Abstract
Bacterial isolates used for the degradation of waste oils has gained greater interest in recent times since these isolates can be immobilized. However, it’s their lack of reproducibility and continual usage that hinders its applicability. The aim of this work is to identify, purify, cultivate and immobilize bacterial isolates that are potential candidates to effectively and selectively degrade waste oils and waste oil blends and reuse them. In an attempt to render bacterium isolates applicable to degradation of lubricating and insulating oil in industrial processes this work investigates the immobilization (through support material, glutaldehyde activated amberlite, polyvinyl alcohol-sodium alginate and chitosan-sodium alginate matrix beads) of bacterial isolates. The data and observed trends show that developed immobilization techniques were successful in immobilizing bacterial isolate strains V2 and D9, with free cell degradation being most efficient (14.7–31.6%) followed by polyvinyl alcohol-sodium alginate (5.3–26.7%), chitosan-sodium alginate (8.8–0.3%) and amberlite-glutaldehyde (2.2–7.0%). The entrapment technique for immobilization of polyvinyl alcohol-sodium alginate and chitosan-sodium alginate proved to be more efficient than adsorption technique used for immobilization of amberlite-glutaldehyde. Free cell degradation was effective in degrading waste oil, but did so upon a single cycle with deterioration. Polyvinyl alcohol-sodium alginate bead matrix can be applied for degradative processes over a wide pH range and short incubation times while chitosan-sodium alginate is best suited for continuous long incubation periods. Reusability of both polyvinyl alcohol-sodium alginate and chitosan-sodium alginate beads are applicable up to a 10-cycle period.
Introduction
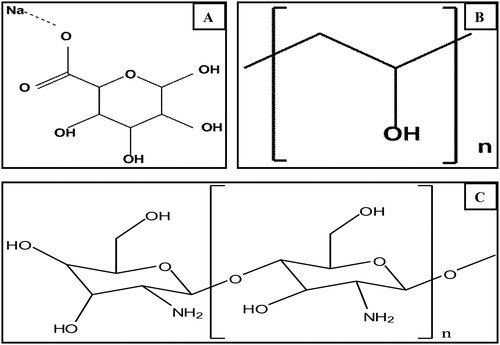
The usage of bacteria in the field of oil degradation has been amplified by the immobilization techniques that offer greater life span and reusability that can prove to be advantageous to industrial processes. Nature provides science with platforms for progress and such is the natural phenomenon of immobilization discovered in microalgae found in the gulf coast. Their natural ability to simultaneously form a biofilm and degrade hydrocarbons sparked an interest in the scientific community, which has led to the rapid usage of bacterial carriers. Immobilization carriers have been identified and used simply to ensure prolonged usage and reusability of the isolates. Key to the usage of such immobilization techniques is the selection of the type of carrier support and immobilization. There are generally two types of immobilization supports, viz., organic and inorganic. Organic supports are usually preferred over the inorganic ones due to availability and examples of organic support include modified cellulose, zeolites and sodium alginates whilst inorganic supports are made up of amberlite resins, polyvinyl alcohol, polyvinyl chloride and polymeric material [Citation1].
The physical and chemical characteristics of a carrier support, must meet the following suitable criteria: high mechanical, biological and chemical stability, low cost and most of all easy handling and regeneration. Four major types of immobilization techniques/protocols are in used currently, namely, (i) adsorption, (ii) entrapment, (iii) encapsulation and (iv) by means of covalent bonding [Citation1].
In this study, two bacterium isolates, Acinetobacter V2 and Paenibacillus D9 were immobilized and used in transformer, truck engine oil and ship oil degradation. Three different support materials was used for immobilization viz., activated amberlite, sodium alginate-polyvinyl alcohol and a sodium alginate-chitosan matrix were investigated to determine their ability to immobilize cells, degrade the substrate, provide good reusability and have good storage with mechanical and chemical stability. Alginates are by far the most used support in immobilization of isolates and this is due mainly because its major advantage in that immobilized cells do not suffer extreme changes such as high temperature and varying pH It is mainly for these advantageous reasons that it was selected as a carrier of choice in this study.
