?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The interest in nanoscale emulsions has considerably grown in recent decades as a consequence of their specific attributes such as high stability, attractive appearance, in addition to high performance and sensorial advantage. In fact, it nanoemulsions are one of the major popular formulation systems in the pharmaceutical and cosmeceutical fields. The thermodynamic and high kinetic stability, besides the minute droplet size of nanoemulsions have spurred their rapid development as a system for delivery of bioactive substances/drugs in cosmetics and dermatological formulations. The composition and the technique of preparation very much define the quality of nanoemulsions. They are mainly targeted at high performance, product distribution to consumers, alongside the prospects of mass production. Formulators, however, do face certain limitations especially regarding the diffusion of active ingredients into the human skin. This review describes the popular techniques used by formulators in recent years to prepare nanoemulsions as final application products for cosmeceutical application. Correspondingly, an overview of characterisation technologies to differentiate between the micro and nanoemulsions – alongside their benchmarks in terms of their physical and thermodynamic stabilities, is also described in this review.
Introduction
The market for cosmeceutical products has grown substantially in recent decades following increasing consumer awareness for dermatologically nutritional products that promote good skin health and disease prevention [Citation1]. Higher demands for improved product efficacy have seen the boundary between cosmetics and topical pharmaceuticals products becoming increasingly difficult to distinguish. ‘Cosmeceuticals’ gain large consumer interest because these products lie between the grey zone of cosmetics and pharmaceuticals [Citation2]. The applications of cosmeceuticals have now extended to skin protection, whitening, tanning, anti-aging and anti-wrinkling, alongside other interesting uses such as deodorants, as well as for nail and hair care. The increased demand for skin care cosmeceutical nanoemulsions is a consequence of their ability for controlled delivery and optimized dispersion of active ingredients into the desired layers of skin. The active ingredients in the form of nanoparticles have a high surface-to-volume ratio, which promotes dispersibility in the emulsion, and are better adapted for multiple functions [Citation3]. As a result of reduced gravity force and Brownian motion, premature destabilisation of this emulsion system is averted; hence, the zero sedimentation during storage. Another destabilisation factor, i.e. flocculation, is also prevented by the minuscule size of nanoemulsions, which prolongs the shelf-life of products [Citation4].
Nanoemulsions can be classified based on their morphology. A ‘water-based’, or oil-in-water (O/W), emulsion has water as the continuous phase and oil as the dispersed phase [Citation5, Citation6], whereas the inversed condition yields an ‘oil-based’ or water-in-oil (W/O) emulsion. The small droplet size of nanoemulsions allows uniform deposition and penetration of active ingredients through the skin surface [Citation7]. Nanoemulsions exhibit better penetration efficacy of the ingredients due to the large surface area and low surface tension of the whole emulsion system [Citation8], thus requiring only 3–10% of surfactants during preparation [Citation9]. Microemulsions, on the other hand, require surfactant concentrations of 20% or higher [Citation10]. As a result, nanoemulsions appear more fluid (at low oil concentrations), with display of appealing physical properties and skin feel, especially in the absence or the small use of thickeners. Destabilisation phenomena such as creaming or sedimentation, flocculation and coalescence that typically affect emulsions are also prevented [Citation11], the consequence of considerable steric stabilisation between the submicrometric droplet size.
One essential point to consider when preparing nanoemulsions is the impact of the order in which the different compounds are mixed during formulation. It is important to emphasize here that the preparation of nanoemulsions requires that surfactants are first mixed with the oily phase. This renders the realisation of conditions that highly favour the formation of nanoemulsions. In contrast, mixing surfactants with water in the initial stages of preparation would favour the development of ‘macroscopic’ emulsions [Citation12]. To date, nanoemulsions are prepared using several methods, which are categorically divided into low-energy or high-energy emulsification approaches or a combination of both [Citation13]. High-energy approaches are characterized by the use of mechanical devices that create intense disruptive forces which break up the oil and water phases to form the oil droplets. Such a technique uses high-pressure homogenizers, microfluidizers and sonication methods [Citation14]. Conversely, low-energy techniques use the internal chemical energy of the system to perform emulsification [Citation15]. This is achieved by diverting the intrinsic physicochemical properties of the surfactant, co-surfactants and excipients in the formulation [Citation16].
Emulsions and nanoemulsions in cosmeceuticals
An emulsion is defined as a system containing two immiscible phases, which consists of a dispersed phase as droplets (internal phase) and a continuous phase (external phase) [Citation17]. Historically, the term ‘microemulsion’ was first used by Schulman et al. [Citation18] in 1959 to describe a transparent solution of a multiphase system consisting of water, oil, surfactant and alcohol. Although not systematically used today, some prefer the names ‘micellar emulsion’ or ‘swollen micelles’ [Citation19] (reviewed by Eastoe [Citation20]). Research on microemulsions continued to remain relatively unknown to the scientific community until a work by Hoar and Schulman was published in 1943 [Citation19]. They described a spontaneous formation of an emulsion of water and oil, upon the addition of a strong surface-active agent. This system was defined by being thermodynamically unstable and kinetically stable systems [Citation4, Citation13, Citation21, Citation22]. For review see [Citation20].
This technology later found its way into commercial preparations of cosmeceutical products. A cosmeceutical is a cosmetic product with the incorporated active ingredient intended for a beneficial physiological effect, resulting from an enhanced pharmacological action when compared to an inert cosmetic [Citation1, Citation23, Citation24]. The major benefits of using nanotechnology in cosmeceutical applications are the enhanced stability of various cosmetic ingredients, viz. unsaturated fatty acids, vitamins, or antioxidants encapsulated within the nanoparticles [Citation23], improved penetration rate of certain ingredients, such as vitamins and other antioxidants [Citation2], improved aesthetics of the product [Citation25], in conjunction with enhanced performance and tolerance of UV filters on the skin surface [Citation26]. For instance, Mayer et al. [Citation27] successfully encapsulated the oil-soluble vitamin (vitamin E) and used a nanoemulsion as the delivery system. In fact in dermatology, W/O emulsions have been shown to be a better system, where the oil-soluble active ingredients are made more favourable by a lipid film formation on the skin [Citation28].
Mechanisms explaining the improved skin penetration by nanoemulsions have so far been associated with their nano-sized range and composition [Citation29]. The diffusional barrier of the stratum corneum may be reduced by the surfactant in the formulation, which leads to high penetration of cosmeceuticals. The water content in the formulation also has an important functional role. When the water content is adequately high in the formulation, percutaneous absorption of cosmeceuticals is improved due to the hydration effect of the stratum corneum. Nano-sized droplets dispersed in the continuous phase of the nanoemulsion can now move smoothly through the stratum corneum and diffuse the active ingredient through the skin barrier [Citation29–31]. Kong et al. [Citation32] reported that hyaluronan (hyaluronic acid, HA)-glycerol α-monostearate (GMS) based nanoemulsion results in an interesting colloidal transdermal carrier suitable for applications in skin care and cosmetic products.
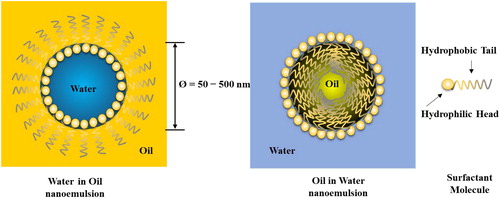
Nanoemulsions are part of a broad class of multiphase colloidal dispersions [Citation5] that do not form spontaneously. The International Union of Pure and Applied Chemistry (IUPAC) has defined nanoemulsions as dispersion made of water, oil, and surfactant(s) that consists of an isotropic and thermodynamically stable system with dispersed domain diameter, with the particles formed at the nano scale by means of mechanical forces [Citation33]. Thus, forces from external shear are needed to rupture larger droplets into smaller ones [Citation5] to form this minute emulsion system. This calls for the use of ultra-high speed mixing devices to overcome effects of surface tension to produce the nano scale droplets [Citation5]. On the contrary, nanoemulsions are defined rather differently by the US Food and Drug Administration (FDA), in which the system is considered as a material or end product engineered in such a way so as to have at least one external dimension, or an internal or surface structure, in the nanoscale range (approximately 1–100 nm); and a material or end product engineered to display properties or phenomena that include physical or chemical properties or biological effects that are attributable to its dimension(s), even if these dimensions fall up to 1 μm (1000 nm) outside the nanoscale range [Citation24, Citation34]. illustrates the differences between nanoemulsion and microemulsion, both of which are composed of oil, water and surfactant. These two types of W/O colloidal dispersions have a similar structure: a hydrophobic shell of oil and surfactant tails and a hydrophilic core of water and surfactant head groups.
Figure 1. Graphic diagram of water-in-oil microemulsions (a) and nanoemulsions (b), consisting of oil, water and surfactant. The structure of the particles in both types of colloidal dispersion is similar, with a hydrophobic shell of oil and surfactant tails and a hydrophilic core of water and surfactant head groups.
From a thermodynamic point of view, nanoemulsions are formed in a non-equilibrium state to afford droplets of remarkably small size in the range of 20–200 nm [Citation35], irrespective of the method used for their preparation. The range of size of particulates in nanoemulsions can substantially vary across different studies, with particulates sizes as low as 20 nm [Citation35] to as high as 1000 nm [Citation24]. Correspondingly, the surface-tension theory of emulsification explains that stabilizers or emulsifiers are required when preparing nanoemulsions, so as to lower the interfacial tension between the two immiscible liquids. Reduction in repulsive forces between the two liquids thus weakens the interactive forces between the molecules of the same liquid [Citation33, Citation36]. Contrariwise, the oriented-wedge theory states that the monomolecular layers of the emulsifying agent are formed by surrounding the droplet in the internal phase of an emulsion. This theory assumes that certain emulsifying agents position themselves around a liquid droplet in accordance to their solubility in that very same liquid [Citation33]. However, according to the plastic-or-interfacial-film theory, the emulsifying agent forms a thin film by adsorption on the surface of water and oil located on the internal phase droplets [Citation33]. The presence of this thin film averts contact and the subsequent coalescence of the dispersed phase. Fundamentally, all three theories would result in the immiscible globules remaining in suspension and continuing to persist in the dispersion medium because of the repulsive forces.
