Abstract
The rhizospheric bacterial community associated with desert shrub is an important part of sandy land ecosystems. In this study, we isolated and characterized culturable bacteria associated with Caragana microphylla from a wide range of habitats in North China. We chose five habitats with different aridity index values and dune types. In both trials, soil physicochemical parameters and culturable bacterial populations showed the rhizosphere effects. The culturable bacterial communities of rhizosphere and bulk soil were determined using 16s rDNA sequencing. We identified that the proportions of beneficial bacterial strains with plant growth promoting (PGP) traits (nitrogen fixation, phosphate solubilization and indole acetic acid production) were significantly greater in rhizosphere soil than in bulk soil. Some of the strains screened in this study had significant effects on the growth of Arabidopsis seedlings. These effects included inhibiting the elongation of primary roots, promoting lateral root formation and increasing the shoot fresh weight. In conclusion, our study provides a comprehensive survey of the culturable bacterial populations of the rhizosphere in C. microphylla habitats and identifies potential PGPBs that may help develop an efficient revegetation strategy to manage desert land.
Introduction
Land desertification is a major environmental problem in arid and semi-arid areas [Citation1]. Desertification is one of the most serious environmental problems in northern China [Citation2]. Shrub vegetation plays a critical role by influencing the physicochemical properties of soil and its microbial activities. Caragana microphylla is a perennial shrub that can resist cold, heat, wind and sand burial stressors. It is widely used in revegetation practice due to its easy breeding and rapid growth in northern China [Citation3].
The root system, the microorganisms and the soil form an underground micro-ecosystem in the rhizosphere [Citation4]. The rhizosphere microbial community varies among plant species, growth stages and environmental habitats [Citation5] and often includes plant growth-promoting bacteria (PGPB), which colonise plant roots [Citation6]. Certain PGPR strains improve the ability of plants to adapt to a stressful environment [Citation7], such as drought and salinity [Citation8, Citation9]. Such PGPB strains can be used as biological inoculants to promote plant growth and development in desert areas.
PGPBs can benefit plant growth in many ways. For example, some studies have indicated that inoculation with Klebsiella oxytoca (Rs-5) containing 1-aminocyclopropane-1-carboxylate (ACC) deaminase enhances the absorption of major nutrients, such as nitrogen (N), phosphorus (P), potassium (K) and calcium (Ca), to promote plant growth [Citation10]. Siderophores from PGPB prevent some phytopathogens from acquiring a sufficient amount of iron, thereby limiting their ability to proliferate [Citation11]. Biological N-fixation is another important role of PGPR. The rhizobia are sensitive to drought stress, resulting in a significant decrease in N-fixation under low soil moisture conditions. Co-inoculation of bean (Phaseolus vulgaris L.) with Rhizobium tropici and two strains of Paenibacillus polymyxa results in increased plant height, shoot dry weight and nodule number [Citation12]. Moreover, phosphate-solubilizing bacteria act as a promising biofertilizer because they can supply plants with P [Citation13]. Indole-3-acetic acid (IAA) is a major phytohormone regulating root development, which plays a very important role in rhizobacteria–plant interactions. PGPR-formulated IAA promotes root initiation, cell division and cell enlargement, thereby increasing root surface area by enhancing the formation of lateral and adventitious roots in crop plants [Citation14, Citation15].
Many studies indicate that plant root systems account for most of the variations in microbial community structure [Citation16]. The rhizosphere of C. microphylla has a strong influence on soil physico-chemical properties and on bacterial population [Citation4]. The environmental effectiveness of rhizospheric bacteria are fundamental issues, including soil nutrition and plant growth promoting (PGP) traits from the perspective of ecosystem conservation. Only a few studies have been conducted on C. microphylla and those were mainly aimed at its biological role [Citation17, Citation18] and its effects on soil improvement [Citation4, Citation19]. However, investigations of culturable bacterial community and environmental effectiveness in terms of C. microphylla have not been reported. In this study, we chose four C. microphylla habitats with an increasing aridity index (AI) to investigate these issues in northern China. Three dune types were chosen to further investigate these issues at another site. The culturable bacterial communities and its environmental benefits in rhizospheric and bulk soil were evaluated using 16s rDNA sequencing. The effect of PGPB on plant growth was verified by the inoculation of Arabidopsis thaliana seedlings. The major objectives of this study were: (1) to isolate and to characterize the bacteria from rhizosphere and bulk soil across C. microphylla habitats; (2) to clarify differences in physicochemical properties and culturable bacteria between rhizosphere soil and bulk soil across four sites with an AI gradient and dune types and (3) to evaluate the potential ability of isolates to promote plant growth. The results are expected to screen the rhizospheric culturable bacteria community and its environmental effectiveness across C. microphylla habitats, which can be used as biofertilizer and biocontrol agents, which affect soil quality and plant growth in a desert region.