Immobilization in this study has been geared or focussed on the remediation of oils viz., used as insulating, automobile (truck) and ship oil, as these particular types have been known to accumulate in large quantities and are difficult to dispose via novel immobilization techniques. To test the immobilization, three oil-based substrate media were used
The novel study of the strain used is manifested in the impact of the morphologies of both bacterial strains producing optimal immobilization. Acinetobacter has rod-like cell morphology and for many years, it has been widely used in the bioremediation of phenols, benzoates and mainly crude oil blends. Paenibacillus species, having coccobacillary shape isolated from diesel contaminated soil, has numerous applications relevant to medicine and commercial industries with its most common application being bioremediation. The impacts of the morphology were investigated to determine its efficiency to become immobilized.
Materials and methods
The experimental design entails the immobilization of two species of bacteria (Acinetobacter V2 and Paenibacillus D9) with three different carriers such as glutaldehyde activated amberlite (immobilization by adsorption) or sodium alginate-polyvinyl alcohol (PVA) beads and sodium alginate-chitosan beads (entrapment immobilization). Further investigation was conducted on the ability to degrade oil, which was monitored by gas chromatography-flame ionization detection (GC-FID), and to gain insight on the bead morphology by Fourier-transform infrared spectroscopy-attenuated total reflectance (FTIR-ATR) and scanning electron microscopy (SEM) techniques. In addition, the chemical and physical of carrier support characterization such as the potential to be re-used, chemical, storage and mechanical stabilities were studied.
Samples
The feedstock samples used in this study comprised: used uninhibited naphthenic based transformer oil supplied by The Oil Centre (Durban, South Africa), waste truck engine lubricating oil obtained from trucks working on a coal mine in Swaziland, and waste ship engine lubricating oil obtained from the ships at the Durban harbour.
Isolation of bacteria strains
Acinetobacter (strain V2) originally isolated from the used engine oil contaminated soil and Paenibacillis (strain D9) was isolated from used diesel oil contaminated soil obtained from the School of Microbiology and Applied Biotechnology at the University of KwaZulu-Natal Westville campus. These isolates were identified based on the pure cultures commercially obtained and biochemically identified.
Preparation of Bushnell and Haas (BH) medium for intended biodegradation of waste oil
The medium was prepared by dissolving, 1.0 g K2HPO4 (Sigma Aldrich), 1.0 g KH2PO4 (Saarchem), 1.0 g NH4NO3 (Merck), 0.02 g CaCl2⋅2H2O (Sigma Aldrich), 0.2 g MgSO4⋅7H2O (Merck) and 0.05 g FeCl3 (Saarchem) to 1.0 L with deionized water. Aliquots of overnight culture of Acinetobacter strain V2 of 1.0 optical density at 600 nm (approx. cell number of 107 to 108), were transferred into 100 mL BH medium for the study described below.
Immobilization of bacterium isolates
For the immobilization of isolates, 2 g PVA and 3 g SA was added to 25 mL of saline, heated at 80 °C for 15 min, and thereafter left to cool to room temperature. 100 mL of 1.0 OD bacterium isolates were added and stirred thoroughly. For the formation of beads, a syringe was used Beads were formed in ice slurry cooled saturated solutions of calcium chloride. Washed with distilled water and stored at 4 °C for complete solidification.
Typical batch bio-degradative process of waste oil
Castrol® 15W40 waste diesel truck engine oil (WTO), ship engine lubricating oil (WSO) as well as uninhibited naphthenic based transformer insulating oil (WIO) was investigated for their biodegradability by two bacterium isolates, Acinetobacter V2 and Paenibacillus D9. The bacterial strains immobilized were added to flasks containing 0.15% (w/w) waste oil and BH medium. These flasks were incubated for 24 h with extracts (samples extracted using Hexane) analyzed by the GC-FID to determine the extent of degradation. Dichloromethane (30 mL) was added to the flasks and extracted, volume was reduced to 5 mL and samples were injected accordingly. A C8–C40 alkane standard was used to determine percentage of alkane present in sample based on retention times. The GC-FID analyzed extracts show that there was significant degradation of oil components during incubation to varying extents.
