?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study investigates in-vitro activities of phytochemicals in Callistemon citrinus against multi-resistant foodborne pathogens, alpha-glucosidase enzyme and MCF-7 cancer cell line. Assays were prepared with lyophilized extracts to determine antioxidant capacity, inhibition of α-glucosidase enzyme and growth of foodborne bacteria. Annexin-V detection kit was used for apoptosis detection and FT-IR spectroscopy to confirm structural and functional groups of phytochemicals. Cytotoxicity of the extracts against MCF-7 cells was monitored with xCELLigence Real-Time Cell Analyser. The result from FT-IR analysis gave a peak at 3295 cm−1 wavenumber, confirming the presence of O–H alcohol functional group. FT-IR analysis also showed the presence of different functional groups such as carboxylic acids, aromatics, alkanes, alcohols, aliphatic amines, alkenes and amine groups in the extracts. Callistemon exhibited strong antioxidant capacities with EC50 values of 0.474 ± 0.03 and 0.787 ± 0.15 mL sample/g of 2,2-diphenyl-1-picrylhydeazyl (DPPH) from leaf and flower extracts, respectively. Growth inhibition of most gram-positive foodborne bacteria by phytochemicals from flower extract appeared more promising as an alternative antimicrobial agent for food preservation. IC50 value of 3.69 ± 0.61 μg/mL obtained from leaf extract showed its inhibitory potential against α-glucosidase enzyme for managing diabetes type-2. A dose response obtained from real-time monitoring with xCELLigence system indicated higher cytotoxicity of the extracts against MCF-7 cell line at ≥200 μg/mL concentrations within 24 h of incubation. The versatility of phytochemicals in Callistemon observed in this study signifies its potential for enhancing feed or food functionality, moderating blood glucose level and inhibiting the growth of foodborne pathogens or invasive carcinoma in man.
Introduction
Life-threatening complications resulting from breast cancer, diabetes and foodborne illnesses are amongst the leading causes of morbidity and mortality worldwide. The debilitating effects of these conditions contribute to soaring epidemic and sporadic health crisis in many countries. In agro-allied industry, foodborne enteric pathogens enter the food chain at any point of attribution from farm to fork. Microbial toxins released in the process, potentially cause food poisoning among vulnerable population [Citation1]. In 2010, 600 (95% uncertainty interval [UI] 420–960) million foodborne illnesses and 420,000 (95% UI 310,000–600,000) deaths were reported owing to foodborne hazards caused by contaminants, invasive infections and diarrhoea causing agents [Citation2]. Bacillus cereus, Listeria monocytogenes, Staphylococcus aureus, Escherichia coli O157:H7, Salmonella typhimurium, Yersinia enterocolitica, Staphylococcus carnosus, Staphylococcus xylosus, Lactobacillus sakei and Lactobacillus plantarum rank high among foodborne organisms of public health concerns [Citation1]. With ‘one-in-eleven adults’ being victims; ‘one-in-two’ undiagnosed and ‘one-in-seven births’ being affected during pregnancy, over 400 million people (20–79 years) are living with diabetes. The number of victims is predicted to increase to 629 million by 2045 [Citation3]. Of the three types of diabetes (type-1, type-2 and gestational diabetes), type-2 diabetes is the most prevalent form. The resultant hyperglycaemia and impairment of pancreatic β-cells from secreting sufficient insulin are comorbid with several complications such as diabetic ketoacidosis, frequent urination (polyuria), increased thirst (polydipsia), increased hunger (polyphagia), renal impairment, hyperosmolar coma, retinopathy, neuropathy, periodontitis, development of diabetic foot (foot ulcer) and risks of dying prematurely [Citation4,Citation5].
Through meta-analysis, co-existence of risk factors contributing to diabetes type-2 and breast cancer was found among Asian, Hispanic and African-American ancestries [Citation6]. Cancer exists in various forms but every tumour exerts different biochemical and painful effects on the victims [Citation7]. As human breast cancer cell line with estrogen, progesterone and glucocorticoid receptors, Michigan Cancer Foundation-7 (MCF-7) is a good model for investigating the effects of various oncogenes on signal transduction pathways [Citation8,Citation9]. Human malignancy caused by breast cancer constitutes one-third of morbidity among women and second highest cause of mortality with low survival rate [Citation10]. While it is difficult to develop an effective ‘one-size-fits-all’ treatments for cancer, monitoring cell viability or toxicity is necessary for preclinical screening to ascertain bioactivities of new compounds vis-à-vis regulatory requirements or induction of apoptosis [Citation11,Citation12]. To date, mono-therapies are seemingly unsuccessful against the virulence of some food pathogens, type-2 diabetes and breast cancer. The use of synthetic antioxidants even promotes carcinogenesis and some side effects [Citation13]. This situation necessitates the use of medicinal plant to provide phytotherapy against type-2 diabetes and breast cancer.
In this study, Callistemon citrinus was chosen for its therapeutic properties in ethno-pharmacology. C. citrinus (Curtis, skeels) is an ornamental medicinal plant from the Myrtaceae family consisting of 132 genera and 5950 species [Citation14,Citation15]. It is commonly known as crimson bottlebrush, red bottlebrush or lemon bottlebrush. Alkaloids, aliphatic acids, flavonoids, polyphenols, tannins, steroids, monoterpenoids, sesquiterpenes and triterpenoids have been isolated from stem backs, leaves, seeds and flowers of this plant [Citation16]. Folkloric use of callistemon include formulation of pills for treating dysentery, bronchitis, haemorrhoids (piles), rheumatism, tuberculosis, urinary incontinence and cleansing genitourinary tract from excessive menstruation or mucosal discharge [Citation17,Citation18]. Essential oils from C. citrinus show higher antibiotic activity over few synthetic antibiotics like micoazole and clotrimazole [Citation19]. In Jamaica, hot drinks are locally prepared from C. viminalis leaves to cure gastroenteritis, diarrhoea and skin infections [Citation20]. Australian Aborigines even suck the nectar from flowers for the production of sweet drinks due to its refreshing flavour [Citation15]. Antimicrobial activity of lyophilized extracts from flowers and leaves of C. citrinus inhibits the growth of Listeria monocytogenes in beef burgers [Citation21]. Either in dietary or non-dietary form, natural antioxidants can reduce oxidative stress [Citation22] and prevent oxidative rancidity in fat-based foods, meat and dairy products [Citation23,Citation24]. Phytochemical screening is effective for predicting antioxidant activity, cytotoxicity, toxicodynamics and therapeutic properties of plants [Citation25]. The objectives of this study were to determine the antioxidant capacity of phytochemicals in the leaves and flowers of C. citrinus and their bioactivities against alpha glucosidase inhibition, some foodborne pathogens and MCF-7 human breast adenocarcinoma cell line.