Materials and methods
Study sites and experimental design
The field investigation was conducted at five sites with an increasing AI: site A (latitude: N38°34′38″; longitude: E102°59′39″), site D (N37°55′39″; E107°26′58″), site H (N42°57′48″; E120°40′42″) and site I (N42°43′60″; E122°32′34″). Aridity index data is the annual average over the 1950–2000 period, which uses the data available from the WorldClim Global Climate Data [Citation20]. AI = MAE/MAP, where MAE is the Mean Annual Potential Evapo-Transpiration and MAP is the Mean Annual Precipitation.
The AI values at sites A, D, H and I were 0.1, 0.26, 0.42 and 0.55, respectively. Thus, site A was an arid desertification area (AI values from 0.03 to 0.2), site D and site H were a semi-arid desertification area (AI values from 2.0 to 5.0), whereas site I was a semi-humid desertification area (AI values from 1.54 to 2.0). Another site (N42°59′38″; E119°39′28″) has three different types of dunes, including a fixed dune (E), a semi-fixed dune (F) and a mobile dune (G). The C. microphylla seedlings of the same age were planted in 2011 at all five study sites (four sites whit different AI, and one site with three dunes). The major aims of plantation were wind-proofing and sand-fixing in the desert land. The height of these shrubs was approximately 1 m after 3 years of growth. Rhizosphere and bulk soil samples of C. microphylla were collected at the four sites and three dunes in mid-May 2014.
Two relatively separate but closely related trials were designed. Both trials were conducted to harvest the physicochemical data of rhizospheric and bulk soil and culturable bacteria community characteristics as well as PGPB types in the rhizospheric soil. Trial 1 compared the differences in the above parameters across the four sites A, D, H and I with different AI values. Trial 2 was focused on evaluating the differences in the above parameters among the three dune types (E, F and G) at the other site. Samples were only taken from fixed dunes at the four sites in trial 1 and fixed, semi-fixed and moving dunes were sampled at Wengniute in trial 2.
Soil material and sampling
Three replicate samples were taken from the rhizosphere and bulk soils. C. microphylla rhizosphere soil was extracted by digging 50 cm deep along the roots and separating the loosely adhering soil as the rhizosphere soil [Citation21]. Each replicate rhizosphere soil sample consisted of three randomly selected adhering soil samples taken from three plants at a distance of 30 to 50 m between single plants depending on the distribution within the vegetation cover (about 300 g from each individual). Each replicate bulk soil sample consisted of three randomly selected soil samples taken from the area (approximately 200 cm from the plants) where the rhizosphere soil samples were obtained. The samples were free of roots and taken from the same depth [Citation22]. All rhizosphere soil samples and bulk soil samples were put in aseptic aluminium cans, immediately transported in a cooling box to the laboratory and stored at −20 °C.
All soil samples were divided into two groups. Samples from the fixed dunes at sites A, D, H and I were considered as trial 1 and samples from the fixed dunes (E), semi-fixed dunes (F) and moving dunes (G) at the other site were considered as trial 2.
Soil physicochemical analyses
All composite rhizosphere soil and composite bulk soil samples were analysed for pH, electronic conductivity (EC), soil total organic carbon (TOC), soil total nitrogen (TN), soil total phosphorus (TP), Ca2+, K+ and Na+. pH and EC were determined with a soil to water ratio of 1:5. TN content was analysed by dry combustion using a C/N analyser (VarioEL GmbH, Germany). TOC was measured using the P-dichromate oxidation method. Total P was measured using the phosphoric acid-molybdenum-antimony colorimetric method. Ca2+, K+ and Na+ contents were determined by flame spectrophotometry. All measurements were taken according to the method of Smith [Citation23].
