal chelator EDTA significantly reduced the activity of the enzyme (). In addition, TritonX-100 used in the protein renaturing process did not affect the activity of the enzyme. Studies [Citation27] related to cold-active enzymes show that the stability provided by Ca2+ binding is higher than that provided by all weak interactions and even higher than that provided by disulphide bonds. Eight to nine ligand bonds involved in this mechanism can be linked to multiple secondary structures or protein domains to increase the stability of substrate binding to enzymes. It has also been reported that the mechanism underlying the reduction of the enzyme activity in the presence of Cu2+ lies in its ability to bind the mercaptan groups of some amino acids or to interact with the carboxyl groups of some amino acids [Citation4].
Potential applications of cold-active cellulase
Cold-active cellulase has a unique role in industrial production and food processing. Compared with medium temperature cellulase, low temperature cellulase has high enzyme activity and catalytic efficiency at room temperature, and allows to quickly reach the temperature required for production. On the other hand, it can save heating and cooling costs in the production process, and can use mild heat treatment to inactivate the enzyme without affecting the quality of the product. In food processing, more and more companies are adopting low-temperature processing methods, because low temperature can not only reduce the consumption of production energy, but also preserve the original quality of food, reduce the loss of some essential flavours and reduce the occurrence of other adverse reactions [Citation5]. In the textile industry, cold active cellulase can participate in low temperature washing, biopolishing at low temperatures to remove fine fluff on the surface of cotton fabric, etc. [Citation28]. In biofuel production, bioconversion of cellulose to industrial ethanol is generally required to be carried out in an environment of 50–60 °C. Saccharomyces cerevisiae and cold-active cellulase complex can produce ethanol from cellulose by coupling saccharification/fermentation at low temperature, saving production costs while increasing ethanol production [Citation31].
Protein modeling
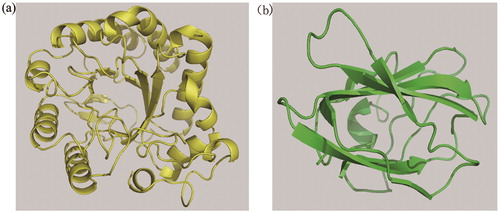
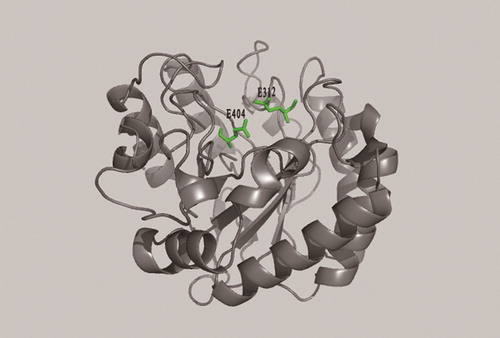
According to the results of the conserved domain analysis, two protein crystal structures with high fitting degree to the two domains of MkCel5 protein: the cellulose endoglucanase protein crystal structure from Thermobofida fusca (PDB code 2CKR) and the crystal structure of chitosan-binding module from Paenibacillus fukuinensis (PDB code 4ZY9). The crystal structures of the two proteins reported were used as templates to simulate the three-dimensional structure of MkCel5 protein, so the positions of the two domains at the tertiary level of the protein were observed more intuitively (). The model image showed that the predicted GH5 module of MkCel5 protein structure () has a typical (β/α)8 barrel structure of the GH5 family and the predicted substrate bonding module () has a shape of a β-sandwich fold. In the structure of the wild-type enzyme, the G312 and G404 residues were located on either side of the protein active pocket (). In the study of the catalytic mechanism of double displacement put forward by Koshland [Citation32], G312 provides hydrogen (H) as a proton donor to catalyze substrate and nearby G404 provides oxygen (O) as a nucleophile to bind substrate [Citation18]. The two single-point mutant proteins constructed had only slight catalytic ability for CMC and their loss of activity also showed the important role of these two catalytic residues.
Conclusions
This is the first report of the cloning, expression and characterization of the endoglucanase gene from Microbacterium kitamiense. The expressed recombinant MkCel5 protein had cellulase activity in a narrow range of pH and temperature. It is worth noting that MkCel5 exhibits good enzyme activity and high stability at low temperature conditions (0–25 °C). Compared with medium-temperature cellulases, this cold-active enzyme could have a unique role in industrial production and food processing. In addition, directed-site mutagenesis experiments provided evidence for the two-displacement mechanism. Through the study of the two glutamic acid catalytic residues, a new idea for the study of the double displacement mechanism of the GH5 can be further provided. We hope to conduct further research on the genetic modification of this protein in the future in order to obtain soluble proteins and gain greater enzyme activity.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This work was supported by the Provincial Key Project of Natural Science Research for Colleges and Universities of Anhui Province of China (KJ2017A321 and KJ2017ZD26), the Provincial Innovation Project for Oversea Talents in Anhui Province, Innovation Team of Scientific Research Platform in Anhui Universities and Anhui Provincial Key Laboratory of the Conservation and Exploitation of Biological Resources.
References
- Zihare L, Blumberga D. Market opportunities for cellulose products from combined renewable resources. Environ Clim Technol. 2017;19:33–38.
- Siqueira F, Filho EXF. Plant cell wall as a substrate for the production of enzymes with Industrial applications. Mini-Rev Org Chem. 2010;7:54–60.
- Klemm D, Heublein B, Fink HP, et al. Cellulose: fascinating biopolymer and sustainable raw material. Angew Chem Int Ed Engl. 2005;44:3358–3393.
- Lin L, Liu X, Zhou Y, et al. A novel pH-stable, endoglucanase (JqCel5A) isolated from a salt-lake microorganism, Jonesia quinghaiensis. Electron J Biotechnol. 2016;24:56–62.