Materials and methods
Plant collection and preparation
Fresh leaves and flowers of C. citrinus were harvested, dried and grounded into coarse powder after botanical authentication. Following simplex lattice design, 40 g of extract from the leaves (CL) was diluted in 960 mL deionized water and 40 g of extract from flowers (CF) in a mixture of 480 mL methanol plus 480 mL deionized water and distillated with Heidolph rotary evaporator (Hei-VAP HL/G3). The distillates from CL and CF were lyophilized with LABCONCO freeze dryer (model 117-A65312906; freezone 2.5) for 24 h and stored in plastic vials at –80 °C until analysis.
Chemicals and extraction
HPLC grade water (18.2 MΩ cm) was prepared using a Millipore Simplicity 185 Direct-Q water purification system (Millipore Corp., Bedford, USA). Gallic acid, methanol, sodium carbonate, KH2PO4, sodium hydroxide, α-glucosidase, p-nitrophenyl α-d-glucoside, l-glutathione, potassium persulfate, sodium chloride, 2,2-diphenyl-1-picrylhydeazyl (DPPH), Trolox, 2,2′Azinobis-(3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) were used for the analyses. These reagents were purchased from Sigma-Aldrich chemical company (St. Louis, USA). Extraction of antioxidants from the samples was first done by screening experiments. Solvent mixtures comprising methanol, acetone, ethanol and water (18.2 MΩ-cm) were screened prior to the determination of total phenolic content (TPC). The aim of screening was to determine the best solvent or combination of solvents that would give optimal extractability of phytochemicals from the samples. After screening, solvents were statistically optimized using simplex lattice design to have the best proportions of solvents for extracting TPC from the extracts (). This design was chosen for its appropriateness in the experimental domain.
Table 1. Simplex lattice design and experimental data for responses from Callistemon extract.
Total phenolic content (TPC)
TPC was determined as described by Aaby et al. [Citation26]. Appropriately diluted extract (0.4 mL) from CL and CF was mixed with 2 mL Folin solution and 1.6 mL of Na2CO3. Blank solution was prepared in the same way using the same amount of water instead of extracts. All samples were incubated for 1 h under room temperature. Absorbance was measured at 765 nm (using Agilent 8453 spectrophotometer, Waldronn, Germany). Five different concentrations of Gallic acid solutions (20–100 mg/L) were used for the calibration. All the extracts were analysed in three replicates. Final results were expressed as Gallic Acid Equivalents (mg GAE) per gram of dry matter.
Fourier transform infrared (FT-IR) spectroscopy
FT-IR spectroscopy was used to identify different chemical bonds or functional groups in phytochemicals as described in literature with minor modification [Citation27]. Few drops of C. citrinus extracts were used for FT-IR analysis and 5 g of the sample was weighed into 250 mL beaker containing distilled water and boiled under continuous stirring for some minutes. Spectroscopic spectra were obtained through FT-IR characterization by using Agilent Technology, UV-1901, and Cary 600 series FT-IR spectrometer, USA in the spectral region of 4000–400 cm−1 and resolution of 4 cm−1.
Antioxidant capacity
Two methods were used to determine antioxidant capacity of the extracts. DPPH• radical scavenging assay was performed according to Brand-Williams et al. [Citation28] with some modifications. Standard solution of 25 mg DPPH• was prepared and the mixtures were vortexed for 10 s and 0.1 mL of 500-fold diluted extracts was pipetted into different tubes. The extracts were diluted at 5-, 25-, 100-, 250- and 500-fold, respectively. The control was prepared following similar procedures by adding 0.1 mL distilled water instead of the extracts. The solutions were then left to stand in the dark for 30 minutes at room temperature. Absorbance of the resulting violet coloured mixture was read at 517 nm after baseline correction with methanol. Radical scavenging capacity was expressed as the inhibition percentage effect and calculated using the formula:
Results were expressed as efficient concentration (EC50), based on percentage of radical inhibition in relation to the control without extract. Trolox Equivalent Antioxidant Capacity (TEAC) was determined by ABTS•+ radical scavenging activity. Stock solution was prepared by dissolving 30 mg ABTS•+ in 7.8 mL of 2.46 mmol/L potassium peroxodisulfate buffer (pH 7.6) to give 0.700 ± 0.005 absorbance at 734 nm. Samples (50 μL) were diluted with the same buffer by 1:20 (v/v); and mixed with ABTS•+ solution. Absorbance was measured after 6 min of incubation. Results were expressed as mg TEAC per gram of the sample.
Alpha-glucosidase inhibition assay
Alpha-glucosidase inhibition activity of the extracts was determined according to the method by Ryu et al. [Citation29] with slight modifications. Briefly, 0.2 unit/mL of α-glucosidase (50 μL) in cold 67 mmol/L KH2PO4 (pH 6.8), 3 mmol/L glutathione solution (50 μL) in order to activate the supply of enzyme activity. Properly diluted extracts were pipetted into a screw capped vial followed by the addition of 1250 μL of phosphate buffer for proper dilution. The mixtures were vortexed and placed in a rack to equilibrate at 37 °C in a water bath. Ten millimoles per litre of p-nitrophenyl α-d-glucoside (125 μL) was pipetted into each mixture to start the enzyme solution. The reaction was stopped with the addition of 100 mmol/L Na2CO3 (2 mL) for 20 min. Control samples were prepared by adding water instead of extracts. Blank samples were also prepared by adding equal volume of water instead of extracts and enzyme solution. Absorbance of the resulting pale yellow colour due to activity of p-nitrophenyl from the substrate was recorded at 400 nm. Enzyme activity of α-glucosidase was determined in the presence of extracts at various concentrations ranging from 1 to 10 mg/L. The IC50 values were calculated based on the graphs constructed by % inhibition vs. sample concentration and expressed as percentage inhibition following the equation below:
Determination of minimum inhibitory concentration (MIC)
Minimal Inhibitory Concentration (MIC) is defined as the lowest concentration which is able to inhibit any visible microbial growth. Agar well diffusion method was used to determine antimicrobial activity of the extracts as described by Sagdic et al. with slight modifications [Citation30]. The following pathogenic gram-positive bacteria strains: B. cereus ATCC 33019, L. monocytogenes ATCC 7644, S. aureus ATCC 25923; gram-negative strains: E. coli O157:H7 ATCC 33150, S. typhimurium ATCC 14028, Y. enterocolitica ATCC 1501 bacteria and non-pathogenic gram-positive: S. carnosus, S. xylosus, L. sakei, L. plantarum, were used as indicators. Pathogenic bacteria and Staphylococcus sp. were maintained in Nutrient broth (Merck, Germany). Inoculum suspensions were prepared by putting the vitalized cells into maximum recovery diluent solution at the ratio of 1% (v/v) to have 105 CFU/mL. Lactic acid bacteria (LAB) were inoculated in MRS broth (Merck, Germany). Bacteria cultures were added to nutrient agar and MRS agar as 1% concentration at 45–50 °C and poured 20 mL of agar into petri dishes. Petri dishes were kept to solidify at 4 °C for 1 h and wells were bored by sterile cork borers (Ø = 6 mm). Meanwhile, extract solutions were prepared at 1, 2, 5 or 10% concentration in distilled water and added to the wells by an automatic pipette. Pathogenic bacteria, Staphylococcus sp. and LAB were incubated at 37 °C for 24 h, respectively. Inhibition zones formed around the wells were measured at the end of incubation and results expressed in millimetres (mm).