Bacterial isolation
A 10-g soil sample was suspended in 100 mL of sterile phosphate-buffered saline (PBS) and agitated on a rotary shaker (200 r/min) for 30 min at room temperature. One millilitre of this soil suspension was used to prepare serial 10-fold dilutions with PBS. A 100-μL aliquot of the 10−3, 10−4 and 10−5 dilutions was used for plating in triplicate on R2A agar to enumerate the total culturable heterotrophic bacteria. The plates were incubated in the dark for 7–12 days at 28 °C to assay for a wide spectrum of bacteria [Citation24]. Bacterial colonies were counted and colony-forming units (CFU) per gram of soil dry mass were determined. Plate counts were performed in triplicate and the results are presented as means of the plates. All isolates were subjected to further purification steps by streaking them onto R2A plates, prepared in glycerol culture stocks and stored at −80 °C.
DNA extraction, polymerase chain reaction (PCR) amplification, and sequencing
Genomic DNA of the bacterial isolates was extracted using the CTAB method. The 16S rRNA genes were amplified from genomic DNA by PCR using the universal primers 27F (5′-AGAGTTTGATCCTGGCTCAG-3′) and 1492R (5′-GGTTACCTTGTTACGACTT-3′) [Citation25]. PCR amplifications were performed using the following conditions: initial denaturation of template DNA at 94 °C for 4 min, then one cycle consisting of denaturation (1 min at 94 °C), annealing (20 s at 55 °C) and extension (50 s at 72 °C), 37 cycles at 94 °C for 20 s, 55 °C for 20 s, 72 °C for 50 s and a final extension at 72 °C for 4 min. A negative control, in which the template was replaced with an equal volume of sterile deionized water, was included in each batch. PCR products were checked by 0.8% agarose gel electrophoresis and sequenced on an ABI 3730 XL Genetic Analyzer (Genewiz Inc., USA).
Molecular analysis
All resulting sequences were subjected to operational taxonomic unit (OTU) partitioning based on a 3% sequence difference using Mothur and representative sequences were selected for subsequent analysis. All sequences were submitted to the GenBank database using the BLAST: BLASTN program version 2.2.20 for alignment and determining the initial phylogenetic relationship. A sequence similarity >97% was considered to be the same genus. MEGA6 was used to establish a phylogenetic tree based on the representative OTUs from the rhizosphere and bulk soil samples. The GenBank accession numbers of the 16S rDNA sequence obtained in this study are: KY649367-KY649395 (bulk soil samples) and KY649396-KY649423 (rhizosphere samples). The community structural component diagram was prepared using Origin version 8.0 software (OriginLab, Northampton, MA, USA).
Screening for potential PGP bacterial isolates
The nitrogen-fixing ability of the isolates was tested on Ashby nitrogen-free medium [Citation26]. Strains grown on Ashby nitrogen-free medium after a 5–7 days incubation at 28 °C indicated N-fixation capacity. Phosphate-solubilizing ability was screened on Pikovskya’s medium [Citation27]. Phosphate-dissolving rings formed around strains with phosphate-solubilizing ability after a 5–7 days incubation at 28 °C. A Chrome Azurol S (CAS) plate [Citation28] was used to test the ability of siderophore production referred to as Alexander’s method [Citation29]. Strains with the ability to produce siderophores turned plates from blue to orange after a 5–7 days incubation at 28 °C. Strains with ACC deaminase activity were screened on plates containing Dworkin–Foster minimal medium [Citation30]. The plates were incubated in the dark for 5–7 days at 28 °C. Strains with ACC deaminase activity can be grown on plates using ACC as the sole nitrogen source. The ability to produce IAA was tested on King’s B medium. The strain was cultured on King’s medium for 5–7 days at 28 °C. A colorimetric solution was added dropwise, and strains capable of producing IAA turned the colorimetric solution from colourless to pink [Citation31]. The distribution of PGP traits was shown by a heat map prepared using Heatmap Illustrator, version 1.0 (Hem1).