Application of nanotechnology in cosmeceuticals
It has been recently reported that the overall global cosmeceuticals market amounted to a whopping USD 46.93 billion in 2017 and is forecasted to jump to USD 80.36 billion by 2023 at a compound annual growth rate (CAGR) of 9.38% [Citation37]. Nonetheless, nanotechnology in cosmeceuticals is not exactly a new technology. As a matter of fact, it was first introduced to the cosmeceutical market in the 1980s in the form of liposomes. Since then, a myriad of other nanotechnology-based formulations that comprise peptides, proteomics, stem cells and epigenetic factors have been formulated and are being sold to consumers [Citation38–40]. Large cosmetic companies publish several nanotechnology-related patents every year. Hence, the relevance of these patents and investments today [Citation41]. Some nanotechnology-based cosmeceutical patents and products available on the market are tabulated in and , respectively.
Table 1. Patent list on nanotechnology-based cosmeceuticals.
Table 2. Examples of cosmeceutical products that used nanotechnology.
It is an undeniable fact that nanomaterials and nanobiotechnology can radically change the way cosmetics and drugs deliver their benefits [Citation42]. In fact, two main uses of nanoparticles in cosmetic products have been focused on UV filtering and delivery of active ingredients, through encapsulation technology to transport a wide range of beneficial ingredients. This was affirmed by a steady increase in patent-protection activities since 1994, as reported by PatBase. Approximately, 900 patent families were classified as ‘nanoemulsions’ for cosmetic applications, representing a total of 1900 granted patent applications over the course of 20 years. About two-thirds of these patent families are for bodycare, skincare and personal care products [Citation43]. Modern day cosmetic companies are now more innovative by adopting a more integrative approach which combines novel formulation processes with marketing strategies that emphasize on consumer perception and meeting their preferences [Citation28].
Patents reviews on nanotechnology based cosmetics
A large number of novel cosmetic formulations have been granted over the past few decades and enlists some of the patents held by several companies. For instance, patent EP1768749B1 held by L’Oreal [Citation44] for aqueous photoprotective composition comprising hydrophilic metal oxide nanopigments and a vinylpyrrolidone homopolymer uses at least a particular hydrophilic inorganic nanopigments/nanoparticles based on metal oxides untreated with an aluminium phosphate. The hydrophilic metal oxide nanoparticles used in sunscreen products, for instance titanium oxides, zinc oxides or their mixtures have a unit particle mean size between 5 nm and 500 nm. The Korea Research Institute of Bioscience and Biotechnology was granted a patent (US 20090022765A1) [Citation45] that employed a cosmetic pigment composition exhibiting colours in the visible region which comprises of gold nanoparticle (red colour), silver nanoparticles (yellow colour), gold-silver alloy nanoparticles (flame colour) and gold nanoparticles (blue colour). They are used in certain cosmetic products, for instance, lipstick.
Patents number US20050276761A1 [Citation46] and US20070166339A1 [Citation47] have formulations that incorporate the nano-sized Zeolite A containing complexes of anionic zeolites and active oxygen donor agents such as organic and inorganic peroxides. These complexes are usually meant for topical compositions for skin whitening, as well as addressing issues related to skin discolouration and age spots. Act Co Ltd (patent number US 20130095157A1) [Citation48] was patented as a method for stabilising retinol (vitamin A) meant for an anti-inflammatory and wrinkle reducing cosmetic prepared by nano-emulsification. The retinol polymer nanocapsule is fashioned by nanoemulsifying retinol in porous polymer particles (size of 50–200 nm). Mung bean medium chain triglyceride extract and lecithin are then used for the stabilisation of retinol.
A Japanese company, Ands Corporation, holds a patent which designates the preparation of a nanoemulsion of size 100 nm that contains nanoparticles of phospholipid and a lysophospholipid, dispersed in an aqueous phase. Iwamoto Shigemi patented a method to formulate a dry collagen face lotion containing a mix of a collagen or gelatin protein with the incorporation of nanoparticle powder of particle sizes between 10 and 40 nm (Patent No.: JP 2005206567A) [Citation49]. Pacific Corp patented the procedure to prepare nanoemulsion by emulsifying main metabolites of ginseng saponin. Glucose and other compounds were converted into liposomes using a dermotropic emulsifier (EP 1327434A1) [Citation50], for improved skin penetration to stimulate proliferation of fibroblast and biosynthesis of collagen.
It is apparent that nanotechnology in cosmetics has become progressively popular, based on the increasing number of patents. It goes to show that consumers prefer nanobased cosmetics, as they become more informed of their advantages as compared to conventional cosmeceutical products.
Sunscreen products
Protection against UV radiation has become an important issue, as we are at risk of sunburn and developing skin cancer due to overexposure to ultraviolet radiation (UVR) from the sun [Citation51]. The technique of nanoencapsulating antioxidant ingredients into sunscreens was developed to combat free radicals generated by excessive exposure to UV radiation. These formulations have been hailed as an innovative photoprotective and chemopreventive strategy to protect the human skin [Citation52, Citation53].
Basically, two basic types of UV filters, originating from organic and inorganic sources, are used as active ingredients in sunscreen products [Citation54]. The ability of organic-based filters to absorb UV radiation of specific wavelengths depends on their chemical structures, while inorganic filters such as titanium dioxide and zinc oxide have wider applicability, more capable of absorbing and scattering UV radiation. These compounds also have a broader UV blocking range than the filters of organic origin. Thus, titanium dioxide and zinc oxide are extensively employed as active components in sunscreens. Nanoformulations containing nanoparticles of titanium dioxide and zinc oxide have been shown to consistently provide better performance in UV protection than larger particles, reflecting visible light and absorbing UV with very high efficiency [Citation54, Citation55].
Nanotechnology is also used to formulate suitable carrier systems for delivering sunscreens and has been found more efficient than conventional delivery systems. The tiny particulates offer better protection of labile organic filters against chemical degradation by entrapping them inside the particle core, instead of molecularly solvating them either in an oil or a water phase [Citation56, Citation57]. For instance, solid lipid nanoparticles (SLN) were introduced by Wissing and Muller [Citation58] as carriers for active cosmetic ingredients and pharmaceutical drugs. They first used SLNs to encapsulate oxybenzone using cetyl palmitate or other crystalline lipids by high pressure homogenisation [Citation56, Citation59, Citation60]. SLNs were also successfully incorporated by Villalobos-Hernández and Müller-Goymann [Citation61] into the carnauba wax-based inorganic filter that used titanium dioxide. Similarly, a mixture of titanium dioxide with organic filters was formulated to produce composite nanoparticles with a synergistic sunscreen effect, enabling the tuning of UV absorption over the UVA and UVB range, with the concomitant scattering of UV rays and intrinsic UV absorption [Citation54, Citation62].
L'Oreal, Paris is among the major manufacturers of nanobased-sunscreen products using inorganic pigments and nanopigments, based on metal oxides, i.e. titanium dioxide. Nano-pigments of titanium dioxide of below 100 nm, generally between 10 nm and 50 nm (Patent 5,607,664), are found in their most popular sunscreen products, for instance, UV Perfect Anti-Dullness SPF 50 and UV Perfect Even Complexion SPF 50.
Anti-ageing products
Human skin is the largest and most complex organ that acts as a physical barrier to defend the body from water loss, as well as from environmental stresses. Invasions by pathogens and coming into contact with chemicals, physical agents and UVR (as mentioned above) are among the factors that damage the human skin [Citation63, Citation64]. These constant bombardments eventually age the skin, manifesting as wrinkles, dryness, sagginess, pigmentations, etc., over the face and body [Citation63, Citation65–67]. However, there is still hope as the human skin can be rejuvenated, and wrinkle formation can be reversed by the use of collagen. Wrinkles become apparent when the collagen structure in our skin becomes deteriorated as a consequence of the factors mentioned earlier [Citation63].
Anti-aging creams typically contain either of two core groups of proxy, the antioxidants and the cell regulators [Citation68]. Antioxidants, viz. vitamins, polyphenols and flavonoids, are added into creams to reduce collagen degradation. These compounds quench the free radicals in the tissues, thus keeping their concentrations low [Citation28, Citation69]. Cell regulators such as retinols, peptides and growth factors, on the other hand, impact collagen metabolism and production [Citation70]. Vitamin A (retinol) containing creams are the most popular on the market, and so are its derivates (retinoid, retinaldehyde and tretinoin) [Citation71]. These compounds are capable of reducing expression of MMP 1 (collagenase 1) and beneficially inducing the biosynthesis of collagen. Retinol is the more preferred ingredient over tretinoin in anti-ageing creams, as retinol causes less skin irritation compare to tretinoin (for review see [Citation72]).
There are a large number of nanotechnology-based cosmeceutical products claiming to have anti-wrinkle and firming effects. The enhanced delivery process of the bioactive ingredients through the skin has to do with their minute particle size and large surface area [Citation2]. For example, the small sized lipid vesicles enable bioactive materials to be absorbed more readily into skin [Citation73]. In fact, one of the most popular anti-wrinkle products using this technology is Revitalift from L’Oreal Paris. This product contains nanosomes with Pro-retinol A (a modified version of vitamin A specially designed to deliver the nutrient to cells). Nanosomes are a highly efficient delivery system that can enter the human bloodstream through inhalation, or oral, topical, dermal, ocular or parenteral routes [Citation74]. Thus, topical delivery of the drug to the core of the skin cells leads to efficient absorption of the active ingredients [Citation75] to combat wrinkles. In addition, nanosomes can promote skin moisturization and slow down collagen breakdown. Studies have shown that long term application of retinol can increase epidermal water content, epidermal hyperplasia, and cell renewal and encourage collagen synthesis [Citation76]. Introducing retinol into the human skin can interfere with melanogenesis and inhibit the action of matrix metalloproteinases. This reduces the appearance of fine lines and wrinkles, and helps in lightening lentigines/pigmentations on the skin [Citation77]. Another antiwrinkle product is marketed by Lancôme by the name of Hydra Zen cream. The product formulation which contains nanoencapsulated Triceramide was marketed for its ability to restore skin softness and renew skin for a healthy look. Reviewed in [Citation78].