Cell culture
MCF-7 human breast adenocarcinoma cell line used was purchased from ATCC (No HTB-22) of the American type culture collection (Manassas VA, USA). Fibroblast healthy cell was obtained from Genome and Stem Cell Centre in Turkey and used as control. Cells were grown in 175 cm2 cell culture flasks to approximately 80–85% confluence. MCF-7 cell line was completely cultured in a high Dulbecco’s Modified Eagle’s Medium (DMEM) containing 10% Foetal Bovine Serum (FBS), 2 mM l-glutamine, 100 g/mL sodium pyruvate, 0.01 mg/mL human insulin and 1% penicillin/streptomycin. Fibroblast normal cell was completely cultured in DMEM but in low medium containing 20% FBS with 2 mM l-glutamine and maintained in an incubator under 5% CO2 atmosphere at 100% humidity and 37 °C. After reaching ≥80% confluence, cells were washed with Dulbecco’s Phosphate-Buffered Saline (DPBS) solution and detached from the flasks with trypsin/EDTA. The cell was centrifuged with the Universal 320 R (Hettich, Zentrifugen, 1406 Germany) at 1000 rpm for 5 min at 25 °C, seeded on E-plate and counted with a haemocytometer.
Cell proliferation and apoptosis detection
VybrantR MTT [(3-4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide)] cell proliferation assay kit which uses standard microplate absorbance readers was used to determine cell population profile. The cells were dispensed in 96-well plates at a density of 1 × 104/well. After 24 h of incubation in the normal growth medium, cells were exposed to different concentrations: 1, 5, 10, 50, 100, 200 and 400 μg/μL of the extracts. After treatment with the extracts at 24, 48 and 72 h time points, cells in the well containing CL extracts were incubated with 10 μL 100% dimethylsulfoxide (DMSO) solution and those with CF extract in Dulbecco’s Phosphate-Buffered Saline (DPBS) solution. Absorbance at 560 nm was determined using 750 nm as the reference wavelength using an ELISA reader (Bio-Tech instrument, Inc., USA). All the experiments were carried out in triplicates and result generated was presented as percent viability of the control. The percentage of cells exhibiting changes in cell death was determined using ‘Annexin V & Dead Cell Assay kit (MCH100105)’ according to manufacturer's instructions (Merck Millipore, Darmstadt, Germany). This assay is based on phosphatidylserine detection on external membrane of apoptotic cells using fluorescently labelled Annexin V in combination with the dead cell marker, 7-Aminoactinomycin D (7-AAD). After harvesting the cells (1 × 106) and washing twice with ice-cold PBS, experiments were set up at 1, 5, 10, 50, 100, 200 and 400 μg/mL concentrations over three time points: 24, 48 and 72 h. Apoptotic ratio was calculated by identifying four populations: (i) non-apoptotic cells: Annexin V (–) and 7-AAD (–); (ii) early apoptotic cells: Annexin V (+) and 7-AAD (–); (iii) late stage apoptotic and dead cells: Annexin V (+) and 7-AAD (+) and (iv) mostly nuclear debris: Annexin V (–) and 7-AAD (+). The samples were counted by the Muse Cell Analyzer (Merck Millipore) and analysed by a software provided by Merck Millipore.
xCELLigence real-time monitoring
Cytotoxicity of the extracts were continuously monitored with xCELLigence Real-Time Cell Analyser (RTCA) as previously described by Urcan et al. [Citation31] with slight modifications. Optimal seeding concentration for the fibroblast and MCF-7 cell lines were first determined and after seeding the cells in E-plate 96 wells, the process of cell proliferation, attachment and spreading was monitored every 15 min with RTCA xCELLigence system. Approximately 24 h post-seeding when the cells were in the log growth phase, a total of 3000 cells/well were seeded in the E-plate 96 wells and impedance was determined. Controls received medium plus DMSO. All the treated groups were replicated 4-times and the experiments were run for 97 h. All calculations were done with the RTCA-integrated software of the xCELLigence system. RTCA software performs the curve-fitting of selected ‘sigmoidal dose-response equation’ and calculated logarithmic half maximum effect of concentration [log (IC50)] values at a given time point based on log concentration producing 50% reduction of cell index (CI) value relative to the control CI value (100%).
Statistical analysis
Data was subjected to one-way analysis of variance (ANOVA) using SAS (2000) software. Differences were considered to be statistically significant at P ≤ 0.05 and means were compared using Tukey’s multiple comparison procedure.
Results and discussion
Solvent optimization, Fourier transform infrared spectroscopy and total phenolic content
In this study, four solvents were optimized for extracting Total Phenolic Content (TPC) from CL and CF samples following simplex lattice design. Results of optimization showed that solvents did not exert the same effect on the tested samples (). Extractability of TPC from Callistemon leaves was in the following order: methanol (CLMT) > water (CLWA) > ethanol (CLET) > acetone (CLAC). The sequence was not the same for extracts from flowers: water (CFWA) > methanol (CFMT) > acetone (CFAC) > ethanol (CFET). As shown in , while methanol extracted the highest amount of TPC from CL, water recorded the highest extractability of TPC from CF. This result indicates that using methanol alone was more efficient than the combination of methanol with water (1:1 v/v) for extracting polyphenols from Callistemon leaf extract. Apparently, hygroscopic nature of extracts from Callistemon flower was responsible for the results obtained in this study. It also signifies the suitability of water for phenolic extraction. In addition to polarity of extracting solvents, the degree of their dissolution or miscibility affected the yield, phenolic content and antioxidant activity of plant extracts. In a similar study on C. viminalis leaves, high polarity of methanol and its efficiency to dissolve endogenous compounds in plant materials also influenced the extractability of phenolics [Citation32]. Our findings therefore agree with previous assertion on correlation expected from the ratio of plant material (by weight) to solvent (by volume) used for extraction [Citation33].
Figure 1. Comparison of extraction methods with aqueous and organic solvents for extracts from leaf and flower of C. citrinus. CLWA, HPLC grade water leaf extract; CLMT, methanol leaf extract; CLAC, acetone leaf extract; CLET, ethanol leaf extract; CFWA, HPLC grade water flower extract; CFMT, methanol flower extract; CFAC, acetone flower extract; CFET, ethanol flower extract.