Effects of PGP bacterial isolates on the growth of Arabidopsis seedlings
A. thaliana col-0 was selected as the plant material in this study. The seeds were treated with surface sterility, then culture at 4 °C for 2 days on the 1/2 MS medium. The seedlings were then transplanted and placed vertically in the growth room, incubated at 22 °C and relative humidity 65–70% for 5 days.
The strain was cultured in R2A liquid medium, 28 °C and 150 rpm for 24 h. After culture for 24 h, the bacteria were centrifuged at 7500 rpm for 10 min, washed twice with sterile water and then suspended in sterile water. The concentration of the bacteria was adjusted to 0.5 (5 × 108 CFU·mL−1) at 600 nm spectrophotometry. Each group was treated with 20 μL bacterial suspension on a 1/2 MS sterile plate (sterile water was added as control). There were 3 replicates in each group and 8 seedlings were co-cultured with bacteria. In order to verify the promoting effect of the bacterial strain on the growth of seedlings, we measured the fresh weight of the shoots co-cultured with the bacterial strain after 7 days of vertical culture. In order to study the effect of bacterial strains on seedling root morphology, the length of primary roots and the number of lateral roots were determined.
Statistical analysis
The least-significant difference test (SPSS version 18.0 for Windows; SPSS Inc., USA) was implemented to explore statistical differences in data. A redundancy analysis (RDA) was used as direct gradient analysis to test specific relationships between the soil properties and microbial biomass as well as community composition. The ordination analysis was performed with CANOCO 5.0 (Microcomputer Power Inc., USA).
Results and discussion
Culturable bacteria of rhizosphere and bulk soils at different habitats
Totals of 334 and 64 morphologically distinct colonies were isolated individually from the rhizosphere and bulk soil samples, respectively. All isolates were sequenced; a search for chimeric or sequencing anomalous sequences did not reveal any ambiguities. After sequence alignment, all strains were divided into 5 phyla and 42 genera.
Firmicutes and Actinobacteria dominated the isolates in rhizosphere and bulk soils across the three dune types. Firmicutes and Actinobacteria are dominant in arid soils worldwide [Citation32]. These groups are well adapted to environmental stress, and can survive in barren soil [Citation33]. The rhizosphere soil contained a higher proportion of Bacteroides. Studies have shown that Bacteroides accounts for a higher proportion of the bacteria in soils with better nutrient conditions [Citation34].
Correlation analysis between the bacterial communities and environmental factors
The RDA results regarding different regions and different types of sand dunes, based on the biotic and abiotic factors of the rhizosphere and bulk soil are presented in . The soil samples at site H and site I in the wetter region were distributed on the left side of the Y-axis and the AI of these two sites was higher than that of the other two sites. Soil samples at these two sites had relatively high soil nutrient contents (including TOC, TN and TP) and CFU. The proportion of Firmicutes and Proteobacteria in soil culturable bacteria was also higher. The drier soil samples at sites A and D were distributed on the right side of the Y-axis and the ion content (including Ca2+, K+ and Na+) and pH values of these soils were relatively high. Actinobacteria accounted for a higher proportion in these soils (). The CFU and diversity of culturable bacteria in the rhizosphere soil of fixed dunes were significantly higher than those in other soils, which was closely related to the significant increase in the contents of TOC and TP, as well as the contents of Ca2+, K+ and Na+ in the rhizosphere soil. The proportion of Actinobacteria, γ-Proteobacteria and Bacteroides in rhizosphere soil also showed significant positive correlation with these properties (). The soil samples in the rhizosphere and bulk soils at each site and the sand dune types were significantly separated. This finding suggests that the rhizosphere is the primary driving force for changes in soil properties, which is consistent with a study of cactuses grown in the desert [Citation35].
Figure 1. Redundancy analysis between the bacterial communities and the environmental factors present in the rhizosphere soil and bulk soil of C. microphylla in four sites with different aridity index (a) and three types of dunes (b).