Morphology of beads used as immobilized support
Fourier-transform infrared-attenuated total reflection spectroscopy (FTIR-ATR) of beads
To confirm that the bead matrix contained both polymers, infrared characterization of the bead samples were analyzed on a Perkin Elmer 100 FTIR with a Universal ATR attachment.
Surface imaging of immobilized support by scanning electron microscopy (SEM)
The SEM-EDX using Joel JSM 6100 instrument with Espirit 1.8.5 software was used to scan high-resolution images of bead samples. Samples of dimensions 5 mm by 5 mm were placed on a sample holder and coated with gold. The samples were loaded into the instrument and images were processed at a magnification of 30 X and EHT of 5.00 kV.
Chemical and physical properties of immobilized support
Reusability of immobilized isolated strains
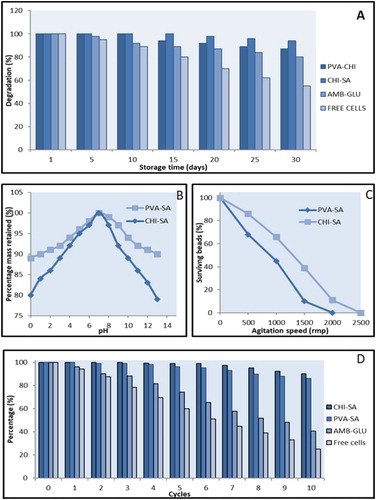
To test the reusability of the beads, 0.1% oil was degraded by strains for 6 h incubation cycles. After 10 cycles of oil degraded was tested [Citation2].
Mechanical stability of immobilized isolated strains
A 4-blade turbine and beaker with four baffles were used to determine the relative mechanical strength of the beads. The baffles were placed 1.2 cm wide each. A given number of beads were placed into the beaker and water was added to approx. 5 cm in height. The speed was 500–3000 rpm for 5 min and the surviving beads were counted. Each run was done in triplicate [Citation3].
Chemical stability of immobilized isolated strains
Using 0.1–1.0 mol dm3 hydrochloric acid and sodium hydroxide, solutions of pH 1–13 were prepared. The beads where soaked in this solution for 48 h, thoroughly rinsed with deionized water and dried in a desiccator until no further change in mass was observed [Citation4].
Storage stability of immobilized isolated strains
Immobilized beads were stored at 4 °C in saline over a period of 0–25 days. During intervals of 5 days the cells were then tested for their degradation ability [Citation2].
Results and discussion
The ability of waste oils to be subjected to degradation by free bacterial cells suffers from several drawbacks including non-reusability, decreasing efficiency due to cell damage and sustaining optimal conditions for survival. It is for these reasons that immobilizations are an important research aspect of such investigations. In this study, three developed immobilization support materials viz. PVA-SA matrix beads, CHI-SA matrix beads and GLU-AMB activated beads, were successful in immobilizing bacterial isolate strains V2 and D9 but to varying extents and with each technique showing advantageous chemical and physical properties. Immobilization via the PVA-SA matrix bead technique showed excellent mechanical strength, good chemical stability and high porosity as shown in, thus leading to the ease of enzyme diffusion and extensive feedstock degradation (). The CHI-SA matrix bead technique proved to be more rigid and less flexible, hence having a lower mechanical stability when subjected to mechanical pressure using a 4-blade mechanical stirrer. From SEM imaging, a film formation by the chitosan acted as a cell release control barrier, thus affording better protection and retaining integrity of the isolate immobilized within the CHI-SA beads during storage. This in turn also retains the integrity of the enzyme produced by the isolate thus increasing the efficacy its reusability in comparison to PVA-SA beads.
Table 1. Band assignments for FTIR-ATR spectra for pure PVA, CHI and SA.