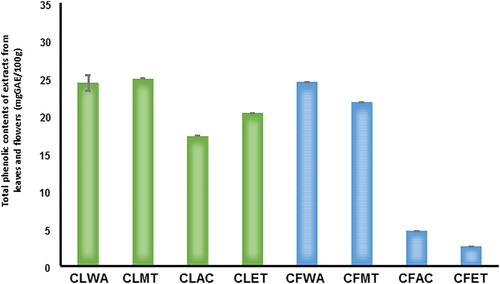
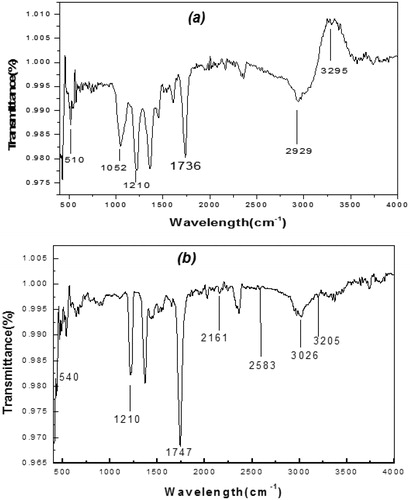
The functional groups of various phytochemicals in the extracts were identified through Fourier Transform Infrared (FTIR) spectroscopy. FTIR measures extracts’ absorbance of infrared light at various wavelengths. The spectra arising from molecular vibrations fall within the spectroscopic regions of 3295–1736 cm−1 (). The results showed unique combination of bands corresponding to the presence of various chemical bonds and functional groups present in the extracts. However, extracts from Callistemon flowers with wavenumbers of 3295 cm−1, 2929 cm−1 and 1736 cm−1 signifying the presence of alcohol/phenol O–H stretch, alkyl C–H stretch and aldehyde, ketone, ester and carboxylic acid CO stretch (). From , the wavenumbers from leaf extracts were 3205 cm−1, 3026 cm−1, 2583 cm−1, 2161 cm−1 and 1747 cm−1, respectively. The result indicates the presence of O–H stretch of carboxylic acid, alkyl and CO stretch of aldehyde, ketone, ester and carboxylic acid respectively. Again, TPC values showed significant variations in the range of 24.985 ± 7.38 and 23.118 ± 1.57 mg GAE/g sample from CL and CF, respectively (). Absolute methanolic extract produced higher amounts of TPC from CL than CF. Although using combinatorial approach can enhance high throughput screening in testing bioactivity, yet technical accuracy of Folin–Ciocalteu’s phenol reagent assay also contribute to the amount of TPC and antioxidant capacity of the samples [Citation34,Citation35]. Based on Gallic Acid Equivalents (GAE), the TPC obtained in this study was in consonance with previous findings where the presence of total soluble phenolics in methanol: chloroform (1:1) extracts revealed a strong similarity with antioxidant activity of Salvia species [Citation36]. Thus, it can be deduced that the type of solvent used and the strength of extract can significantly affect total extractable compounds from plant extracts.
Table 2. Total phenolic contents, antioxidant capacity and α-glucosidase inhibition potential of extracts from Callistemon leaves and flowers.
Antioxidant capacities of Callistemon extracts
A number of assays have been developed to determine antioxidant activity of phytochemicals due to protective action of these naturally occurring compounds against chronic conditions caused by oxidation [Citation37]. Of these, Trolox Equivalent Antioxidant Capacity serves as a benchmark assay for antioxidant screening for its operational simplicity, endpoint and biological relevance [Citation38]. Oxygen radical absorbance capacity (ORAC), ferric reducing ability of plasma (FRAP), DPPH and ABTS decolourization are common antioxidant capacity assays that use Trolox as standard. The UV–vis spectrophotometry test in this study showed that half-maximal effective concentration (EC50) of Callistemon extracts induced 50% antiradical effect after a specified exposure time from DPPH assay. Radical scavenging activity of extracts against stable DPPH produced EC50 values of 0.474 ± 0.03 and 0.787 ± 0.15 mL sample/g DPPH for CL and CF, respectively. These results imply that extracts from the leaves demonstrated better antioxidant capacity than those from flowers. In other words, CL with a lower EC50 value had higher free radical scavenging capacity than CF and thus conforming to established principle that the lower the EC50 value, the higher its effectiveness in scavenging DPPH free radicals [Citation39].
Few ideas have been proposed to explain the rationale behind the disparities in EC50 values. The polarity index of various solvents used for extraction are not the same. Methanol, for instance has a polarity index (P′) of 5.1. Marxen et al. [Citation40] pointed out that DPPH is stable in methanolic solution. Thus, the stability of DPPH in methanolic solution, its concentration and time taken to reach a steady state in reducing the initial DPPH concentration by 50% might be responsible for a lower EC50 value obtained from CL. A similar EC50 pattern was reported by Zubair et al. [Citation19] under an in vitro study on antiradical activity in which methanol was used to extract antioxidants from the leaves of C. viminalis. Under in vivo condition, methanolic extract from the leaves of C. lanceolatus equally had similar free radical scavenging effect on DPPH radicals in wistar rats [Citation41]. Fundamentally, TEAC is an electron transfer assay based on scavenging of a relatively stable blue–green ABTS radical and its conversion into a colourless product. As shown in , both extracts showed measurable disappearance kinetics indicating a distinct free radical-scavenging activity. The degree of decolourization induced by the extracts was in the range of 426.604 ± 12.54 and 196.269 ± 6.54 TEAC mg/g sample for CL and CF. TEAC value from CL is more than double the amount found in CF which implies that the antioxidant potential and ABTS□•+ scavenger activity of extract from Callistemon leaves is superior to the flowers. Essentially, ABTS is not ionic-strength dependent but soluble in organic and aqueous solvents. Therefore, it can be used in multiple media to determine both hydrophilic and lipophilic antioxidant capacities of extracts and body fluids [Citation35]. Generally, our findings agree with the existing evidence that phytochemicals in Callistemon extracts demonstrate strong in vitro antioxidant activity which can further be utilized to neutralize free radicals [Citation42].
Alpha-glucosidase inhibitory activity of Callistemon extracts
In , the alpha-glucosidase inhibition of Callistemon extracts are expressed as half-maximal inhibitory concentration (IC50). Although both extracts inhibited the activity of α-glucosidase enzyme, the IC50 value from CL was significantly (P ˂ 0.05) lower than the value from CF as expressed with IC50 values of 3.69 ± 0.61μg/mL for CL and 4.83 ± 0.00μg/mL for CF, respectively. The results showed that phytochemicals in CL are more potent as inhibitors of α-glucosidase enzyme than those in CF. In comparison with a previous study, the IC50 value of 2.58 μg/mL obtained from the aqueous extract of Pouteria caimito is a bit lower than the values obtained from both extracts [Citation41]. Both IC50 values from C. citrinus were lower than 5.56 ± 0.00 μg/mL obtained from pomegranate peels [Citation34]. Also, the values were approximately four-times lower than the α-glucosidase inhibitory activity (IC50=14.72 μg/mL) of black tea pomace reported by Striegel et al. [Citation43]. In their findings, Rani et al. [Citation44] found that anti-diabetic activity of fruit extract from strawberry during the ripening stage, was likewise more than 31 and 41 times higher than the IC50 values from Callistemon extracts. In literature, biguanides, meglitinides, peroxisome proliferator activated receptor γ agonists, sulfonylureas and other α-glucosidase inhibitors, have been identified as oral agents for type-2 diabetes. This is because the α-glucosidase inhibitors competitively inhibit the activities of maltase, isomaltase, sucrase, and glucoamylase enzymes in small intestine by delaying the breakdown of polysaccharides without causing nutrient malabsorption [Citation44]. Hydrolysis of dietary carbohydrates is the main source of blood glucose. Several medicinal plants have α-glucosidase inhibitory capacity to reduce gastrointestinal glucose production and absorption of carbohydrate-digesting enzymes such as α-amylase and α-glucosidase. Therefore, inhibitors of these enzymes may be effective for lowering glucose absorption and rise of postprandial blood glucose in the luminal side of intestine. By application, the glucose-lowering potential of α-glucosidase inhibitors may be used as monotherapy or in combination with other oral anti-diabetic agents for treating severe hyperglycaemia [Citation45–47]. Our findings suggest that α-glucosidase inhibitor from C. citrinus leaf and flower extracts can offer a novel alternative intervention for lowering glucose level in the blood or treating metabolic disorder caused by abnormal insulin secretion and correct defective insulin signalling in patients suffering from type-2 diabetes.