Types of nanoemulsion systems
Cosmeceutical formulations are basically categorized into O/W emulsions and W/O emulsions, where the latter are the dermatologically better delivery systems. There is also the double emulsion (oil-water-oil or water-oil-water) [Citation28] but these more complex systems will not be discussed in the following subsections. The fundamental characteristics between the two basic nanoemulsion systems are discussed below.
Water in oil (W/O)
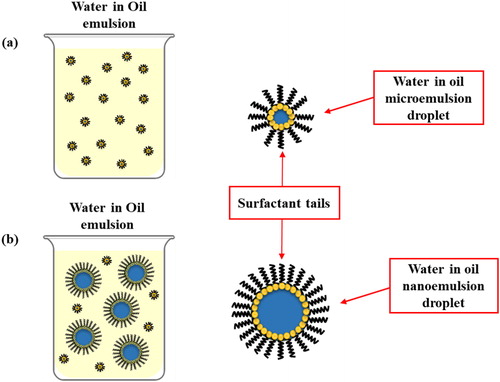
Water in oil nanoemulsion (W/O) is a class of emulsion that has nano-sized water droplets dispersed in organic media through the action of surfactants [Citation79]. Preparation of any given type of emulsion must consider hydrophile-lipophile balance (HLB). HLB is a semi-empirical scale that aids formulators to select surfactants [Citation80]. It shows the ratio of the hydrophilic portion of the non-ionic surfactant to the lipophilic portion [Citation81], to yield the ‘best emulsion’ and, not undergo flocculation or coalescence [Citation82]. The HLB values suited for particular types of emulsion are listed in [Citation83]. Most importantly, surfactants of a final HLB value of 4–6 are ideal for preparation of W/O nanoemulsions.
Table 3. HLB value for various applications.
W/O nanoemulsions are industrially useful in micro-reactors to control nanoparticles growth [Citation84] such as CdS nanoparticles [Citation85] and titania–silica nanoparticles [Citation86]. Ceramic nanoparticles [Citation87] are produced using various W/O emulsions as the reaction media. In the pharmaceutical field, W/O emulsions are valuable as adjuvants for vaccines with unusual antigens, for instance synthetic peptides, recombinant proteins or DNA [Citation88].
Oil-in-water (O/W)
Oil-in-water, or water-based, nanoemulsions are comprised of small lipid droplets dispersed within an aqueous phase, with normal mean droplet diameter of <200 nm [Citation89, Citation90]. While nanoemulsions and conventional O/W emulsions are thermodynamically unstable systems, the homogenisation technique used in their production dictates their droplet sizes [Citation91, Citation92]. According to Winsor [Citation93], there are four types of emulsion phases existing in equilibrium, referred to as Winsor phases. An O/W type is classified as Winsor I, a two phase system whereby the upper oil layer exists in equilibrium with the lower (O/W) emulsion phase [Citation33]. The schematic diagram of W/O and O/W nanoemulsions consisting of surfactant micelles is shown in .
Techniques to prepare nanoemulsions
The non-equilibrium nature of nanoemulsions means that they cannot be prepared spontaneously [Citation92], but the process critically requires a sufficient energy input from mechanical equipment [Citation94]. For the droplet size to be small, a greater amount of mechanical energy is necessary. The work required (W) to increase an interface is represented as:
(1)
(1)
where ΔA is the increase in the total interfacial area and γ is the interfacial tension. This relationship advocates that higher amount of work is required when ΔA is large (i.e. smaller droplet size), or if the interfacial tension is high [Citation95]. Alternatively, spontaneous techniques are also employed to prepare nanoemulsions [Citation95]. Having said that, techniques used to formulate nanoemulsions are conveniently divided into two main groups: the low-energy and high-energy methods.
Low energy techniques
‘Low-energy’ techniques involve preparing nanoemulsions via spontaneous emulsification without the use of any device or energy [Citation96]. Low-energy emulsification methods are dependent on the chemical energy stored in the components of the system to be emulsified [Citation97]. These methods basically take advantage of the intrinsic physicochemical properties of the components to generate submicronic droplets [Citation12]. Phase transitions that occur in the low energy method involve equilibrium phases, such as microemulsion, lyotropic liquid crystalline and/or micellar phases, and happen during emulsification by condensation [Citation35, Citation98]. The process requires the mixing of two liquid phases, a lipophilic phase into which a hydrophilic surfactant is added and then solubilized to form a homogeneous liquid at room temperature. The aqueous phase is usually made up of pure water. Next, the hydrophilic species contained in the oily phase (i.e. surfactants) is solubilized into an aqueous phase to initiate the un-mixing of the oil to form the nano-droplets. The formed nanodroplets are then instantly stabilized by the amphiphiles [Citation12]. Phase inversion composition (PIC) and phase inversion temperature (PIT) are examples of low energy methods used to form nanoemulsions, with the former being less energy intensive.
Phase inversion composition (PIC)
Phase inversion composition transpires where the change of curvature in the low-energy emulsification methods is attained under a constant temperature by changing the composition [Citation97]. Preparing nanoemulsions by the PIC method requires the gradual dilution of the oil phase with the water phase, or vice versa [Citation16]. The hydrophilic/lipophilic balance of this system changes at a constant temperature, causing the hydration degree of the surfactant to fluctuate as per the dilute phase. Upon exceeding the transition composition by slight variations of the water-to-oil ratio, the emulsion becomes destabilized and then ruptures to afford the kinetically stabilized nanoemulsion [Citation24, Citation99, Citation100]. Likewise, nanoemulsion formulation in PIC method is also producible with the support of a titration chart [Citation101]. For instance, isopropylmyristate-in-water nanoemulsions with droplet sizes below 200 nm are formed above the critical micelle concentration of surfactant by the PIC method. This was achieved by simply varying the ethanol concentrations using the surfactant, PEG-60 hydrogenated castor oil [Citation102].
The PIC method is done by slowly adding the components of the continuous phase into the components of the dispersed phase [Citation103]. This consequently invokes a phase inversion process in certain sections along the emulsification route [Citation104]. During the emulsification route, a zone where a liquid crystal (lamellar or cubic) or bicontinuous phase exists has to be determined to cross, as to favour formation of small and uniform droplets. This is to ensure that mostly small and uniform droplets are formed from this process [Citation105]. The preparation protocol (addition and mixing rate) should ensure a complete integration of the final dispersed phase (water for W/O and oil for O/W emulsions) in these phases. The dispersed phase must be judiciously mixed with the continuous phase, before more continuous phase is added till the final composition of the nanoemulsion is reached. Only then, the reorganisation of the dispersed phase into small droplets is favoured [Citation104, Citation106]. Usón et al. [Citation103] successfully formulated several batches of W/O nanoemulsions using a low internal phase content using the PIC method. In their work, a water/mixed Cremophor EL-Cremophor WO7 surfactant/isopropyl myristate systems was prepared. Another study prepared W/O nanoemulsions using a simple PIC method at an elevated temperature. The resultant emulsion was stabilized by Span 80-Brij 35, which yielded an emulsion exhibiting a high internal phase [Citation107].
Being less energy intensive, the low energy method has shortcomings related to the use of large amounts of surfactant and the requirement of a full control of the physicochemical parameters. Thus, the method is unsuitable for industrial-scale preparations of emulsions [Citation4], the products are prone to coalescence and creaming [Citation4, Citation108].
Phase inversion temperature (PIT)
Unlike the PIC method, PIT is a temperature dependent process that allows a certain degree of flexibility in preparing the nanoemulsion. The process can be repeated several times by tuning the mixing temperature to achieve nanodroplet quality [Citation109]. The emulsification method to stimulate emulsification rests on the principal of the extremely low interfacial tensions achieved at the HLB temperature of the order of 10−2–10−5 mN m−1 [Citation92, Citation110]. However, this self-emulsification process tends to yield nanoscale droplets that lamellar layers on their surfaces, which become increasingly less stable over long over long term storage [Citation5, Citation111].
In [Citation112], the solid black line marks the inversion locus and the dotted lines mark the hysteresis zone. The interfacial tension is usually minimal within the optimum formulation zone and at the inversion locus. During low-energy emulsification, this ultralow interfacial tension is engaged to form finely dispersed droplets, whereas the final emulsion should be far away from these regions to improve emulsion stability [Citation112]. When using non-ionic surfactants, the stability of the emulsion can be achieved by changing the temperature of the system. This forces a transition from an O/W emulsion at low temperatures to a W/O emulsion at higher temperatures, a process called the transitional phase inversion. During the cooling process, the system crosses a point of zero spontaneous curvature and minimal surface tension, to promote the formation of finely dispersed oil droplets (vertical arrow in ) [Citation113]. A work by Shinoda and Arai described the importance of suitable phase inversion temperatures to achieve various combinations of hydrocarbons and emulsifiers [Citation114]. For instance, phenytoin-loaded nanoemulsions with droplet sizes between 11 and 15 nm were produced spontaneously by using the phase inversion method to promote topical wound healing through enhanced proliferation of epidermal cells [Citation110, Citation115, Citation116].
Figure 3. Schematic drawing of catastrophic and transitional phase inversion for the preparation of oil in water emulsions [Citation113].
![Figure 3. Schematic drawing of catastrophic and transitional phase inversion for the preparation of oil in water emulsions [Citation113].](/cms/asset/f5abf6cc-c2ac-43a0-bcb7-5868a1ef5f13/tbeq_a_1620124_f0003_c.jpg)
High energy methods
In general, nanoemulsions formulated using ‘high-energy’ methods require the use of specific devices to supply enough energy to increase the water/oil interfacial area for generating sub-micronic droplets. The powerful mechanical energy (stirring, pressure or wave equipments such as microfluidization, high-pressure homogenisation, or sonication) breaks up macroscopic phases or droplets into smaller droplets [Citation117]. Nanoemulsion formed by the so-called dispersion or high-energy emulsification methods is well-reported in the literature [Citation118–120]. The use of high-energy process to prepare nanoemulsions requires two consecutive steps: (i) deformation and disruption of macrometric droplets into the smaller droplets; (ii) adsorption of surfactant at their interface (to ensure the steric stabilisation) [Citation22, Citation24]. It is found that equipment supplying energy in the shortest time and producing the most homogeneous flow, also generates the smallest sizes of particulates [Citation121]. To formulate nanoemulsions, the generated force must exceed the interfacial energy by several orders of magnitude. Only then large interfacial areas to form nanoscale emulsion will be achieved. Under such extreme forces, larger droplets are ruptured into smaller ones by the generated fluid stresses. The strong forces rupture the interfacial tension between the two immiscible liquids to form smaller droplets [Citation122].