In recent studies by Singh et al. [Citation6], synthetic organic carriers have gained a lot of attention mainly due to their effectiveness in protection from harsh environments such as high temperature or pressure. Furthermore, Zhan et al. [Citation7] indicated that sodium alginate-PVA based matrices boast excellent properties for parameters such as reusability and stability. PVA contributes to the improvement of durability, whereas sodium alginate is said to improve surface properties such as diffusibility and porosity, which in turn increases interaction. Sodium alginate-PVA beads were previously used by Idris et al. [Citation8] and Takei et al. [Citation5] in aqueous media and proved to be successful and efficient. Another common polysaccharide biopolymer, chitosan, has been widely used in numerous applications for the immobilization of bacteria by Lawrie et al. [Citation9].
Many previous researchers have studied the chemical and physical properties of the polymer matrix to gain a fundamental understanding of the morphology, workings and characteristics [Citation3,Citation4, Citation10]. In this study, the polymer matrix is moulded into a spherical bead with the bacteria entrapped on in the inside.
Monitoring of immobilization techniques through morphological studies of beads
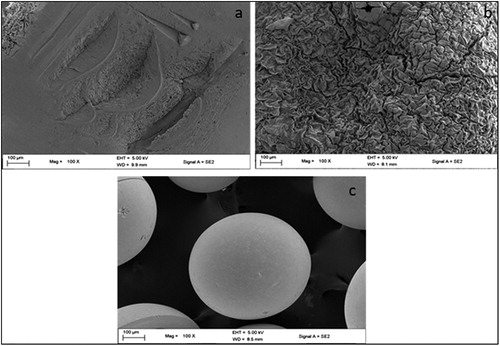
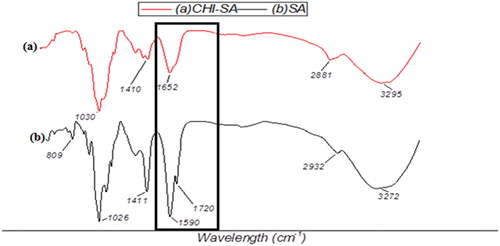
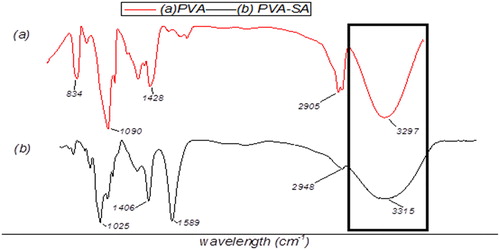
The structure of the beads needs to be monitored in order to follow its ability to function effectively and give an indication of its deterioration. The FTIR was useful in monitoring the successful formation of bead matrix for immobilization as shown in , whilst SEM proved to be a successful in gaining visual indication of bacterium isolates within the bead matrix .
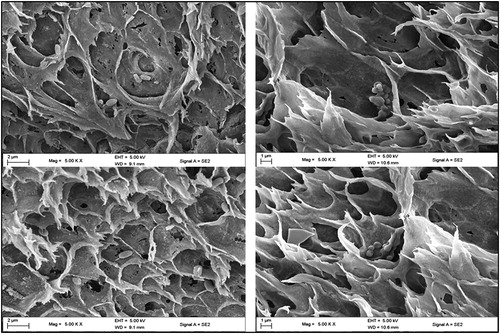
Figure 2. Cross-section SEM images of PVA-SA (top and bottom left) and CHI-SA beads (top and bottom right).
SEM imaging of beads
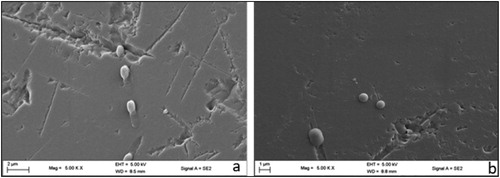
For detailed surface and structural morphology of immobilized PVA-SA, CHI-SA and AMB-GLU beads were analyzed by SEM imaging, which showed the isolates, entrapped or adsorbed during immobilization (). Firstly, it was noticed that D9 was rod-like whilst V2 was coccobacillary shaped. Poor immobilization, difficulty in attachment and inferior cell loading could be attributed to the shape of V2.