Bioactivities of Callistemon extracts against some foodborne pathogens
In the present study, nutrient agar showed varying antimicrobial activities of Callistemon extracts from the leaves (CL) and flowers (CF) against most of the selected bacteria strains except for S. typhimurium, E. coli 0157:H7, L. sakei and L. plantarum. This finding further buttresses the potential relevance of Callistemon extracts to tackle the outbreak of foodborne zoonosis in food and nutraceutical industries. Again, antimicrobial activity of Callistemon extracts compared with their zone of inhibition produced exciting inhibitory effects against all the gram-positive pathogenic bacteria and Y. enterocolitica, which is a gram-negative bacterium (). The overall results exposed the sensitivity of test pathogens to activities of phytochemicals in the extracts and solvents used for extraction. Again, the combination of water with methanol (1:1, v/v) evidently showed that CF produced relatively higher growth inhibitions against the gram-positive strains which perhaps underscores the efficacy of bioactive compounds in CF and effectiveness of solvents mixture for extraction process. Further result shows that, at the highest (10%) and lowest (1%) concentrations, CF produced the widest inhibition zones of 19.50 ± 0.17 mm and 14.00 ± 0.17 mm against the growth of Bacillus cereus (). Although, the values obtained were not statistically different but the results indicated that CF was more effective for inhibiting the growth of Bacillus cereus than CL. The growth inhibitions previously reported against B. cereus, S. aureus and S. pyogenes were lower than values obtained in this study. Basically, Bacillus cereus is a gram-positive aerobic endospore-forming rod that has been isolated from water, raw and processed foods. All populations are susceptible to food poisoning strains from B. cereus and consumption of foods containing more than 105 viable toxigenic B. cereus per gram of food results in foodborne illness in form of emetic or diarrheal symptoms. Thus, efficient incorporation of Callistemon extracts in food might curtail incidence of food poisoning caused by B. cereus.
Table 3. Antibacterial activity of Callistemon extracts against pathogenic gram-positive and gram-negative bacteria.
Again, Callistemon extracts produced inhibition zones ranging from 12.50 ± 071 mm to 20.00 ± 00mm against the growth of S. aureus. While no significant difference was observed at the highest concentration of 10% for both extracts, CF recorded a numerically higher value of 1 mm above CL. Our results were not opposed to the one reported on antibacterial effect of alkaloids in Callistemon leaf extracts against the growth of S. aureus [Citation48]. These results unravel the possibility of exploiting the antimicrobial properties of Callistemon extracts for suppressing the growth of this foodborne organism. Enterotoxins produced by S. aureus in fermented foods, dairy products, eggs and meat are metabolites posing serious health risks to consumers. L. monocytogenes was another gram-positive foodborne pathogen that was detected in the present study. The growth of L. monocytogenes was remarkably inhibited by CL and CF but antimicrobial activity of CF was strongest (P < 0.05) with 35.00 ± 2.83 mm at the highest concentration of 10%. L. monocytogenes has been isolated from variety of dairy products, meat, eggs, seafood and fresh produce [Citation49]. Hence, efficient inclusion of these extracts in food can enhance safety and reduce the fatality rate of listeriosis with its associated syndromes: gastroenteritis, abortion, septicaemia, encephalitis, meningitis, pneumonia and stillbirths among immuno-compromised individuals.
Moreover, at the highest concentration of 10%, CL produced maximum inhibition diameter of 18.50 ± 0.71 against the growth of Y. enterocolitica. In comparison with extracts from Callistemon leaves, extracts from flowers consistently recorded lower growth inhibitions at 1%, 2%, 5% and 10%, respectively. Thus, the antimicrobial effects of phytochemicals in CL and solvents used for extraction were more effective than CF against this invasive enteric gram-negative bacterium. Y. enterocolitica is a well-known psychotropic pathogen that causes foodborne illnesses in refrigerated and/or frozen foods. Its capacity to release endotoxin and survive in frozen foods under frequent refrigeration, freezing and thawing makes it an organism of serious concern in food industry. Inclusion of Callistemon extracts in refrigerated or frozen foods may hinder the growth of this organism and its side effects in cold-preserved foods. In addition, none of the methanolic and hydro-methanolic extracts was active against S. typhimurium and E.coli 0157:H7 (). An outbreak of salmonellosis caused by S. typhimurium O157:H7 has been linked with the consumption of meat products such as biltong, ground beef, duck meat and eggs [Citation50]. The use of Callistemon extracts is therefore proposed for biosecurity measures to prevent cross-contamination of this multi-resistant serotype. Although, no growth inhibition was detected against L. sakei and L. plantarum yet, CL demonstrated better antimicrobial activity than CF against S. xylosus and S. xylosus (). In their study, Adwan et al. [Citation51] attributed low sensitivity of the gram-negative bacteria strains in C. viminalis extracts to permeability barrier imposed by extra-lipopolysaccharide and protein cell wall. To a large extent, our findings were consistent with a previous study where the growth of both gram-positive and gram-negative bacteria strains were inhibited by extracts from C. citrinus with gram-positive strains being more susceptible [Citation52]. It is also similar to the results on crude aqueous and methanolic extracts from C. viminalis leaves, which exhibited significant antibacterial activity against gram-positive and gram-negative bacteria strains [Citation53]. Apart from the aforementioned factors, the effect of some unidentified compounds such as anthocyanin could have contributed to variations in growth inhibitions of various bacterial strains.
Table 4. Antibacterial activity of Callistemon extracts against non-pathogenic bacteria.