High-shear stirring using a rotor/stator system
Hydrodynamic shear is the driving force for this high speed stirring technique [Citation123]. Emulsions have been prepared on an industrial scale by a variety of emulsification equipment. The manufacturing protocol retained the similar operating principle which is agitation, and the rotor/stator homogenisation belongs to this category [Citation124]. The emulsification process has two steps: first, the high shear stress deforms the droplets and increases their specific surface area for disruption. Second, the new interface is stabilized by emulsifiers [Citation125]. A few factors that affect control droplet size during the process include shear stress level, nature and concentration of emulsifier, and the order of the preparation during the process [Citation125, Citation126]. The applied shear forces encourage production of narrower particle size, only when the forces are homogeneously applied to the particles. The shear forces are measured as in Reynolds number, which signify the shear forces from high speed stirring. Pertinently, higher Reynolds numbers favour formation of smaller particles [Citation127, Citation128]. The dimensionless Reynolds number is typically used in fluid mechanics to identify whether fluid flowing past a body or in a duct, is steady or turbulent [Citation129]. Shear rates in the range of 108 s−1 are necessary to form nanoemulsions of approximately 100 nm mean size [Citation5, Citation127]. However, this sort of shear is not applicable in most blenders, which require very high speed/sheer equipment. An ART MICCRA D27 system was found highly effective to produce nanoemulsions (135 nm in size) showing narrow size distribution within 5 min of production [Citation127]. Al-Sabagh et al. prepared W/O nanoemulsions using a ultraturrax homogenizer (Ultraturrax pro 200, USA) and achieved particle sizes between 19.3 and 39 nm [Citation14].
High-pressure homogenization
High pressure homogenization (HPH) is the most popular method for preparation of nanoemulsions [Citation127, Citation130]. The technique relies on the powerful cavitation phenomenon to disrupt and produce smaller sizes oil droplets. Other factors such as homogenisation pressure and number of cycles can profoundly influence the mean droplet size and particle distributions [Citation127]. A high-pressure homogenizer is used to produce high pressure over the mixture of oil phase, aqueous phase and surfactant or co-surfactant [Citation131].
While the HPH technique may be popular, it also has inherent issues of poor productivity and component deterioration, as a consequence of too much heat. HPH is only suitable to prepare O/W liquid nanoemulsion containing less than 20% oil phase. This technique becomes unsuitable when formulating high viscosity or creamy nanoemulsions of mean droplet diameters below 200 nm [Citation132]. Conversely, HPH is useful for decreasing droplet size and the polydispersity of oil droplet [Citation133]. For example, Sakulku et al. [Citation133] reported that encapsulated citronella oil nanoemulsion prepared by HPH produced a stable and small droplet size. Nanoemulsion produced from the extract of jackfruit (Artocarpus heterophyllus Lam) pulp was processed twice by HPH at 800 bar and yielded small oil droplets (<200 nm). The resultant cream showed low viscosity and high stability during storage at 4 °C or 20 °C [Citation118].
Ultrasonication generator/sonication
Nanoemulsion preparation by ultrasonication is gaining the interest of formulators due to its exceptional energy efficiency, requirement of low-end mixing instruments, easy system manipulation and most importantly, its low production cost [Citation134, Citation135]. Ultrasound emulsification uses an acoustic field to disperse one liquid into another immiscible liquid [Citation136]. The key effect of ultrasound is cavitation, which involves the rapid formation of vapour bubbles in a liquid under reduced pressure at ambient temperature [Citation137]. The produced bubbles rapidly collapse and generate pressurized shock waves. This, in turn creates highly localized turbulence and great shear forces [Citation136] that traverse the liquid, forming high velocity liquid jets [Citation137]. The mixing of the emulsion in the vicinity of a collapsing bubble is promoted by the disruption of the droplets [Citation136].
The produced ultrasonic waves efficiently disperse the oil phase into water phase through a simple process [Citation138], in which monodisperse droplets of diameters of less than 100 nm are formed [Citation138]. Canselier et al. [Citation139] proposed a two-step mechanism that occurs during ultrasound-assisted emulsification. First, combination of interfacial waves and instability of the system cause the explosion of dispersed phase droplets into the continuous phase. In the second step, the droplets are further broken up by cavitation close to the interface [Citation140, Citation141]. For review see [Citation142]. Preparation of nanoemulsions by this method is limited to only small batches each time [Citation143], hence inappropriate for the industrial scale. Despite its great potential, the method remains limited to only laboratory investigations [Citation132, Citation144].
Microfluidization
The instrument for formulating nanoemulsion is called a microfluidizer [Citation145]. The first generation microfluidizer was designed by the Arthur D. Little Co., but was later taken over by the Microfluidics Corp [Citation146]. Formulating by microfluidization demands the use of high-energy inputs and powerful equipment to produce ultrafine emulsions at much lower surfactant-to-oil ratio (SOR < 0.1) [Citation90]. High pressure is used to drive the fluid through specifically configured microchannels, and a combined effects of shear, impact and cavitation superbly emulsifies the fluid [Citation147]. The process begins when the mixture of the water phase and oil phase is forced into an inline homogenizer to produce a course emulsion. The resultant emulsion is then forced into an interaction chamber lined with micro channels by a high-pressure positive displacement pump (500–200 psi). The flow of the emulsion through an impingement area then turns the viscous mixture into very fine submicron or nano-sized droplets, to finally achieve a stable nanoemulsion [Citation132]. Smaller size emulsions can be produced by increasing the pressure up to ∼700 MPa [Citation148]. It is thought that a microfluidizer is more efficient in producing higher quality nanoemulsions showing smaller and narrower particle size distributions as compared to the HPH [Citation142, Citation149]. Towbin et al. [Citation150] used a Microfluidizer® Processor to prepare a nanoemulsion containing an anti-inflammatory agent, i.e. aspirin. Data from the croton oil-induced (CD-1) mouse ear oedema model exhibited reduced inflammation based on ear lobe thickness, as well as an accumulated auricular cytokine levels as biomarkers for inflammation [Citation150]. Thus, a noteworthy aspect to highlight here is that emulsions produced by different methods can exhibit varying efficacies, connected directly to the produced droplet size distributions. compares the higher energy and low energy methods for formulating nanoemulsion and highlights the advantages and disadvantages of each method.
Table 4. Comparison between high and low energy methods to formulate nanoemulsions.
Technologies for characterisation of nanoemulsions
Zeta potential
This is a parameter that describes the function of surface charge on droplets and is usually measured using a zeta sizer. The sample of nanoemulsion is placed in a zeta cuvette and the reading of the droplet is recorded in mV [Citation151]. Zeta potential is often a better representation of the electrical characteristics of an emulsion droplet, as it inherently accounts for the adsorption of any charged counter ions. Thus, it is a reflection of the electro kinetic potential of in a colloidal dispersion [Citation152, Citation153]. The rule of thumb for zeta potential of nanoemulsion is that values between −5 mV to +5 mV indicate fast aggregation, −20 mV or +20 mV refers to short-term stability, and readings exceeding +30 mV or below −30 mV indicate a stable nanoemulsion. Excellently stabilized nanoemulsions have zeta potentials higher than +60 mV or below − 60 mV [Citation154–156].
It is worth mentioning here that zeta potential between |−25 mV| to |−30 mV| has an energy barrier between the droplets that is strong enough to prevent destabilisation of the emulsion by coalescence [Citation64]. Maruno and Rocha-Filho [Citation11] observed significant production of negative zeta potentials when non-ionic surfactants were used in the formulation. This was presumably due to certain desirable chemical properties of the polyoxyethylene chains in the surfactants used to formulate the nanoemulsion. In any case, zeta potentials of formulations showing initial values greater than −30 mV before and after the electro kinetic potential test, indicate that the formulations were sufficiently stable to undergo the subsequent accelerated stability tests [Citation64]. This range of absolute value (> −30 mV) is a conjecture for stability and conveys enhanced uniformity, as a consequence of repulsive forces between particles in the nanoemulsion which prevent aggregation. The absolute values of zeta potential are high when more hydrophobic domains become exposed on the surface of the nanoemulsion, thereby increasing the inter-droplet repulsive forces [Citation157].
Droplet size and polydispersity (intensity based size distributions) index
Particle size distribution, mean particle diameter (Z-averages), and polydispersity index (PDI) are imperative indicators that describe the quality, stability, uniformity and dispersibility of nanoemulsions [Citation5]. Droplet size is a key factor to gauge the self-nano-emulsification performance because it determines the rate and extent of release and the absorption of the active ingredient. Photon correlation spectroscopy (PCS) and light scattering techniques like static light scattering (SLS), dynamic light scattering (DLS) are the methods typically employed to measure droplet size of a nanoemulsion [Citation158]. A small droplet size will prevent flocculation because of the high curvature and Laplace pressure that opposes the deformation of large droplets. Likewise, coalescence of droplets in nanoemulsion can be prevented by a thick multilamellar surfactant film adsorbed over the interface of droplets. As for most nanoemulsion, destabilisation of this system is the result of Ostwald ripening [Citation159]. This phenomenon of destabilisation occurs when small droplets with high radius of curvature are converted into larger droplets with low radius of curvature, for instance, two droplets diffuse and become one large droplet. Given enough time, droplets size distribution gradually shifts to larger sizes and the nanoemulsion becomes increasingly turbid [Citation160].