It is visible from the images that the cell loading of V2 onto amberlite looked much inferior to D9 (). Poor immobilization of V2 onto amberlite may be the reason for low degradation rates observed with V2 compared to D9 (). To propel forward, bacteria cells must detect and follow chemical gradient of various strengths and distances with most cells travelling in close proximity to surfaces that are solid [Citation11]. From studies conducted by previous researchers the energetics and physics for dealing with these conditions strongly favour rod-like shaped bacteria [Citation11]. From the above mentioned findings it is possible to link the ability of rod-like shaped D9 to favour attachment to AMB-GLU beads rather than coccobacillary shaped V2. Not much information may be gathered on the structure of the polymer matrix but successful immobilization can be seen in the images above. It can be noted that both PVA-SA and CHI-SA polymer bead matrix are capable of immobilizing both D9 and V2 by entrapment methods. It may not be possible to gather information about the loading capacity of the beads; however, it can be seen that there was a subsequent amount of bacteria visible within the beads.
Table 2. Percentage degradation of oil by isolates in different immobilization carriers.
With regard to the topology (shown in SEM images, ) of the beads, PVA-SA and CHI-SA showed porosity but to a varying extent. CHI-SA seemed to show a film barrier as compared to PVA-SA, this could also prove to be a negative attribute of the chitosan matrix bead, since a relatively higher rate of substrate degradation was observed with PVA-SA () due to an increase in the diffusivity of the substrate and enzymes through the polymer matrix. This increase is attributed to a comparable lesser film barrier and a greater porosity as compared to CHI-SA. This was confirmed in a study by Partovinia and Koosha [Citation12], whereby PVA-alginate coated nanofibrous webs as immobilization carriers proved to achieve 1.4 -2.4 times greater degradation extents attributing this to the greater surface area and porosity of PVA. Furthermore, the topology of both beads showed pores which appeared to be coarse (see SEM image, ) and uneven, which may increase the specific surface area and increase interaction ability.
Monitoring of immobilization through FTIR-ATR analysis
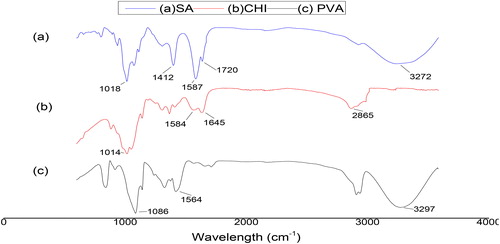
Successful crosslinking of polyvinyl alcohol with sodium alginates (PVA-SA), chitosan with sodium alginate CHI-SA to form bead matrix and amberlite with glutaldehyde by activation (AMB-GLU) in this study was monitored by FTIR-ATR spectra ().
The PVA-SA matrix showed that the hydroxyl stretching vibration of PVA increased to a high band (3315 cm−1), which exhibited that SA and PVA interact through molecular winding, destroy their intra- and intermolecular hydrogen bonds, increase the system density and limit the hydroxyl movement [Citation13].
CHI-SA matrix spectrum showed that the 1720 cm−1 peak decreased; this could be an indication of the majority of alginate in the matrix being deprotonated and therefore could be a reason to prove the blending of sodium alginate and chitosan in the CHI-SA beads to be successful [Citation9, Citation13]. Overall, there cannot be much critical information gathered from the FTIR-ATR spectrum; however, it provided the study with adequate insight on the blending of the matrix beads.
Investigating degradation of waste oils with immobilized bacterial isolates
Due to the above confirmation of successful immobilization, subsequent degradation studies were conducted and the percentages thereof are shown in . For the degradation of transformer oil, V2 and D9 immobilized in PVA-SA achieved 14.7 67 and 26.7% degradation, respectively; immobilized in CHI-SA achieved 11.47 and 20.26%, respectively, and immobilized in AMB-GLU 2.69 and 4.72%, respectively. The same trend in degradation was observed for truck oil and ship oil ().