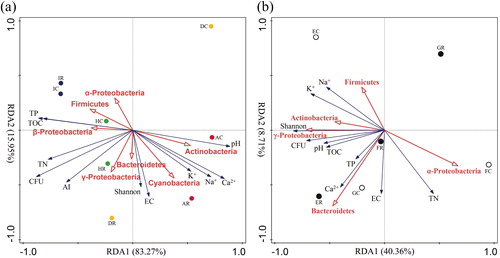
Both biotic and abiotic factors can bring about adaptive changes in quantity and diversity of soil bacterial populations and communities [Citation36]. Microbial communities are frequently affected by various environmental filtration conditions related to soil properties, meteorological factors, host type and random forces [Citation35, Citation37].
Distribution characteristics of the PGP traits of the isolated strains
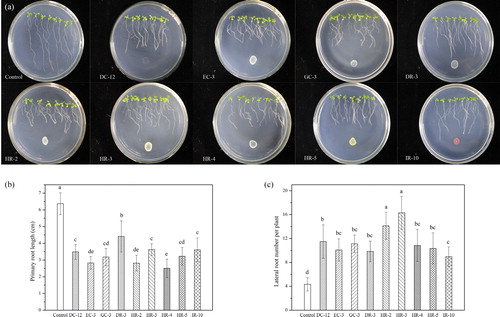
Five different PGP activities were tested in vitro for all representative strains isolated from C. microphylla rhizosphere and bulk soil. The screening results for the multiple PGP traits are presented in and . In general, the proportion of strains with multiple PGP traits in rhizosphere soil was higher than that in bulk soil. A total of 19 strains with more than four PGP traits were screened. Among them, 14 were isolated from rhizosphere and 5 from bulk soil. Whether these strains could promote plant growth needs to be tested by inoculation of plants. After 7 days of culture, the growth of Arabidopsis seedlings, especially the development of roots, changed significantly under the influence of the tested strains (). Among these strains, DR-3, HR-2, HR-3, HR-4, HR-5 and IR-10 from rhizosphere, as well as DC-12, EC-3 and GC-3 from bulk soil had the most significant influence on the roots structure of seedlings (). The primary root length of A. thaliana seedlings affected by these strains ranged from 2.51 ± 0.55 cm to 4.41 ± 0.93 cm, which was significantly lower than that of the control group (6.37 ± 0.65 cm) (). In order to study the influence of the test strain on the lateral roots of seedlings, we counted the lateral roots of each seedling under the microscope. The results showed that the number of lateral roots in the control group was 4.34 ± 1.11/plant, while the number of lateral roots after inoculation with PGPB was 8.95 ± 1.64/plant to 16.31 ± 2.75/plant ().
Figure 2. Phylogenetic relationships among bacterial isolates with multiple PGP traits from bulk soil of C. microphylla based on the partial 16S rRNA gene sequences. The potential PGP traits of bacterial isolates are indicated with the following symbols: ▲ nitrogen fixation, ▼ phosphate solubilization, ● siderophore production, ◆ ACC production, ■ IAA production.
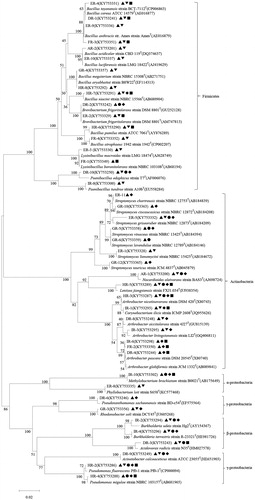
Figure 3. Phylogenetic relationships among bacterial isolates with multiple PGP traits from rhizosphere soil of C. microphylla based on the partial 16S rRNA gene sequences. The potential PGP traits of bacterial isolates are indicated with the following symbols: ▲ nitrogen fixation, ▼ phosphate solubilization, ● siderophore production, ◆ ACC production, ■ IAA production.