In contrast, polydispersity in a nanoemulsion refers to the ratio of standard deviation to mean droplet size, demonstrating the uniformity of droplet size within the formulation. PDI show the deviation from the average size. PDI of lower than 0.22 is desirable as it suggests that the droplets in the nanoemulsion were well-dispersed, and in most part are free from adhesion and aggregation. A high PDI implies an undesirable feature of a less uniform droplet size [Citation161], and PDI < 0.25 indicates good stability of the emulsion [Citation162, Citation163]. Most importantly, PDI closer to zero specifies a monodisperse droplet population, while PDI that is closer to 1 (one) indicates a wide-ranging droplet size [Citation46]. PDI that remains relatively constant at 0.2, despite an extended duration of storage indicates a homogeneous droplet population in the formulation [Citation4]. A nanoemulsion that has small particle size (P > 0.05) and low PDI is inclined to produce a narrow size distribution. Nevertheless, nanoemulsion of slightly small particle size (P ≤ 0.05) with lower PDI advocates a much narrower size distribution [Citation157].
Nanoemulsions are often recorded as a single peak or multiple peaks that may be narrow or broad. A colloidal dispersion showing a single narrow peak could either be a microemulsion or nanoemulsion, but presence of multiple peaks or broad peaks may be indicative of a nanoemulsion. Multiple peaks can be seen in a system that is made up of a mixture of microemulsion and nanoemulsion droplets [Citation90]. Correspondingly, preparation of an emulsion by using higher numbers of homogenisation cycles, for instance, 3–4 or 10 cycles, tends to produce a smaller particle size. Deciding on the number of cycles to be used when preparing a nanoemulsion can be done by just observing the PDI of the drug or active ingredient after each cycle [Citation160]. As the particle size decreases, the stability of nanoemulsions against coalescence and flocculation usually improves. The strength of the attractive forces decreases more rapidly than the strength of the repulsive forces, when the particle size decreases [Citation15]. Other phenomena such as coalescence and flocculation also tend to broaden the particle size distribution and yield a higher PDI.
Viscosity
Viscosity is a parameter crucial to exemplify and gauge stability liquid and semi-solid preparations, on top of the efficient release of active ingredients from the carrier in formulations. In fact, viscosity is very much dependent on the compositions of surfactant, water and oil components of the emulsion along with their concentrations. Increasing the water content during formulation usually lowers viscosity. On the contrary, reducing the amounts of surfactant and co-surfactant can lead to an increase in interfacial tension between water and oil, as a consequence produce a more viscous emulsion. Typically, viscosity of any given nanoemulsion is measured under different shear rates and temperatures, using a Brookfield type rotary viscometer immersed in a thermo bath at 37 ± 0.2 °C [Citation160].
Also, parameters viz. viscosity, conductivity and dielectric methods offer critical insights into the macroscopic level of the formulated emulsion. Data from these parameters can indicate the presence of rod-like or worm-like reverse micelles, or for determining whether it is an oil-continuous or water-continuous nanoemulsion. This allows for the monitoring of phase inversion phenomena during formulation [Citation158]. Nanoemulsions consisting of O/W often possess lower apparent viscosities. This warrants information on the rheological properties of nanoemulsion carriers, in order to estimate the rates of release of active ingredients [Citation158]. However, lower viscosity emulsions are preferred by manufacturers and also users following the easier handling and packing, particularly if the nanoemulsion is designated for oral use [Citation151, Citation161]. It is worth mentioning here that viscosity determination becomes important when the percentage of the oil component is increased. This is because viscosity of an emulsion increases correspondingly with further addition of oil [Citation164], which may affect the sensory attribute of the resultant formulation.
Entrapment efficiency
Entrapment efficiency (EE) is used to estimate the efficacy of a nanocarrier to retain the drug/active ingredient, to ensure delivery of an adequate amount of the component to the targeted site [Citation165]. Key factors that can have a profound impact on EE include the technique of formulation, type of formulation ingredients and nature of the encapsulated bioactive compound in the vesicles [Citation166]. Moreover, particle size tends to expand with higher loading of the active ingredient into the nanoemulsion [Citation167], thus reducing EE of the nanoemulsion. The estimation of EE was successfully demonstrated using a microdialysis technique for nanocapsules, nanospheres and nanoemulsions [Citation166, Citation168]. Other strategies for estimating EE of different nanocarriers include gel filtration, dialysis bag diffusion, ultrafiltration and ultracentrifugation. EE estimation by gel filtration is in actual, a type of exclusion chromatography. An aqueous suspension of porous gel particles is used to separate the nanoparticles according to their molecular weight. Dialysis, on the other hand, can isolate nanoparticles from a mixture of other nanoparticles or free drugs. The free active ingredient diffuses out of the dialysis bag, while the nanoparticles are retained within. In contrast, the centrifugation technique separates free molecules from the micelles based on their different ability to traverse membrane of a certain pore size during centrifugation [Citation166]. The general equation to determine the EE is as follows:
(2)
(2)
where W1 is the amount of active ingredient added in the formulation and W2 is the amount of active ingredient in the supernatant.
Future outlook
Modern day consumers are now more well-informed and insist on the quality and safety of cosmetics that are used on a daily basis. From their standpoint, the positive effects of cosmetics on their health are as germane as the impact on the environment when developing the cosmetics, in addition to the manufacturing and quality control procedures. The trend seen here corresponds to rising public concerns on the ecology or animal welfare with respect to all activities related to manufacturing such products. Recent studies have connected the toxicity of cosmetic ingredients present in wastewater effluents, surface water and fish tissues with bioaccumulation and biomagnification in living tissues of animals higher up the food chain [Citation28]. It is believed that the issue can be circumvented by replacing synthetic cosmetic ingredients with natural ones. But it is rather premature to say that the alternative is safer and more sustainable, thereby necessitating long-term impact studies to confirm its viability. As a matter of fact, the US Environmental Protection Agency (EPA) have outlined a research strategy which requires the proactive inspection on nanoparticles in cosmetics, sunscreens, paints, in relation to their effect on environment and human health [Citation169]. On the same note, members of the scientific committee on Consumer Products (SCCP) has cautioned on the possible toxicity of topical use of insoluble nanoparticles in cosmetics. This is because the minute particles could enter the bloodstream and be taken up by cells [Citation170]. Comparably, the Europe Cosmetics have made efforts in convincing cosmetic manufacturing companies to participate in sustainable practices. The manufacturers are encouraged to adopt the Life Cycle Assessment initiative and eco-design their products, in hopes of minimising the impact of their manufacturing activities on the environment [Citation28].
The technology for development of cosmetics must also be tailored to new market trends and regulations, while being able to incorporate novel active ingredients in the formulations. Most importantly, manufactures should well deliberate and document the behaviour and interactions of the materials that make up the delivery system in the cosmetic formulations. This requires a comprehensive understanding of the science behind the design of novel encapsulating agents. This is to facilitate more cost-effective cosmetic developments in the future by employing rationally designed procedures, instead of wasteful and time-consuming trial and error strategies. Moreover, safety evaluations on new encapsulating agents must step up to allow wider public acceptance. Modern day consumers are expecting delivery systems to have a dual purpose, and therefore, cosmeceutical products are increasingly required to have some health benefits, too [Citation28]. This brings us to the matter of the blurring line between cosmetic and pharmaceutical products, as delivery systems typically used for drug delivery are being used more than ever in cosmetics. In fact, it is becoming the go-to system for enhancing product performance. The once drug-based delivery systems are now used to increase the stability of labile ingredients, i.e. natural antioxidants in cosmeceutical products. In addition to improving control and targeting the delivery, this form of delivery system requires low dosages of the active ingredients [Citation28], which is advantageous when the active ingredients are costly or available in limited quantities. To address this recent demand for cosmeceutical products, the US FDA has clarified the definition of anti-ageing skin care products. Such products claiming to counteract, retard or control the aging process by ‘molecules [that] absorb and expand, exerting upward pressure to lift wrinkles upward’, in reality, imply that the products can invoke inner structural change within the human skin. Such a claim is usually applicable to drugs, and would, therefore, mandate a battery of safety and health quality tests viz. toxicology and microbiology test, chronic toxicity and carcinogenic test, and conducting safe-for-human-use trials for cosmetics claiming to have anti-ageing properties [Citation78]. In lieu of this, research at the global level in the cosmetic field is urgently needed to assess the possible serious effects, as a result of long-term use of anti-ageing cosmetic products by consumers.
It is becoming apparent that the sustainability of future cosmetics and cosmetic dermatology industry will depend on the preparedness of manufacturers to embrace the advancements in the fields of nanotechnology and nanobiotechnology, in line with changes in consumer trends and increasing awareness on the versatility of such technologies. Equally, we should seek new ways of using nanotechnology and nanobiotechnology to formulate nano-products that could ameliorate the well-being of the general population [Citation42]. These products must be marketed in a way that fully respects and values consumer health, as well as the environment. Therefore, stringent laws should be levied on the regulation and safety of nanoparticles in cosmeceuticals, considering that clinical trials are not mandatory for the approval of cosmeceuticals to enter the open market [Citation171].
Conclusions
The aspects surrounding the available techniques of preparation and the physical characterisation of nanoemulsions detailed in this review will furnish the formulator with a wide basis on the available techniques for preparation of nanoemulsions, as well as the technologies for characterisation of the prepared nanoemulsions, according to specific needs. Nanoemulsions are commonplace in many applications including in pharmaceutical and cosmeceutical industries. This is because of their versatility as an efficient carrier system to deliver active ingredients to the targeted delivery sites. Therefore, a firm understanding to correctly prepare nanoemulsions and their important characterisation features is required, in order to achieve a stable nano-sized emulsion. As a conclusion, the usage of nanotechnology for cosmeceutical applications is a promising technology of the future. It has garnered considerable attention from the scientific community and is well reflected in higher number of publications in this area. Imperatively, this technology promises sustainability in cosmetic formulations and, can aid the industry to remain relevant, by delivering consumers cutting-edge, effective cosmeceutical products.
Acknowledgements
We would like to acknowledge valuable help and suggestions provided by our colleagues.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Royer M, Prado M, García-Pérez ME. Study of nutraceutical, nutricosmetics and cosmeceutical potentials of polyphenolic bark extracts from Canadian forest species. PharmaNutrition. 2013;1:158–167.
- Katz LM, Dewan K, Bronaugh RL. Nanotechnology in cosmetics. Food Chem Toxicol. 2015;85:127–137.
- Singh R, Lillard JW. Nanoparticle-based targeted drug delivery. Exp Mol Pathol. 2009;86:215–223.