- Ueda M, Maruyama T, Kawasaki K, et al. Purification, characterization, and gene cloning of a cold-adapted endo-1,4-β-glucanase from Bellamya chinensis laeta. Mol Biotechnol. 2016;58:241–250.
- Irfan M, Guler HI, Belduz AO, et al. Cloning, purification and characterization of a cellulase-free xylanase from Geobacillus thermodenitrificans AK53. Appl Biochem Microbiol. 2016;52:277–286.
- Shinoda S, Kanamasa S, Arai M. Cloning of an endoglycanase gene from Paenibacillus cookii and characterization of the recombinant enzyme. Biotechnol Lett. 2012;34:281–286.
- Rubini MR, Dillon AJP, Kyaw CM, et al. Cloning, characterization and heterologous expression of the first Penicillium echinulatum cellulase gene. J Appl Microbiol. 2010;108:1187–1198.
- Zheng F, Tu T, Wang X, et al. Enhancing the catalytic activity of a novel GH5 cellulase GtCel5 from Gloeophyllum trabeum CBS 900.73 by site-directed mutagenesis on loop 6. Biotechnol Biofuels. 2018;11:76.
- Shahid ZH, Irfan M, Nadeem M, et al. Production, purification, and characterization of carboxymethyl cellulase from novel strain Bacillus megaterium. Environ Prog Sustain Energy. 2016;35:1741–1749.
- Lee JP, Kim YA, Kim SK, et al. Characterization of a multimodular endo-β-1,4-Glucanase (Cel9K) from Paenibacillus sp. X4 with a potential additive for saccharification. J Microbiol Biotechnol. 2018;28:588–596.
- Tan H, Miao R, Liu T, et al. A bifunctional cellulase-xylanase of a new Chryseobacterium strain isolated from the dung of a straw-fed cattle. Microb Biotechnol. 2018;11:381–398.
- Song JM, Hong SK, An YJ, et al. Genetic and structural characterization of a thermo-tolerant, cold-active, and acidic endo-β-1,4-glucanase from Antarctic Springtail, Cryptopygus antarcticus. J Agric Food Chem. 2017;65:1630–1640.
- Iyo AH, Forsberg CW. A cold-active glucanase from the ruminal bacterium Fibrobacter succinogenes S85. Appl Environ Microbiol. 1999;65:995–998.
- Bai X, Yuan X, Wen A, et al. Cloning, expression and characterization of a cold-adapted endo-1, 4-β-glucanase from Citrobacter farmeri A1, a symbiotic bacterium of Reticulitermes labralis. PeerJ. 2016;4:e2679.
- Zeng R, Xiong P, Wen J. Characterization and gene cloning of a cold-active cellulase from a deep-sea psychrotrophic bacterium Pseudoalteromonas sp. DY3. Extremophiles. 2006;10:79–82.
- Matsuyama H, Kawasaki K, Yumoto I, et al. Microbacterium kitamiense sp. nov. a new polysaccharide-producing bacterium isolated from the wastewater of a sugar-beet factory. Int J Syst Bacteriol. 1999;49:1353–1357.
- Valérie D, Czjzek M, Belaich A, et al. Crystal stucture of the catalytic domain of a bacterial cellulase belonging to family 5. Structure. 1995;3:939–949.
- Heckman KL, Pease LR. Gene splicing and mutagenesis by PCR-driven overlap extension. Nat Protoc. 2007;2:924–932.
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254.
- Arnold K, Bordoli L, Kopp J, et al. The SWISS-MODEL workspace: a web-based environment for protein structure homology modelling. Bioinformatics. 2006;22:195–201.
- Bordoli L, Kiefer F, Arnold K, et al. Protein structure homology modeling using SWISS-MODEL workspace. Nat Protoc. 2009;4:1–13.
- Shinya S, Nishimura S, Kitaoku Y, et al. Mechanism of chitosan recognition by CBM32 carbohydrate-binding modules from a Paenibacillus sp. IK-5 chitosanase/glucanase. Biochem J. 2016;473:1085–1095.
- Marchler-Bauer A. CDD: a conserved domain database for protein classification. Nucleic Acids Res. 2005;33:192–196.
- Lombard V, Golaconda Ramulu H, Drula E, et al. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014;42:490–495.
- Margesin R, Schinner F. Low-temperature bioremediation of a waste water contaminated with anionic surfactants and fuel oil. Appl Microbiol Biotechnol. 1998;49:482–486.
- Yang J, Dang H. Cloning and characterization of a novel cold-active endoglucanase establishing a new subfamily of glycosyl hydrolase family 5 from a psychrophilic deep-sea bacterium. Fems Microbiol Lett. 2011;325:71–76.
- Bhat A, Riyaz-Ul-Hassan S, Ahmad N, et al. Isolation of cold-active, acidic endocellulase from Ladakh soil by functional metagenomics. Extremophiles. 2013;17:229–239.
- Ghadikolaei KK, Gharechahi J, Haghbeen K, et al. A cold-adapted endoglucanase from camel rumen with high catalytic activity at moderate and low temperatures: an anomaly of truly cold-adapted evolution in a mesophilic environment. Extremophiles. 2018;22:315–326.
- Fu X, Liu P, Lin L, et al. A novel endoglucanase (Cel9P) from a marine bacterium Paenibacillus sp. BME14. Appl Biochem Biotechnol. 2010;160:1627–1636.
- Deshpande MV. Ethanol production from cellulose by coupled saccharification/fermentation using Saccharomyces cerevisiae and cellulase complex from Sclerotium rolfsii UV-8 mutant. Appl Biochem Biotechnol. 1992;36:227–234.
- Koshland D. Stereochemistry and the mechanism of enzymatic reactions. Biol Rev. 1953;28:416–436.