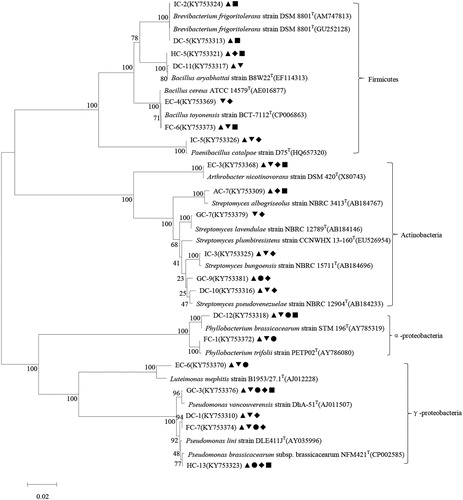
Figure 4. Effects of PGPB inoculation on the growth of Arabidopsis seedlings. (a) Representative images of 12-day-old seedlings after 7 days of growth under control and PGPB inoculated conditions; (b) Effects of PGPB on primary root length; (c) Effects of PGPB on lateral root numbers. Data represent mean values with standard deviation (±SD) of three groups of seedlings each consisting of 8 seedlings. Different letters indicate statistically significant differences (Tukey’s HSD test; p < 0.05). The experiment was repeated three times with similar results.
In order to verify the effect of PGPB on the growth of Arabidopsis seedlings, the roots of all the experimental groups were cut from the shoots, and the fresh weights of these cut shoots were determined respectively. The results showed that the average shoot fresh weight per plant in the control group was 3.21 ± 0.52 mg. Under the influence of PGPB, the shoot fresh weight per plant increased significantly to 3.84 ± 0.54 mg to 5.32 ± 0.65 mg ().
Figure 5. Effects of PGPB inoculation on shoot fresh weight. Data represent mean fresh weights ± SD of three groups of seedlings each consisting of 8 excised shoots. Different letters indicate statistically significant differences (Tukey’s HSD test; p < 0.05). The experiment was performed in triplicate.
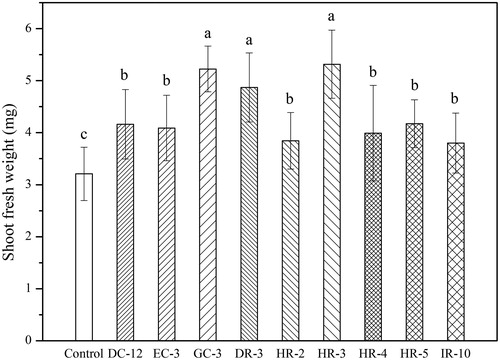
Studies have shown that under the influence of PGPB, the shoot fresh weight and root structure of Arabidopsis seedlings were changed [Citation38, Citation39]. The strains screened in this study had similar effects on the growth of Arabidopsis seedlings, which included inhibiting the elongation of primary roots, promoting lateral root formation and increasing the shoot fresh weight. Since the surface area of root system was greatly increased after the inoculation of PGPB, the uptake of nutrients and water in soil by plants was improved; thus, the growth of plants was promoted [Citation40]. Our study showed that strains DC-12, EC-3, DR-3, HR-4 and IR-10 had significant effects on the growth and root structure of Arabidopsis seedlings. Moreover, the strains GC-3, HR-2, HR-3 and HR-5 with five PGP traits showed excellent plant growth promotion. These strains had the value of further research and application.
A disturbance in a natural plant community is often accompanied or preceded by loss of physicochemical and biological properties of the soil, such as soil structure, plant nutrient availability, organic matter content and microbial activity. The cooperation of microbial symbionts inoculated in the rhizosphere of target indigenous plant species is a successful biotechnological tool to aid the recovery of desertified ecosystems. This approach can be used as an initial step to restore a self-sustaining ecosystem [Citation7, Citation41]. The use of microorganisms and the exploitation of beneficial plant-microbe interactions offer promising and environmentally friendly strategies to prevent and control desertification worldwide.
Conclusions
In this study, the rhizosphere and bulk soils of C. microphylla at four sites with different AI and in different dune types were investigated and compared. We isolated and screened the culturable bacteria. The relationship between culturable bacterial populations and soil properties demonstrated environmental effectiveness across contrasting C. microphylla habitats. We also found a large number of strains with multiple PGP traits, and verified their plant-promoting effect. In conclusion, this study provides a novel understanding of population characteristics and the environmental benefits of rhizosphere bacteria in C. microphylla habitats, which may help the development of a strategy for revegetation management in desertified land.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Reynolds JF, Smith DMS, Lambin EF, et al. Global desertification: building a science for dry land development. Science. 2007;316:847–851.