Free-cell degradation was most efficient (14.7–31.6%, ), followed by PVA-SA (5.3–26.7%), CHI-SA (8.8–20.3%) and AMB-GLU (2.2–7.0%). In a study by Wu et al. [Citation14] achieved a 48–58% biodegradation of crude oil by a consortium of bacterium isolates after 8 weeks of incubation. This was fair inferior to that achieved in this study over a 24-hour incubation period (14.7–31.6%, ) [Citation14]. Free-cell degradation has many limitations such as low reusability and loss of cells, which deems it unfit to be used in industrial processes, since it is not cost effective. Entrapment (PVA-SA and CHI-SA) techniques were superior to adsorption (AMB-GLU), since isolates degraded substrates more efficiently when being entrapped rather than adsorbed (). For instance, it was seen that D9 degraded (percentage of initial amount degraded) truck oil 16.9% (PVA-SA), 14.5% (CHI-SA) and 7.0% (AMB-GLU). Entrapment may prove to be the preferred technique, since it is effective, durable, quick and cost effective; nonetheless, the practical use of this method is limited as it tends to incur mass transfer limitations of substrate or analyte to the enzyme active site, deactivation during immobilization, low loading capacity and abrasion of support material during usage [Citation1]. Despite adsorption immobilization using AMB-GLU not being as effective and efficient as entrapment, unlike entrapment, this technique has the ability to be reloaded [Citation1]. In a review article by Varjani et al. [Citation15], the ability of a carrier to be recollected and reloaded is an important characteristic for application in industrial processes to save both money and time.
Physical and chemical properties of beads
The discussion that follows examines and compares the mechanical, chemical and storage stabilities of PVA-SA and CHI-SA matrix beads along with AMB-GLU activated beads used in the successful degradation of waste oils, mainly for industrial applications.
Storage stability of matrix beads
After storage duration of 5–30 days, at 4 °C with subsequent incubation at 30 °C, the beads were tested for their stability and ability to cause degradation that influences the application of such an immobilized cell system. The initial signal of a significant decrease in the degradative ability after storage was observed after 15 and 25 days for PVA-SA (94%) and CHI-SA (98%), respectively. After storage for 30 days CHI-SA achieved 94% degradation followed by PVA-SA beads matrix (87%), AMB-GLU (80%) and free cells (55%). Borne out of this decreasing trend, it is evident that CHI-SA beads are identified as effective immobilized beads for industrial applications that require long storage durations, simply due improving the process with shorter residence degradation times and less costs incurred. The ability of CHI-SA beads to provide better degradation ability after storage may stem from the chitosan forming a thicker film, hence protecting the cells, as shown by the SEM images in , which concurs with the results obtained by Wang et al. [Citation10].
Chemical stability and mechanical stability of beads
For the immobilization of bacterium isolates to be applicable in various processes used in industries, it is necessary that these polymer matrices be stable over a wide pH range, pH 1–13. The PVA-SA matrix beads show a greater stability (>90%, based on an active polymer bead matric mass retention) over the pH range 1–13 as compared to CHI-SA (>80% based on an active polymer bead matric mass retention) when immersed in varying pH solutions. This may be so due to chitosan that is more susceptible to acid and base hydrolysis due to being a carbohydrate based compound ( and ).
CHI-SA beads showed a lower threshold for disintegration at a centrifugal speed of 1500 rpm, whilst PVA-SA matrix beads showed greater mechanical stability with disintegration first observed at a centrifugal speed of 2000 rpm. It was observed that 90% of the CHI-SA beads survived after 1500 rpm with continual decrease until 2500 rpm where only 10% remained. PVA-SA beads are best suited to industrial application withstanding higher mechanical strain.
Reusability studies of beads
The efficiency of reusability of immobilized beads to degrade waste oils was investigated over several cycles of incubation () to monitor any signs of deactivation through loss of functionality or loss of isolates through detachment (leakage) from entrapment or adsorption. The CHI-SA and PVA-SA beads showed effective degradation of waste oils over the 10-cycle incubation period as shown in . A minimal decrease in levels of isolates was observed from cycle 5 onwards for both CHI-SA and PVA-SA bead matrices; however, this loss is deemed not to have a significant effect on the degradation as the system still maintained a degradation capacity exceeding 80% as shown in . On the other hand, AMB-GLU and free cells showed effective degradation only in the first application with significant decrease in counts of isolates from cycle 2 onwards as shown by the negative gradient in . Entrapment techniques such as those used in the formation of PVA-SA and CHI-SA beads, protect isolates within the persisting matrix, thus somewhat preventing leakage. Adsorption techniques such as those used in the formation of AMB-GLU beads, allowed for isolates to attach as opposed to being entrapped to the surface, which may be a contributing factor to a weaker adherence allowing for easy detachment. Hence, CHI-SA and PVA-SA show efficient degradation ability without much loss in isolates over a 10-cycle incubation as compared to the free cells and AMB-GLU.