- Tadros T, Izquierdo P, Esquena J, et al. Formation and stability of nano-emulsions. Adv Colloid Interface Sci. 2004;108–109:303–318.
- Mason TG, Wilking JN, Meleson K, et al. Nanoemulsions: formation, structure, and physical properties. J Phys: Condens Matter. 2006;18:R635–R666.
- Singh Y, Meher JG, Raval K, et al. Nanoemulsion: concepts, development and applications in drug delivery. J Control Release. 2017;252:28–49.
- Sonnevilleaubrun O. Nanoemulsions: a new vehicle for skincare products. Adv Colloid Interface Sci. 2004;108-109:145–149.
- Bouchemal K, Briancon S, Perrier E, et al. Nano-emulsion formulation using spontaneous emulsification: solvent, oil and surfactant optimisation. Int J Pharm. 2004;280:241–251.
- Tan SF, Masoumi HR, Karjiban RA, et al. Ultrasonic emulsification of parenteral valproic acid-loaded nanoemulsion with response surface methodology and evaluation of its stability. Ultrason Sonochem. 2016;29:299–308.
- Dehghani F, Farhadian N, Golmohammadzadeh S, et al. Preparation, characterization and in-vivo evaluation of microemulsions containing tamoxifen citrate anti-cancer drug. Eur J Pharm Sci. 2017;96:479–489.
- Maruno M, Rocha-Filho PAd. O/W Nanoemulsion after 15 years of preparation: a suitable vehicle for pharmaceutical and cosmetic applications. J Dispers Sci Technol. 2009;31:17–22.
- Anton N, Vandamme TF. Nano-emulsions and micro-emulsions: clarifications of the critical differences. Pharm Res. 2011;28:978–985.
- Ngan CL, Basri M, Lye FF, et al. Comparison of Box–Behnken and central composite designs in optimization of fullerene loaded palm-based nano-emulsions for cosmeceutical application. Ind Crops Prod. 2014;59:309–317.
- Al-Sabagh AM, Emara MM, Noor El-Din MR, et al. Formation of water-in-diesel oil nano-emulsions using high energy method and studying some of their surface active properties. Egypt J Pet. 2011;20:17–23.
- Saberi AH, Fang Y, McClements DJ. Fabrication of vitamin E-enriched nanoemulsions: factors affecting particle size using spontaneous emulsification. J Colloid Interface Sci. 2013;391:95–102.
- Anton N, Benoit J-P, Saulnier P. Design and production of nanoparticles formulated from nano-emulsion templates – a review. J Control Release. 2008;128:185–199.
- Becher P. Emulsions: theory and practice. New York (NY): Reinhold; 1965.
- Schulman JH, Stoeckenius W, Prince LM. Mechanism of formation and structure of micro emulsions by electron microscopy. J Phys Chem. 1959;63:1677–1680.
- Hoar T, Schulman J. Transparent water-in-oil dispersions: the oleopathic hydro-micelle. Nature. 1943;152:102.
- Eastoe J. Surfactant chemistry. Bristol (UK): University of Bristol; 2003. Chapter 3, Microemulsions. Available from: http://www.chm.bris.ac.uk/eastoe/Surf_Chem/Surfactant.htm
- Fofaria NM, Qhattal HS, Liu X, et al. Nanoemulsion formulations for anti-cancer agent piplartine – characterization, toxicological, pharmacokinetics and efficacy studies. Int J Pharm. 2016;498:12–22.
- Solans C, Morales D, Homs M. Spontaneous emulsification. Curr Opin Colloid Interface Sci. 2016;22:88–93.
- Care O, Corner C, Listing J, et al. Nanotechnology in cosmetics. Nanotechnology. 2017.
- Yukuyama MN, Ghisleni DD, Pinto TJ, et al. Nanoemulsion: process selection and application in cosmetics – a review. Int J Cosmet Sci. 2016;38:13–24.
- Mu L, Sprando RL. Application of nanotechnology in cosmetics. Pharm Res. 2010;27:1746–1749.
- Weir A, Westerhoff P, Fabricius L, et al. Titanium dioxide nanoparticles in food and personal care products. Environ Sci Technol. 2012;46:2242–2250.
- Mayer S, Weiss J, McClements DJ. Vitamin E-enriched nanoemulsions formed by emulsion phase inversion: factors influencing droplet size and stability. J Colloid Interface Sci. 2013;402:122–130.
- Costa R, Santos L. Delivery systems for cosmetics – from manufacturing to the skin of natural antioxidants. Powder Technol. 2017;322:402–416.
- Boonme P, Junyaprasert VB, Suksawad N, et al. Microemulsions and nanoemulsions: novel vehicles for whitening cosmeceuticals. J Biomed Nanotechnol. 2009;5:373–383.
- Barry BW. Lipid-protein-partitioning theory of skin penetration enhancement. J Control Release. 1991;15:237–248.
- Boonme P. Applications of microemulsions in cosmetics. J Cosmet Dermatol. 2007;6:223–228.
- Kong M, Chen XG, Kweon DK, et al. Investigations on skin permeation of hyaluronic acid based nanoemulsion as transdermal carrier. Carbohydr Polym. 2011;86:837–843.
- Kale SN, Deore SL. Emulsion micro emulsion and nano emulsion: a review. Syst Rev Pharm. 2016;8:39–47.
- Talegaonkar S, Azeem A, Ahmad FJ, et al. Microemulsions: a novel approach to enhanced drug delivery. Recent Pat Drug Deliv Formul. 2008;2:238–257.
- Gutiérrez JM, González C, Maestro A, et al. Nano-emulsions: new applications and optimization of their preparation. Curr Opin Colloid Interface Sci. 2008;13:245–251.
- Siepmann J, Florence AT. Modern pharmaceutics. New York (NY): Informa Healthcare; 2009.
- Mordor I. Cosmeceuticals market – segmented by product type (skin care, hair care, injectable, oral care), active ingredients (antioxidants, botanicals, exfoliants, peptides, retinoids), and regions – growth, trends, and forecast (2019–2024). Gachibowli, Hyderabad (India): Mordor Intelligence; 2018 [cited 2018 Nov 10]. Available from: https://www.mordorintelligence.com/industry-reports/globalcosmeceuticals-market-industry
- Mihranyan A, Ferraz N, Strømme M. Current status and future prospects of nanotechnology in cosmetics. Prog Mater Sci. 2012;57:875–910. DOI:10.1016/j.pmatsci.2011.10.001.
- Shah S, Solanki A, Lee K-B. Nanotechnology-based approaches for guiding neural regeneration. Acc Chem Res. 2016;49:17–26.
- Elbadry MI, Espinoza JL, Nakao S. Induced pluripotent stem cell technology: a window for studying the pathogenesis of acquired aplastic anemia and possible applications. Exp Hematol. 2017;49:9–18.
- Muñoz-Espí R, Álvarez-Bermúdez O. Nanoemulsions. Amsterdam (Netherlands): Elsevier; 2018. Application of nanoemulsions in the synthesis of nanoparticles; p. 477–515.
- Morganti P. Use and potential of nanotechnology in cosmetic dermatology. Clin Cosmet Investig Dermatol. 2010;3:5–13.
- Pathak K, Pattnaik S, Swain K. Nanoemulsions. Amsterdam (Netherlands): Elsevier; 2018. Application of nanoemulsions in drug delivery; p. 415–433.
- L'Alloret F, Simonnet J-T. Aqueous photoprotective compositions comprising hydrophilic metal oxide nanopigments and vinylpyrrolidone homopolymers. Google Patents. 2010.
- Chung BH, Lim YT, Kim JK, et al. Cosmetic pigment composition containing gold or silver nano-particles. Google Patents. 2009.
- Gupta S. Zeolite based UV absorbing and sunscreen compositions. Google Patents. 2005.
- Gupta S. Skin whitening methods and compositions based on zeolite-active oxygen donor complexes. Google Patents. 2007.
- Jeong S-H, Son J-h, Jang S-J, et al. Cosmetic composition containing retinol stabilized by porous polymer beads and nanoemulsion. Google Patents. 2015.
- Nanbu T. Skin-revitalizing cosmetic composition. Google Patents. 2009.
- Yoo B, Kang B, Yeom M, et al. Nanoemulsion comprising metabolites of ginseng saponin as an active component and a method for preparing the same, and a skin-care composition for anti-aging containing the same. Google Patents. 2003.
- Watson M, Holman DM, Maguire-Eisen M. Ultraviolet radiation exposure and its impact on skin cancer risk. Semin Oncol Nurs. 2016;32:241–254.
- Oliveira CAd, Peres DDA, Graziola F, et al. Cutaneous biocompatible rutin-loaded gelatin-based nanoparticles increase the SPF of the association of UVA and UVB filters. Eur J Pharm Sci. 2016;81:1–9. DOI:10.1016/j.ejps.2015.09.016.
- Morabito K, Shapley NC, Steeley KG, et al. Review of sunscreen and the emergence of non-conventional absorbers and their applications in ultraviolet protection. Int J Cosmet Sci. 2011;33:385–390.
- Shi L, Shan J, Ju Y, et al. Nanoparticles as delivery vehicles for sunscreen agents. Colloids Surf A: Physicochem Eng Aspects. 2012;396:122–129.
- Lu P-J, Huang S-C, Chen Y-P, et al. Analysis of titanium dioxide and zinc oxide nanoparticles in cosmetics. J Food Drug Anal. 2015;23:587–594.
- Muller RH, Petersen RD, Hommoss A, et al. Nanostructured lipid carriers (NLC) in cosmetic dermal products. Adv Drug Deliv Rev. 2007;59:522–530.
- Das S, Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech. 2011;12:62–76.
- Wissing SA, Müller RH. Cosmetic applications for solid lipid nanoparticles (SLN). Int J Pharm. 2003;254:65–68.
- Wissing SA, Müller RH. Solid lipid nanoparticles as carrier for sunscreens: in vitro release and in vivo skin penetration. J Control Release. 2002;81:225–233.
- Wissing SA, Müller RH. The development of an improved carrier system for sunscreen formulations based on crystalline lipid nanoparticles. Int J Pharm. 2002;242:373–375.