- Zhao HL, Zhou RL, Su YZ, et al. Shrub facilitation of desert land restoration in the Horqin Sand Land of Inner Mongolia. Ecol Eng. 2007;31:1–8.
- Li XH, Jiang DM, Luo YM. Soil fertile islands of shrub canopy and impacts on vegetation in chronosequence of Caragana microphylla. J Liaoning Tech Univ. 2010;49:830–838.
- Cao C, Abulajiang Y, Zhang Y, et al. Assessment of the effects of phytogenic nebkhas on soil nutrient accumulation and soil microbiological property improvement in semi-arid sandy land. Ecol Eng. 2016;91:582–589.
- Hussain Q, Liu Y, Zhang A, et al. Variation of bacterial and fungal community structures in the rhizosphere of hybrid and standard rice cultivars and linkage to CO2 flux. FEMS Microbiol Ecol. 2011;78:116–128.
- Hayat R, Ali S, Amara U, et al. Soil beneficial bacteria and their role in plant growth promotion: a review. Ann Microbiol. 2010;60:579–598.
- Tabassum B, Khan A, Tariq M, et al. Bottlenecks in commercialisation and future prospects of PGPR. Appl Soil Ecol. 2017;121:102–117.
- Baha N, Bekki A. An approach of improving plant salt tolerance of Lucerne (Medicago sativa) grown under salt stress: use of Bio-inoculants. J Plant Growth Regul. 2015;34:169–182.
- Delshadi S, Ebrahimi M, Shirmohammadi E. Effectiveness of plant growth promoting rhizobacteria on Bromus tomentellus Boiss seed germination, growth and nutrients uptake under drought stress. S Afr J Bot. 2017;113:11–18.
- Yue HT, Mo WP, Li C, et al. The salt stress relief and growth promotion effect of Rs-5 on cotton. Plant Soil. 2007;297:139–145.
- Rajkumar M, Ae N, Prasad MN, et al. Potential of siderophore-producing bacteria for improving heavy metal phytoextraction. Trends Biotechnol. 2010;28:142–149.
- Figueiredo M, Seldin L, de Araujo FF, et al. Plant growth promoting rhizobacteria: fundamentals and applications. In: Maheshwari DK, editor. Plant growth and health promoting bacteria. Berlin: Springer; 2011. p. 21–43.
- Zaidi A, Khan MS, Ahemad M, et al. Plant growth promotion by phosphate solubilizing bacteria. Acta Microbiol Immunol Hung. 2009;56:263–284.
- Kudoyarova GR, Vysotskaya LB, Arkhipova TN, et al. Effect of auxin producing and phosphate solubilizing bacteria on mobility of soil phosphorus, growth rate, and P acquisition by wheat plants. Acta Physiol Plant. 2017;39:253.
- Werner D. Production and biological nitrogen fixation of tropical legumes. In: Werner D, Newton WE. Nitrogen fixation in agriculture, forestry, ecology, and the environment. Netherlands: Springer; 2005. p. 1–13.
- Massimo NC, Devan MMN, Arendt KR, et al. Fungal endophytes in above ground tissues of desert plants: infrequent in culture, but highly diverse and distinctive symbionts. Microb Ecol. 2015;70:61–76.
- Su H, Li YG, Lan ZJ, et al. Leaf-level plasticity of Salix gordejevii in fixed dunes compared with lowlands in Hunshandake Sandland, North China. J Plant Res. 2009;122:611–622.
- Yan QL, Liu ZM, Ma JL, et al. The role of reproductive phenology, seedling emergence and establishment of perennial Salix gordejevii in active sand dune fields. Ann Bot-Lond. 2007;99:19–28.
- Zhao HL, Liu RT. The “bug island” effect of shrubs and its formation mechanism in horqin sand land, Inner Mongolia. Catena. 2013;105:69–74.