Effects of Callistemon extracts on apoptosis and proliferation of MCF-7 cell line
Based on the outcome of flow cytometry, fluorescence signals from Annexin V staining of various samples produced different binding patterns of conjugates to phosphatidyl serine (). Apoptotic ratio which was calculated by identifying four populations showed that within 24 h, cells that were treated with 400 μg/mL extract from CF recorded the highest live or non-apoptotic [Annexin V (–) and 7-AAD (–)] ratio. While late stage apoptotic and dead cells [Annexin V (+) and 7-AAD (+)] was almost absent in all the groups, cells that were treated with extract from CL had the highest total apoptosis of 46.80%. Also, findings on cell survival showed that different concentrations of Callistemon extracts exhibited unequal effects on MCF-7 cell line (). The result implies that MCF-7 cells that were treated with different concentrations of the extracts and exposed to varying incubation periods of 24 h, 48 h and 72 h, did not proliferate evenly. On the contrary, the control sample containing only MCF-7 cells had approximately 100% rate of proliferation in all the groups. The dose-and-time dependent nature of this study revealed that the group with the lowest concentration of 1 µg/mL from leaf extract (CL) produced the least proliferation of MCF-7 cells of 8.2 ± 0.02, 5.4 ± 0.06 and 15.2 ± 0.04 at 24 h, 48 h and 72 h incubation periods, respectively. Similarly, the group with the lowest concentration of 1 µg/mL from flower extract (CF) also had the least proliferation of MCF-7 cells of 5.4 ± 0.10, 63 ± 0.20 and 40.8 ± 0.08 at 24 h, 48 h and 72 h incubation periods. Generally, from both extracts, 400 µg/mL CL recorded the highest cell proliferation value of 476.1 ± 0.12 within 24 h of incubation. The results show that phytochemicals in Callistemon exerted heterogeneous time and concentration-dependent activity on the proliferation of MCF-7 cells. From foregoing, the results on MTT proliferation and Annexin V assays did not conform to a dose-response pattern, therefore, confirmatory tests were performed using RTCA xCELLigence system. The intention was to ascertain the reason for inconsistent cellular proliferations obtained from cytometry analysis. In this study, treating healthy cell with 10–100 μg/mL extracts from CL and CF did not show any cytotoxic effects on fibroblast cell line under real-time monitoring with xCELLigence system (). The cell index of healthy cells experienced a sharp linear decline within 24 h after treatment with 400 μg/mL extracts from CL. A dose dependent toxicity elicited by CL and CF suggests that the higher the concentration of the extract, the lower the cell index becomes. Again, cell index of MCF-7 treated with extract from CF shows a dose-dependent pattern which is similar to the result obtained for extracts from CL (). While the cell index for wells containing 1 μg/mL and 10 μg/mL for CF were increasing, a decrease in cell index was noticed for wells containing 50 μg/mL and above. By repeated xCELLigence measurements, quantification through RTCA software made it possible to plot a sigmoidal dose-response curve and calculate the half inhibitory concentration (IC50) (). Further result shows that half inhibitory concentration (IC50) of 29.199 μg/mL was obtained within 24 h 11 min 37 s for CL extracts against MCF-7 cell line.
Figure 3. Population and apoptosis profiles for MCF-7 cell treated with leaf (CL) and flower (CF) extracts.
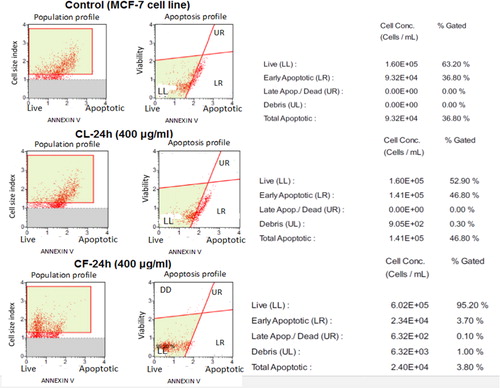
Figure 4. Cell index profiles of fibroblast normal cell line treated with different concentrations of leaf (CL) and flower (CF) extracts from C. citrinus.
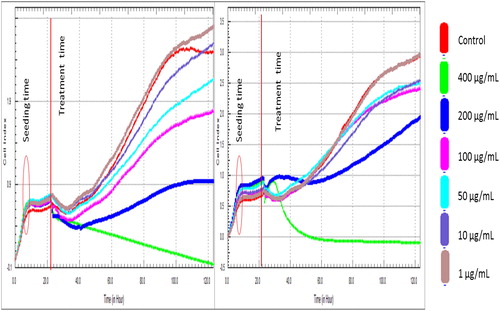
Figure 5. Cell index profiles of MCF-7 cell line treated with different concentrations of leaf (CL) and flower (CF) extracts from C. citrinus.
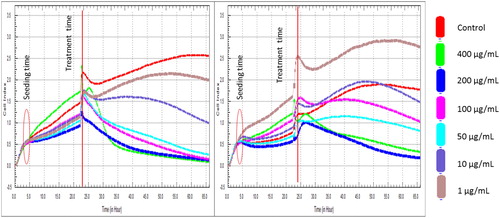
Figure 6. Dose-response curve of MCF-7cell line and IC50 level at 24 h of leaf (CL) and flower (CF) extracts from C. citrinus.
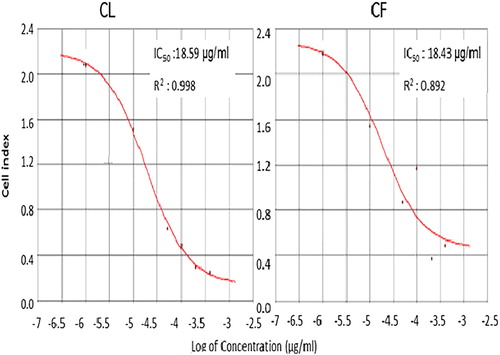
Table 5. MTT cell proliferation rate of MCF-7 cells cultured with and without Callistemon extracts.
Fundamentally, determination of cell proliferation profiles is important for monitoring cell viability. This is because of its involvement in physiological and pathological processes under in vitro and in vivo conditions. MTT proliferation assay which is non-radioactive in nature explains spectrophotometric quantification of cell population by reducing the yellow tetrazolium salt [3-{4, 5-dimethylthiazol-2yl}-2, 5-diphenyl-tetrazolium bromide (MTT)] by metabolically active cells to generate intracellular purple formazan. MTT is a water-soluble reagent used for rapid quantitation of viable adherent or non-adherent cells in 96 well plates. The tetrazolium ring of MTT is cleaved by mitochondrial dehydrogenases in viable cells to yield a water insoluble purple formazan product. MTT based viability assay is used to measure growth or cytotoxicity resulting from either drug treatment or ectopic gene expression after transfection or retroviral infection. Our findings are comparable with similar studies where phenolic hydroxyl groups from green tea extracts interfered with MTT by reducing it to formazan and consequently influencing the outcome of screening and mitochondrial metabolic rate [Citation54,Citation55]. The outcome of xCELLigence real-time measurement clearly corroborates its worth as an efficient analytical tool for describing the relationship between cellular behaviour and strength of cell adhesion. Our results further confirm a previous report where a decrease in net adhesion produced a similar decline in the cell index of different cell lines [Citation56]. While it is known that family history, age, early menarche, late menopause, postmenopausal obesity, alcohol consumption, genetic mutations, inherited conditions [Citation57], susceptibility genes (BRCA1 and BRCA2) and mammographic breast density are commonly connected with the risk of breast cancer [Citation58,Citation59], the use of xCELLigence can enhance the experimental design to identify the responsiveness and effects of phytochemicals on the cytotoxicity or viability of healthy or cancerous cells in real-time.xCELLigence biosensor technology has now been validated by a range of research groups to investigate multiple complex cellular behaviours and drug responses. This includes drug effects on the viability and migration of tumour cells [Citation1,Citation2] and cell toxicity to drugs [Citation3–5].