- Villalobos-Hernández JR, Müller-Goymann CC. Physical stability, centrifugation tests, and entrapment efficiency studies of carnauba wax–decyl oleate nanoparticles used for the dispersion of inorganic sunscreens in aqueous media. Eur J Pharm Biopharm. 2006;63:115–127.
- Smijs TG, Pavel S. Titanium dioxide and zinc oxide nanoparticles in sunscreens: focus on their safety and effectiveness. Nanotechnol Sci Appl. 2011;4:95.
- Lephart ED. Skin aging and oxidative stress: Equol's anti-aging effects via biochemical and molecular mechanisms. Ageing Res Rev. 2016;31:36–54.
- Ribeiro RC, Barreto SM, Ostrosky EA, et al. Production and characterization of cosmetic nanoemulsions containing Opuntia ficus-indica (L.) mill extract as moisturizing agent. Molecules. 2015;20:2492–2509.
- Tobin DJ. Introduction to skin aging. J Tissue Viability. 2017;26:37–46.
- Chang AL. Expanding our understanding of human skin aging. J Invest Dermatol. 2016;136:897–899.
- Kammeyer A, Luiten RM. Oxidation events and skin aging. Ageing Res Rev. 2015;21:16–29.
- Masaki H. Role of antioxidants in the skin: anti-aging effects. J Dermatol Sci. 2010;58:85–90.
- Menaa F, Menaa A, Tréton J. Polyphenols against skin aging. Polyphen Hum Health Dis. 2014;1:819–830.
- Boldyrev AA, Gallant SC, Sukhich GT. Carnosine, the protective, anti-aging peptide. Biosci Rep. 1999;19:581–587.
- Sorg O, Antille C, Kaya G, et al. Retinoids in cosmeceuticals. Dermatol Ther. 2006;19:289–296.
- Ganceviciene R, Liakou AI, Theodoridis A, et al. Skin anti-aging strategies. Dermatoendocrinology. 2012;4:308–319.
- Pardeike J, Hommoss A, Muller RH. Lipid nanoparticles (SLN, NLC) in cosmetic and pharmaceutical dermal products. Int J Pharm. 2009;366:170–184.
- Harisa GI, Badran MM, Alanazi FK, et al. An overview of nanosomes delivery mechanisms: trafficking, orders, barriers and cellular effects. Artif Cells Nanomed Biotechnol. 2018;46:669–679.
- Chung YJ, Kim YD, Kim CR. Peptides for promoting hair growth and improving wrinkle and cosmetic compositions comprising the same. Google Patents. 2012.
- Kafi R, Kwak HR, Schumacher WE, et al. Improvement of naturally aged skin with vitamin a (retinol). Arch Dermatol. 2007;143:606–612.
- Mukherjee S, Date A, Patravale V, et al. Retinoids in the treatment of skin aging: an overview of clinical efficacy and safety. Clin Intervent Aging. 2006;1:327–348.
- Lohani A, Verma A, Joshi H, et al. Nanotechnology-based cosmeceuticals. ISRN Dermatol. 2014;2014:1.
- Peng L-C, Liu C-H, Kwan C-C, et al. Optimization of water-in-oil nanoemulsions by mixed surfactants. Colloids Surf A: Physicochem Eng Aspects. 2010;370:136–142.
- Kubitschek KA, Zero JM. Development of jojoba oil (Simmondsia chinensis (Link) CK Schneid.) based nanoemulsions. Lat Am J Pharm. 2014;33:459–463.
- Gursoy RN, Benita S. Self-emulsifying drug delivery systems (SEDDS) for improved oral delivery of lipophilic drugs. Biomed Pharmacother. 2004;58:173–182.
- Morais GG, Santos ODH, Oliveira WP, et al. Attainment of O/W emulsions containing liquid crystal from annatto oil (Bixa orellana), coffee oil, and tea tree oil (Melaleuca alternifolia) as oily phase using HLB system and ternary phase diagram. J Dispers Sci Technol. 2008;29:297–306.
- Americas I. The HLB system: a time-saving guide to emulsifier selection. Wilmington (DE): ICI Americas, Incorporated; 1984.
- Uskoković V, Drofenik M. Synthesis of materials within reverse micelles. Surf Rev Lett. 2005;12:239–277.
- Tata M, Banerjee S, John VT, et al. Fluorescence quenching of CdS nanocrystallites in AOT water-in-oil microemulsions. Colloids Surf A: Physicochem Eng Aspects. 1997;127:39–46.
- Liu M, Gan L, Pang Y, et al. Synthesis of titania–silica aerogel-like microspheres by a water-in-oil emulsion method via ambient pressure drying and their photocatalytic properties. Colloids Surf A: Physicochem Eng Aspects. 2008;317:490–495.
- Porras M, Martínez A, Solans C, et al. Ceramic particles obtained using W/O nano-emulsions as reaction media. Colloids Surf A: Physicochem Eng Aspects. 2005;270:189–194.
- Aucouturier J, Dupuis L, Deville S, et al. Montanide ISA 720 and 51: a new generation of water in oil emulsions as adjuvants for human vaccines. Expert Rev Vaccines. 2002;1:111–118.
- Chen F, Liang L, Zhang Z, et al. Inhibition of lipid oxidation in nanoemulsions and filled microgels fortified with omega-3 fatty acids using casein as a natural antioxidant. Food Hydrocolloids. 2017;63:240–248.
- McClements DJ. Nanoemulsions versus microemulsions: terminology, differences, and similarities. Soft Matter. 2012;8:1719–1729.
- Ahmed K, Li Y, McClements DJ, et al. Nanoemulsion- and emulsion-based delivery systems for curcumin: encapsulation and release properties. Food Chem. 2012;132:799–807.
- Solans C, Izquierdo P, Nolla J, et al. Nano-emulsions. Curr Opin Colloid Interface Sci. 2005;10:102–110.
- Winsor P. Hydrotropy, solubilisation and related emulsification processes. Trans Faraday Soc. 1948;44:376–398.
- Kumar S, Singh V. Nanoemulsification – a novel targeted drug delivery tool. Journal of Drug Delivery and Therapeutics. 2012;2:40–45.
- López-Montilla JC, Herrera-Morales PE, Pandey S, et al. Spontaneous emulsification: mechanisms, physicochemical aspects, modeling, and applications. J Dispers Sci Technol. 2002;23:219–268.
- Solans C, Solé I. Nano-emulsions: formation by low-energy methods. Curr Opin Colloid Interface Sci. 2012;17:246–254.
- Maestro A, Solè I, González C, et al. Influence of the phase behavior on the properties of ionic nanoemulsions prepared by the phase inversion composition method. J Colloid Interface Sci. 2008;327:433–439.
- D'Arrigo J. Aspects of future R&D regarding targeted lipid nanoemulsions. Stud Interface Sci. 2011;25:333–342.
- Koroleva MY, Yurtov EV. Nanoemulsions: the properties, methods of preparation and promising applications. Russ Chem Rev. 2012;81:21–43.
- Maali A, Mosavian M. Preparation and application of nanoemulsions in the last decade (2000–2010). J Dispers Sci Technol. 2013;34:92–105.
- Kotta S, Khan AW, Ansari SH, et al. Formulation of nanoemulsion: a comparison between phase inversion composition method and high-pressure homogenization method. Drug Deliv. 2015;22:455–466.
- Yang HJ, Cho WG, Park SN. Stability of oil-in-water nano-emulsions prepared using the phase inversion composition method. J Ind Eng Chem. 2009;15:331–335. DOI:10.1016/j.jiec.2009.01.001.
- Usón N, Garcia MJ, Solans C. Formation of water-in-oil (W/O) nano-emulsions in a water/mixed non-ionic surfactant/oil systems prepared by a low-energy emulsification method. Colloids Surf A: Physicochem Eng Aspects. 2004;250:415–421.
- Solè I, Pey CM, Maestro A, et al. Nano-emulsions prepared by the phase inversion composition method: Preparation variables and scale up. J Colloid Interface Sci. 2010;344:417–423.
- Solè I, Maestro A, Pey CM, et al. Nano-emulsions preparation by low energy methods in an ionic surfactant system. Colloids Surf A: Physicochem Eng Aspects. 2006;288:138–143.
- Heunemann P, Prévost S, Grillo I, et al. Formation and structure of slightly anionically charged nanoemulsions obtained by the phase inversion concentration (PIC) method. Soft Matter. 2011;7:5697–5710.
- Pan H, Yu L, Xu J, et al. Preparation of highly stable concentrated W/O nanoemulsions by PIC method at elevated temperature. Colloids Surf A: Physicochem Eng Aspects. 2014;447:97–102.
- Agrawal N, Maddikeri GL, Pandit AB. Sustained release formulations of citronella oil nanoemulsion using cavitational techniques. Ultrason Sonochem. 2017;36:367–374. DOI:10.1016/j.ultsonch.2016.11.037.
- Rao J, McClements DJ. Stabilization of phase inversion temperature nanoemulsions by surfactant displacement. J Agric Food Chem. 2010;58:7059–7066.
- Izquierdo P, Esquena J, Tadros TF, et al. Phase behavior and nano-emulsion formation by the phase inversion temperature method. Langmuir. 2004;20:6594–6598.
- Miller C. In: Sjoblom J, editor. Emulsions and emulsion stability. Boca Raton (FL): CRC Press; 2006.
- Fernandez P, André V, Rieger J, et al. Nano-emulsion formation by emulsion phase inversion. Colloids Surf A: Physicochem Eng Aspects. 2004;251:53–58.
- Bucak S, Rende D. Colloid and surface chemistry: a laboratory guide for exploration of the nano world. Boca Raton (FL): CRC Press; 2013.
- Shinoda K, Arai H. The correlation between phase inversion temperature in emulsion and cloud point in solution of nonionic emulsifier. J Phys Chem. 1964;68:3485–3490.
- Izquierdo P, Esquena J, Tadros TF, et al. Formation and stability of nano-emulsions prepared using the phase inversion temperature method. Langmuir. 2002;18:26–30.
- Teo SY, Yew MY, Lee SY, et al. In vitro evaluation of novel phenytoin-loaded alkyd nanoemulsions designed for application in topical wound healing. J Pharm Sci. 2017;106:377–384.