- Hijmans RJ, Cameron SE, Parra JL, et al. TheWorldClim interpolated global terrestrial climate surfaces [Internet]. Version 1.3. Berkeley: University of California; 2004 [cited 2019 May 31]. Available from: http://biogeo.berkeley.edu/worldclim/worldclim.htm
- Koranda M, Schnecker J, Kaiser C, et al. Microbial processes and community composition in the rhizosphere of European beech-The influence of plant C exudates. Soil Biol Biochem. 2011;43:551–558.
- Gomes NCM, Fagbola O, Costa R, et al. Dynamics of fungal communities in bulk and maize rhizosphere soil in the tropics. Appl Environ Microbiol. 2003;69:3758–3766.
- Smith KA. Soil analysis: instrumental techniques and related procedures. New York (NY): Dekker; 1983.
- Trivedi P, Spann T, Wang N. Isolation and characterization of beneficial bacteria associated with citrus roots in Florida. Microb Ecol. 2011;62:324–336.
- Moreno C, Romero J, Espejo RT. Polymorphism in repeated 16S rRNA genes is a common property of type strains and environmental isolates of the genus Vibrio. Microbiology. 2002;148:1233–1239.
- Wertz JT, Kim E, Breznak JA, et al. Genomic and physiological characterization of the verrucomicrobia isolate Diplosphaeracolitermitum gen. nov., sp nov., reveals microaerophily and nitrogen fixation genes. Appl Environ Microbiol. 2012;78:1544–1555.
- Subba Rao NS. Advances in agricultural microbiology. Kent: Butterworth Scientific; 1982.
- Schwyn B, Neilands JB. Universal chemical assay for the detection and determination of siderophores. Anal Biochem. 1987;160:47–56.
- Alexander DB, Zuberer DA. Use of chrome azurol S reagents to evaluate siderophore production by rhizosphere bacteria. Biol Fert Soils. 1991;12:39–45.
- Dworkin M, Foster JW. Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol. 1958;75:592–603.
- O'Hara GW, Goss TJ, Dilworth MJ, et al. Maintenance of intracellular pH and acid tolerance in Rhizobium meliloti. Appl Environ Microbiol. 1989;55:1870–1876.
- Neilson JW, Quade J, Ortiz M, et al. Life at the hyperarid margin: novel bacterial diversity in arid soils of the Atacama Desert, Chile. Extremophiles. 2012;16:553–566.
- Singh BK, Munro S, Potts JM, et al. Influence of grass species and soil type on rhizosphere microbial community structure in grassland soils. Appl Soil Ecol. 2007;36:147–155.
- Fierer N, Lauber CL, Ramirez KS, et al. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 2012;6:1007–1017.
- Fonseca-García C, Coleman-Derr D, Garrido E, et al. The cacti microbiome: interplay between habitat-filtering and host-specificity. Front Microbiol. 2016;7:150–166.
- Nicolitch O, Colin Y, Turpault MP, et al. Tree roots select specific bacterial communities in the subsurface critical zone. Soil Biol Biochem. 2017;115:109–123.
- Coleman-Derr D, Desgarennes D, Fonseca-García C, et al. Plant compartment and biogeography affect microbiome composition in cultivated and native Agave species. New Phytol. 2016;209:798–811.
- Zamioudis C, Mastranesti P, Dhonukshe P, et al. Unraveling root developmental programs initiated by beneficial Pseudomonas spp. Bacteria. Plant Physiol. 2013;162:304–318.
- Wang J, Zhang Y, Li Y, et al. Endophytic microbes Bacillus sp. LZR216-regulated root development is dependent on polar auxin transport in Arabidopsis seedlings. Plant Cell Rep. 2015;34:1075–1087.
- Walker V, Couillerot O, Felten A, et al. Variation of secondary metabolite levels in maize seedling roots induced by inoculation with Azospirillum, Pseudomonas and Glomusconsortium under field conditions. Plant Soil. 2012;356:151–163.
- Barea JM, Pozo MJ, Azcon R, et al. Microbial co-operation in the rhizosphere. J Exp Bot. 2005;56:1761–1778.