Conclusions
This study demonstrates the versatility of phytochemicals in C. citrinus extracts as a strong alternative to other natural products for tackling the menace of oxidation, foodborne organisms, diabetes and breast cancer. Antimicrobial activities of Callistemon extracts evidently showed its potentials for inhibiting the growth of some foodborne bacteria in livestock feeds and food consumed by man. The result on alpha-glucosidase inhibition by the extracts indicates better glucose-lowering activity for managing metabolic disorder associated with type-2 diabetes. The IC50 value of 2.29 μg/mL from CF extract was apparently 10 times more promising than CL for antineoplastic purpose against MCF-7 cell line. Further studies are therefore recommended to elucidate the mechanisms of action or molecular pathways on how bioactive compounds in these extracts interfere with the growth of foodborne pathogens, alpha-glucosidase enzymes and the process of carcinogenesis. It is advisable to determine the allergenicity of these extracts before using it in nutraceutical, beverages, food and livestock industries for developing functional products.
Author contributions
FPO (conceived the idea, designed the experiments, worked on all experimental phases and wrote the manuscript); OI (co-designed the microbial analyses); MBY and AHD (co-designed the xCELLigence RTCA analyses); CD and ÖS (co-designed the Annexin V, Cytometry and MTT proliferation analyses); ÖC (assisted in research activities); GEU and OEF (co-designed FTIR analysis) & YH (co-designed the study). All the authors have read and approved the manuscript.
| Abbreviations | ||
| ABTS | = | 2,2′Azinobis-(3-ethylbenzthiazoline-6-sulfonic acid) |
| DPPH | = | 2,2-diphenyl-1-picrylhydeazyl |
| DMEM | = | Dulbecco’s Modified Eagle’s Medium |
| DPBS | = | Dulbecco’s Phosphate-Buffered Saline |
| MCF-7 | = | Michigan Cancer Foundation-7 |
| TEAC | = | Trolox Equivalent Antioxidant Capacity |
| MTT | = | [(3-4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
Acknowledgements
Authors are grateful to the Scientific and Research Council of Turkey (TUBITAK) for supporting this study. We also thank Associate Professor Çam Mustafa for his assistance on antioxidant and alpha-glucosidase analyses.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Torgerson PR, Devleesschauwer B, Praet N. World Health Organization estimates of the global and regional disease burden of 11 foodborne parasitic diseases, 2010: a data synthesis. PLOS Med. 2015;12:e1001920.
- Manguiat LS, Fang TJ. Evaluation of DASTM kits for detection of food-borne pathogens in chickens and meat-based street-vended foods. J Food Drug Anal. 2013;21:198–205.
- International Diabetes Federation, Diabetes Atlas (IDFDA). IDF Diabetes Atlas. 8th ed. Brussels: IDFDA; 2018.
- Holman N, Young B, Gadsby R. Current prevalence of type 1 and type 2 diabetes in adults and children in the UK. Diabet Med. 2015;32:1119–1120.
- Oyedemi SO, Oyedemi BO, Ijeh II, et al. Alpha-amylase inhibition and antioxidative capacity of some antidiabetic plants used by the traditional healers in South Eastern Nigeria. Sci World J. 2017;2017:1–11.
- Maskatinec G, Fontaine A, Torfadottir JE, et al. The relation of type 2 diabetes and breast cancer incidence in Asian, Hispanic and African American Populations—a review. Cancer J Diab. 2018;42:100–105.
- Nikolova I, Marinov L, Georgieva A, et al. Metamizole (dipyrone)-cytotoxic and antiproliferative effects on HeLa, HT-29 and MCF-7 cancer cell lines. J Biotechnol. 2018;32:1327–1337.
- Osunderu O. Medicinal plants used in the management of cancer among the Ijubus of Southwest Nigeria. Nat Prod Chem Res. 2017;5:1–9.
- Rajesh D. Identification of molecular pathways affected by treatment with heartwood water extract of Pterocarpus marsupium in MCF-7 cancer cell line. J Herbal Med. 2018;9:42–52.
- Ferlay J, Soerjomataram I, Ervik M, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Inter J Cancer. 2015;136:359–386.
- Parasuraman S. Toxicological screening. J Pharmacol Pharmacother. 2011;2:74–79.
- Weinstein-Oppenheimer CR, Burrows C, Steelman LS, et al. The effects of β-estradiol on Raf activity, cell cycle progression and growth factor synthesis in the MCF-7 breast cancer cell line. Cancer Bio Therapy. 2002;1:254–260.
- Saxena M, Saxena J, Nema R, et al. Phytochemistry of medicinal plants. J Pharmacol Phytochem. 2013;1:168–182.
- Christenhusz MJ, Byng JW. The number of known plants species in the world and its annual increase. Phytotaxa. 2016;261:201–217.
- Sutar N, Sutar R, Kumar M. Callistemon citrinus (Bottle brush), an important medicinal plant: a review of its traditional uses, phyto-constituents and pharmacological properties. Indian J Pharma Sci. 2014;1:70–77.
- Sumitra SS. Genus callistemon: an update review. World J Pharm Pharmaceut Sci. 2014;3:291–307.
- Afrah JA. Studying of antibacterial effect of leaves extract of Callistemon vininalis in vitro and in vivo (urinary system) for rabbits. J Kerbala Univ. 2012;10:1–9.
- Tabuti JRS, Kukunda CB, Waako PJ. Medicinal plants used by traditional medicine practitioners in the treatment of tuberculosis and related ailments in Uganda. J Ethnopharma. 2010;127:130–136.
- Zubair M, Hassan S, Rizwan K, et al. Antioxidant potential and oil composition of Callistemon viminalis leaves. The Sci World J. 2013;2013:1.
- Cowan MM. Plant products as antimicrobial agents. Clin. Microbiol. Rev. 1999;12:564–582.
- Fayemi PO, Ozturk I, Özcan C, et al. Antimicrobial activity of extracts from Callistemon citrinus flowers and leaves against Listeria monocytogenes in beef burgers. Food Meas. 2017;11:924–929.
- Andrade JE, Ju YH, Baker C, et al. Long-term exposure to dietary sources of genistein induces estrogen-independence in human breast cancer (MCF-7) xenograft model. Mol Nutr Food Res. 2015;59:413–423.
- Çam M, Hışıl Y, Durmaz G. Classification of eight pomegranate juices based on antioxidant capacity measured by four methods. Food Chem. 2009;112:721–726.
- Falowo AB, Fayemi PO, Muchenje V. Natural antioxidants against lipid-protein oxidative deterioration in meat and meat products: a review. Food Res Int. 2014;64:171–181.