- Rai VR, Bai JA. Nanotechnology applications in the food industry. Boca Raton (FL): CRC Press; 2018.
- Ruiz-Montañez G, Ragazzo-Sanchez JA, Picart-Palmade L, et al. Optimization of nanoemulsions processed by high-pressure homogenization to protect a bioactive extract of jackfruit (Artocarpus heterophyllus Lam). Innov Food Sci Emerg Technol. 2017;40:35–41.
- Ricaurte L, Perea-Flores MJ, Martinez A, et al. Production of high-oleic palm oil nanoemulsions by high-shear homogenization (microfluidization). Innov Food Sci Emerg Technol. 2016;35:75–85.
- Kaur K, Kumar R, Arpita, et al. Physiochemical and cytotoxicity study of TPGS stabilized nanoemulsion designed by ultrasonication method. Ultrason Sonochem. 2017;34:173–182.
- Floury J, Desrumaux A, Axelos MA, et al. Effect of high pressure homogenisation on methylcellulose as food emulsifier. J Food Eng. 2003;58:227–238.
- Meleson K, Graves S, Mason TG. Formation of concentrated nanoemulsions by extreme shear. Soft Mater. 2004;2:109–123.
- Utomo AT, Baker M, Pacek AW. Flow pattern, periodicity and energy dissipation in a batch rotor–stator mixer. Chem Eng Res Des. 2008;86:1397–1409.
- Maa Y-F, Hsu C. Liquid-liquid emulsification by rotor/stator homogenization. J Control Release. 1996;38:219–228.
- Perrier-Cornet JM, Marie P, Gervais P. Comparison of emulsification efficiency of protein-stabilized oil-in-water emulsions using jet, high pressure and colloid mill homogenization. J Food Eng. 2005;66:211–217.
- Walstra P. Principles of emulsion formation. Chem Eng Sci. 1993;48:333–349.
- Scholz P, Keck CM. Nanoemulsions produced by rotor-stator high speed stirring. Int J Pharm. 2015;482:110–117.
- Jasińska M, Bałdyga J, Cooke M, et al. Specific features of power characteristics of in-line rotor–stator mixers. Chem Eng Process: Process Intensif. 2015;91:43–56.
- Kraichnan RH. The structure of isotropic turbulence at very high Reynolds numbers. J Fluid Mech. 1959;5:497–543.
- Stang M, Schuchmann H, Schubert H. Emulsification in high‐pressure homogenizers. Eng Life Sci. 2001;1:151–157.
- Zahi MR, Wan P, Liang H, et al. Formation and stability of D-limonene organogel-based nanoemulsion prepared by a high-pressure homogenizer. J Agric Food Chem. 2014;62:12563–12569.
- Sharma N, Mishra S, Sharma S, et al. Preparation and optimization of nanoemulsions for targeting drug delivery. Int J Drug Dev Res. 2013;5:37–48.
- Sakulku U, Nuchuchua O, Uawongyart N, et al. Characterization and mosquito repellent activity of citronella oil nanoemulsion. Int J Pharm. 2009;372:105–111.
- Mehmood T, Ahmad A, Ahmed A, et al. Optimization of olive oil based O/W nanoemulsions prepared through ultrasonic homogenization: a response surface methodology approach. Food Chem. 2017;229:790–796.
- Mehmood T. Optimization of the canola oil based vitamin E nanoemulsions stabilized by food grade mixed surfactants using response surface methodology. Food Chem. 2015;183:1–7.
- Tang SY, Shridharan P, Sivakumar M. Impact of process parameters in the generation of novel aspirin nanoemulsions–comparative studies between ultrasound cavitation and microfluidizer. Ultrason Sonochem. 2013;20:485–497.
- Kentish S, Wooster TJ, Ashokkumar M, et al. The use of ultrasonics for nanoemulsion preparation. Innov Food Sci Emerg Technol. 2008;9:170–175.
- Shi Y, Li H, Li J, et al. Development, optimization and evaluation of emodin loaded nanoemulsion prepared by ultrasonic emulsification. J Drug Deliv Sci Technol. 2015;27:46–55.
- Canselier J, Delmas H, Wilhelm A, et al. Ultrasound emulsification – an overview. J Dispers Sci Technol. 2002;23:333–349.
- Jafari SM, He Y, Bhandari B. Production of sub-micron emulsions by ultrasound and microfluidization techniques. J Food Eng. 2007;82:478–488.
- Zhang S, Zhang M, Fang Z, et al. Preparation and characterization of blended cloves/cinnamon essential oil nanoemulsions. LWT – Food Sci Technol. 2017;75:316–322.
- Mahdi Jafari S, He Y, Bhandari B. Nano-emulsion production by sonication and microfluidization – a comparison. Int J Food Prop. 2006;9:475–485.
- Basha SP, Rao KP, Vedantham C. A brief introduction to methods of preparation, applications and characterization of nanoemulsion drug delivery systems. Indian J Res Pharm Biotechnol. 2013;1:25.
- Özgün S. Nanoemulsions in cosmetics. Anadolu Univ. 2013;1:3–11.
- Jaiswal M, Dudhe R, Sharma PK. Nanoemulsion: an advanced mode of drug delivery system. 3 Biotech. 2015;5:123–127.
- Reineccius G. Flavour manufacturing. In: Source book of flavours. London: Chapman & Hall; 1994; p. 572–576.
- Shen L, Tang C-H. Microfluidization as a potential technique to modify surface properties of soy protein isolate. Food Res Int. 2012;48:108–118.
- Thakur A, Walia MK, Kumar S. Nanoemulsion in enhancement of bioavailability of poorly soluble drugs: a review. Pharmacophore. 2013;4:15–25.
- Lee L, Norton IT. Comparing droplet breakup for a high-pressure valve homogeniser and a Microfluidizer for the potential production of food-grade nanoemulsions. J Food Eng. 2013;114:158–163.
- Towbin H, Pignat W, Wiesenberg I. Time-dependent cytokine production in the croton oil-induced mouse ear oedema and inhibition by prednisolone. Inflamm Res. 1995;44:S160–S161.
- Ghareeb MM, Neamah AJ. Formulation and characterization of nimodipine nanoemulsion as ampoule for oral route. Int J Pharm Sci Res. 2017;8:591.
- McClements DJ. Critical review of techniques and methodologies for characterization of emulsion stability. Crit Rev Food Sci Nutr. 2007;47:611–649.
- Choi A-J, Kim C-J, Cho Y-J, et al. Characterization of capsaicin-loaded nanoemulsions stabilized with alginate and chitosan by self-assembly. Food Bioprocess Technol. 2011;4:1119–1126.
- Honary S, Zahir F. Effect of zeta potential on the properties of nano-drug delivery systems – a review (Part 1). Trop J Pharm Res. 2013;12:255–264.
- Honary S, Zahir F. Effect of zeta potential on the properties of nano-drug delivery systems – a review (Part 2). Trop J Pharm Res. 2013;12:265–273.
- Wissing S, Kayser O, Müller R. Solid lipid nanoparticles for parenteral drug delivery. Adv Drug Deliv Rev. 2004;56:1257–1272.
- He W, Tan Y, Tian Z, et al. Food protein-stabilized nanoemulsions as potential delivery systems for poorly water-soluble drugs: preparation, in vitro characterization, and pharmacokinetics in rats. Int J Nanomed. 2011;6:521.
- Chime SA, Kenechukwu FC, Attama AA. Nanoemulsions – advances in formulation, characterization and applications in drug delivery. In: Sezer AD, editor. Application of nanotechnology in drug delivery. Rijeka: InTech; 2014. p. Ch. 03.
- Sharma N, Bansal M, Visht S, et al. Nanoemulsion: a new concept of delivery system. Chron Young Sci. 2010;1:2.
- Mishra RK, Soni G, Mishra R. A review article: on nanoemulsion. World J Pharm Pharm Sci. 2014;258–276.
- Kumar M, Pathak K, Misra A. Formulation and characterization of nanoemulsion-based drug delivery system of risperidone. Drug Dev Ind Pharm. 2009;35:387–395.
- Kelmann RG, Kuminek G, Teixeira HF, et al. Carbamazepine parenteral nanoemulsions prepared by spontaneous emulsification process. Int J Pharm. 2007;342:231–239.
- Kabri T-h, Arab-Tehrany E, Belhaj N, et al. Physico-chemical characterization of nano-emulsions in cosmetic matrix enriched on omega-3. J Nanobiotechnol.. 2011;9:41.
- Azeem A, Rizwan M, Ahmad FJ, et al. Nanoemulsion components screening and selection: a technical note. AAPS PharmSciTech. 2009;10:69–76.
- da Rocha Neto AC, de Oliveira da Rocha AB, Maraschin M, et al. Factors affecting the entrapment efficiency of β-cyclodextrins and their effects on the formation of inclusion complexes containing essential oils. Food Hydrocolloids. 2018;77:509–523. DOI:10.1016/j.foodhyd.2017.10.029.
- Yue P-F, Lu X-Y, Zhang Z-Z, et al. The study on the entrapment efficiency and in vitro release of puerarin submicron emulsion. AAPS PharmSciTech. 2009;10:376–383.
- Rachmadi UW, Permatasari D, Rahma A, et al. Self-nanoemulsion containing combination of curcumin and silymarin: formulation and characterization. Res Dev Nanotechnol Indones. 2015;2:37–48.
- Michalowski C, Guterres S, Dalla Costa T. Microdialysis for evaluating the entrapment and release of a lipophilic drug from nanoparticles. J Pharm Biomed Anal. 2004;35:1093–1100.
- Hammes C. Cosmeceuticals: The cosmetic-drug borderline. Drug discovery approaches for developing cosmeceuticals: advanced skin care and cosmetic products. Southborough: IBC Library Series. 1997.
- Millikan LE. Cosmetology, cosmetics, cosmeceuticals: definitions and regulations. Clin Dermatol. 2001;19:371–374.
- Kaul S, Gulati N, Verma D, et al. Role of nanotechnology in cosmeceuticals: a review of recent advances. J Pharm. 2018;2018:3420204.