Conclusions
The immobilization of D9 and V2 was successful in PVA-SA, CHI-SA and AMB-GLU, with confirmation seen by SEM imaging, and showed capability to degrade waste oils to varying extents. The entrapment technique for immobilization of PVA-SA and CHI-SA proved to be more efficient than the adsorption technique used for immobilization of AMB-GLU, which was clearly observed from their ability to effectively degrade waste oil. Degradation by way of free cells was most effective in degrading waste oil; however, it did so upon a single cycle only, with significant deterioration in its ability post its initial usage. Application of immobilized beads made up of PVA-SA are best suited for degradation over a wide pH range and short incubation times while CHI-SA is best suited for continuous long periods of incubation. The reusability of both PVA-SA and CHI-SA beads is applicable up to a 10-cycle period.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Mohamad NR, Marzuki NHC, Buang NA, et al. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol Biotechnol Equip. 2015;29:205–220.
- Wang Y, Tian Y, Han B, et al. Biodegradation of phenol by free and immobilized Acinetobacter sp. strain PD12. J Environ Sci (China). 2007;19:222–225.
- Chang C-C, Tseng S-K. Immobilization of Alcaligenes eutrophus using PVA crosslinked with sodium nitrate. Biotechnol Tech. 1998;12:865–868.
- Khoo K-M, Ting Y-P. Biosorption of gold by immobilized fungal biomass. Biochem Eng J. 2001;8:51–59.
- Takei T, Ikeda K, Ijima H, et al. Fabrication of poly (vinyl alcohol) hydrogel beads crosslinked using sodium sulfate for microorganism immobilization. Process Biochem. 2011;46:566–571.
- Singh AN, Singh S, Dubey VK. Immobilization of procerain B, a cysteine endopeptidase, on amberlite MB-150 beads. PLoS One. 2013;8:e66000.
- Zhan JF, Jiang ST, Pan LJ. Immobilization of phospholipase A1 using a polyvinyl alcohol-alginate matrix and evaluation of the effects of immobilization. Braz J Chem Eng. 2013;30:721–728.
- Idris A, Zain NAM, Suhaimi MS. Immobilization of Baker's yeast invertase in PVA–alginate matrix using innovative immobilization technique. Process Biochem. 2008;43:331–338.
- Lawrie G, Keen I, Drew B, et al. Interactions between alginate and chitosan biopolymers characterized using FTIR and XPS. Biomacromolecules. 2007;8:2533–2541.
- Wang X-B, Chi C-Q, Nie Y, et al. Degradation of petroleum hydrocarbons (C6–C40) and crude oil by a novel dietzia strain. Biores Technol. 2011;102:7755–7761.
- Young KD. The selective value of bacterial shape. Microbiol Mol Biol Rev. 2006;70:660–703.
- Partovinia A, Koosha M. Fabrication of novel nanocomposite nanofibrous matrices retaining high concentration of microbial cells for heavy crude oil biodegradation. Express Polym Lett. 2019;13:484–499.
- Li ZQ, Li Z, Zhao H, et al. Preparation and characterization of sodium alginate-polyvinyl alcohol wound dressing system containing ciprofloxacin hydrochloride. AMR. 2013;749:388–393.
- Wu M, Li W, Dick WA, et al. Bioremediation of hydrocarbon degradation in a petroleum-contaminated soil and microbial population and activity determination. Chemosphere. 2017;169:124–130.
- Varjani SJ. Microbial degradation of petroleum hydrocarbons. Biores Technol. 2017;223:277–286.