- Nyman AM, Schirmer K, Ashauer R. Importance of toxicokinetics for interspecies variation in sensitivity to chemicals. Environ Sci Technol. 2014;48:5946–5954.
- Aaby K, Skrede G, Wrolstad RE. Phenolic composition and antioxidant activities in flesh and achenes of strawberries (Fragaria ananassa). J Agric Food Chem. 2005;53:4032–4040.
- Kanagasubbulakshmi S, Kadirvelu K. Green Synthesis of iron oxide nanoparticles using Lagenariacineraria and evaluation of its antimicrobial activity. Def Life Sci J. 2017;2:422–427.
- Brand-Williams W, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. Lebensm Wiss Tech. 1995;28:25–30.
- Ryu HW, Cho JK, Curtis-Long MJ, et al. A-glucosidase inhibition and antihyperglycemic activity of prenylated xanthones from Garcinia mangostana. Phytochem. 2011;72:2148–2154.
- Sagdic O, Kuscu A, Ozcan M, et al. Effects of Turkish spice extracts at various concentrations on the growth of Escherichia coli 0157:H7. Food Microbiol. 2002;19:473–480.
- Urcan E, Haertel U, Styllou M, et al. Real-time xCELLigence impedance analysis of the cytotoxicity of dental composite components on human gingival fibroblasts. Dent Mater.. 2010;26:51–58.
- Paluri V, Ravichandran S, Kumar G, et al. Phytochemical composition and in vitro antimicrobial activity of methanolic extract of Callsitemon lanceolatus D.C. Int J Pharm Pharmac Sci. 2012;4:699–702.
- European Pharmacopoeia. Belladonna leaf dry extract standardised. European Pharmacopoeia, 4th ed. Strasbourg Cedex: Council of Europe;2002; p. 700.
- Vaishali RM, Vinitha RP, Pratapchandra KH, et al. Preliminary phytochemical screening of members of Lamiaceae Family: Leucas linifolia, Coleus aromaticus and Pogestemon patchouli. Int J Pharm Sci Rev. 2013;21:131–137.
- Çam M, Içyer NC. Phenolics of pomegranate peels: extraction optimization by central composite design and alpha glucosidase inhibition potentials. J Food SciTech. 2013;1:1–7.
- Huang DJ, Ou BX, Prior RL. The chemistry behind antioxidant capacity assays. J Agric Food Chem. 2005;53:1841–1856.
- Kamatou GPP, Viljoen AM, Steenkamp P. Antioxidant, anti-inflammatory activities and HPLC analysis of South African Salvia species. Food Chem. 2010;119:684–688.
- Garcia EJ, Oldoni TLC, Alencar S. M D, et al. Antioxidant Activity by DPPH Assay of Potential Solutions to be applied on bleached teeth. Braz Dent J. 2012;23:22–27.
- Sharma P, Singh RP. Evaluation of antioxidant activity in foods with special reference to TEAC method. Am J Food Technol. 2013;8:83–101.
- Marxen K, Vanselow KH, Lippemeier S, et al. Determination of DPPH Radical oxidation caused by methanolic extracts of some microalgal species by linear regression analysis of spectrophotometric measurements. Sensors (Basel). 2007;7:2080–2095.
- Kumar S, Kumar V, Prakash O. Anti-diabetic, hypolipidemic and antioxidant activities of Callistemon lanceolatus leaves extract. J Herbs Spices Med Plant. 2011;17:144–153.
- Radulović NS, Randjelović PJ, Stojanović NM, et al. Aboriginal bush foods: a major phloroglucinol from Crimson Bottlebrush flowers Callistemon citrinus, Myrtaceae) displays strong anti-nociceptive and anti-inflammatory activity. Food Res Int. 2015;77:280–289.
- Striegel L, Kang B, Pilkenton SJ, et al. Effect of black tea and black tea pomace polyphenols on α-glucosidase and α-amylase inhibition, relevant to type 2 diabetes prevention. Frontiers Nutr. 2015;2:1–6.
- Rani S, Mandave P, Kuvalekar A, et al. Antiglycation, antioxidant and antidiabetic activity of strawberry (Fragariax ananassa Duch.) fruits during ripening stages. Res J Pharm Biol Chem Sci. 2014;5:193–203.
- Singh R, Devi S, Gollen R. Role of free radical in atherosclerosis, diabetes and dyslipidaemia: larger-than-life. Diabetes Metab Res Rev. 2015;31:113–126.
- de Souza PM, de Sales PM, Simeon LA, et al. Inhibitory activity of α-amylase and α-glucosidase by plant extracts from the Braz Cerrado. Planta Med. 2012;78:393–399.
- Tundis R, Loizzo MR, Menichini F. Natural products as alpha-amylase and alpha-glucosidase inhibitors and their hypoglycaemic potential in the treatment of diabetes: an update. MRMC. 2010;10:315–531.
- Mabhiza D, Chitemerere T, Mukanganyama S. Antibacterial properties of alkaloid extracts from Callistemon citrinus and Vernonia adoensis against Staphylococcus aureus and Pseudomonas aeruginosa. Intl J Med Chem. 2016;2016:6304163.
- Zhu Q, Gooneratne R, Hussain MA. Listeria monocytogenes in fresh produce: outbreaks, prevalence and contamination levels. Foods. 2017;6:1–11.
- Saitanu K, Jerngklinchan JK. Incidence of Salmonella in dug eggs in Thailand. Southeast Asian J Trop Med Pub Health. 1994;25:328–331.
- Adwan K, Abu-Hasan N, Essawi T. Isolation and characterization of Shiga toxigenic Escherichia coli strains from northern Palestine. J Med Microbiol. 2002;51:1–3.
- Liu Y, Nguyen N, Colditz GA. Links between alcohol consumption and breast cancer: a look at the evidence. Womens Health (Lond Engl). 2015;11:65–77.
- Delahaye C, Rainford L, Nicholson A, et al. Antibacterial and antifungal analysis of crude extracts from the leaves of Callistemon viminalis. J Med Bio Sci. 2009;3:1–7.
- Wang P, Henning SM, Heber D. Limitations of MTT and MTS-based assays for measurement of antiproliferative activity of green tea polyphenols. PLOS One. 2010;5:e10202.
- Boyd NF, Martin LJ, Rommens JM, et al. Mammographic density: a heritable risk factor for breast cancer. Methods Mol Biol. 2009;472:343–360.
- Kho D, MacDonald C, Johnson R, et al. Application of xCELLigence RTCA Biosensor Technology for Revealing the Profile and Window of Drug Responsiveness in Real Time. Biosensors (Basel). 2015;5:199–222.
- Levenson AS, Jordan VC. MCF-7: the first hormone-responsive breast cancer cell line. Cancer Res. 1997;57:3071–3078.
- Han M, Li JF, Sun YY, et al. Limitations of the use of MTT assay for screening in drug discovery. J Chinese Pharma Sci. 2010;19:195–200.
- Özdemir A, Ark M. xCELLigence real time cell analysis system: a new method for cell proliferation and cytotoxicity. Niche. 2014;2:15–17.