Abstract
Halogenated compounds pose long-term potential risks to the well-being of humans due to their recalcitrance and persistent toxicity. The quest for microorganisms capable of degrading such perilous substances merits urgent consideration. In this study, a new dehalogenase-producing bacterium was isolated from a hypersaline environment (TuzGölü Lake, Turkey), and identified as Bacillus megaterium strain CTBmeg1 (Accession number MK128900). Under culture conditions (pH 8.0, NaCl 20%, 30 °C, 200 rpm, 9 days), the B. megaterium strain CTBmeg1 showed an optimum growth on 10 mmol/L of 2,2-dichloropropionic acid with a doubling time of 26.41 h. Furthermore, the presence of a putative halotolerant dehalogenase gene (dehCTBmeg1) of B. megaterium strain CTBmeg1 was detected and amplified via PCR technique. Bio-prospecting for microorganisms in a highly saline environment capable of utilizing halogenated compounds as the sole carbon may prove to be a practical and safer means for bioremediation of contaminated coastal areas, an increasingly common place predicament faced by many nations.
Introduction
Hypersaline environments, like all other ecosystems, are impacted by industrial activities and frequently contaminated with harmful organic compounds following an escalation in the number of manufacturing activities carried out so close to water bodies [Citation1]. Many naturally occurring hypersaline environments are increasingly threatened by environmental pollution as the result of unscrupulous dumping of industrial effluents into these ecosystems. Moreover, it was estimated that 5% of industrial effluents are saline and hypersaline in nature [Citation2], which could potentially increase the concentration of solutes in affected water bodies. Since food sources, such as fishes and prawns, from marine environments form a substantial proportion of human diet [Citation3], pollution of these ecosystems with antropogenic effluents may lead to higher consumption of contaminated food by humans. This is due to the bioaccumulation of various pollutants in the affected marine life that feeds on contaminated food sources [Citation4, Citation5]. In this context, microbial prospecting may prove as one of the better alternatives for bioremediation of contaminated water bodies [Citation6].
Studies have indicated that hypersaline habitats tend to harbour unique and ancient microbial life capable of surviving or thriving under this harsh environment; such microorganisms are known as halophiles. Halophiles are salt loving and have adapted to thrive in extreme conditions of salinity (35% NaCl), for instance, in specific areas around the world such as the Dead Sea, Jordan [Citation7], Urmia salt lake, Iran [Citation8], Tunisian Solar Saltern [Citation9], Tuzkoy salt mine, Turkey [Citation10], alongside hypersaline environments in south Spain [Citation11] and the Great Salt Lake, USA. [Citation12]. Previous studies have shown that halophilic microorganisms isolated from the hypersaline TuzGölü Lake (Turkey) are capable of synthesizing highly efficient halo-stable enzymes which included amylase, cellulase, caseinase, gelatinase, lipase, catalase and oxidase [Citation13, Citation14]. Consequently, halophilic microorganisms may be categorized into five groups: (I) non-halophiles, which grow optimally in less than 0.2 mol/L (< 1% w/v) NaCl, (II) slight halophiles with optimum growth at 0.2–0.5 mol/L (1–3%) NaCl, (III) halotolerant, which are non-halophilic microorganisms but can tolerate high concentrations of salt, in some cases as high as 5.5 mol/L (25% NaCl), (IV) moderate halophiles, growing best between 0.5 and 2.5 mol/L (3–15% NaCl), (V) extreme halophiles with optimum growth between 2.5 and 5.5 mol/L (15–25% NaCl) and ability to grow even at saturated salt concentrations [Citation15].
Pertinently, survival of microorganisms in hypersaline conditions requires specialized cellular adaptations to help preserve the osmotic balance with their external environment. These adaptations involve the existence of organic compatible solutes (osmoprotectants) or the accumulation of variably high concentrations of KCl in their cytoplasms [Citation16, Citation17]. Similar evolution was extended to the enzymes secreted by halophiles, which invariably are metabolically and physiologically stable in a hypersaline environment [Citation18, Citation19]. This is related to the considerably higher proportions of acidic residues in the halophilic protein sequences that confer superior water-binding capacity, thereby better preserving the solubility of their proteins compared to that of the non-halophilic homologues [Citation20].
It has been reported that halogenated compounds, such as 2,2-dichloropropionic acid (2,2-DCP) or commercially known as Dalapon, is the prevalent pollutant found in the environment, typically originating from both natural and human activities. This substance is now widespread in the environment due to our reliance on biocides for weed management in the agricultural industry [Citation21]. The problem is further exacerbated by the fact that 2,2-DCP is highly toxic and resistant to degradation [Citation22], for that reason, this toxic substance could remain in the ecosystem for an extended period of time, long enough to adversely affect marine habitats including commercial and marine fisheries located along the coastal areas [Citation23]. Considering the recalcitrance of such substance, it is as much a local problem as well as a global concern [Citation24–26] and the development of safer means to eliminate the seperilous substances from the environment deserves special consideration.
Over the past decade, bacteria isolated from contaminated marine environments have been reported [Citation27–29], viz. thermophiles [Citation30] and psychrophiles [Citation31, Citation32]. However, very little is known about the halophiles capable of degrading halogenated compounds [Citation15, Citation20]. These microorganisms are deemed as a good source of biocatalysts and are slowly gaining popularity among the environmental biotechnologists as the cost effective means to clean up polluted environments [Citation33]. The innate stability of halophilic microbes at high salt concentrations justifiably supports their possible use for bioremediation of marine ecosytems polluted with halogenated compounds. Therefore, this present study that aimed at providing insights into the availability of 2,2-DCP degrading bacteria at TuzGölü Lake (Turkey) merits special consideration.
Materials and methods
Sampling and reagents
The samples were collected from the contaminated hypersaline TuzGölü Lake, Turkey (latitude: 39°06′30.8.N; longitude: 33°18′58.4E) at 1,665 km2 surface area and it is one of the largest hypersaline lake in the world () [Citation34]. Using aseptic techniques, the sample was collected and brought back to the laboratory. Chlorinated hydrocarbon, 2,2-DCP was obtained from Sigma Aldrich (USA). Distilled water was used in all media preparation. Analytical grade organic solvents and other chemical reagents were procured from Sigma Aldrich (USA).
Media preparation
The liquid media involved the preparation of two different stock solutions. The first stock solution contained basal salts: NaH2PO4·2H2O (10.0 g/L), K2HPO4·3H2O (42.5 g/L), (NH4)2SO4 (25.0 g/L) and the second stock solution contained trace metal salts: nitrilotriacetic acid (NTA) (1.0 g/L), MgSO4 (2.0 g/L), FeSO4.7H2O (120.0 mg/L), MnSO4·4H2O (30.0 mg/L), ZnSO4·H2O (30 mg/L) and CoCl2·6H2O (10 mg/L); both stocks were prepared in distilled water [Citation35].
Minimal media was prepared in a 250 mL sterile Erlenmeyer flask which contained basal salts (10 mL), trace metals (10 mL) and distilled water to a final volume of 100 mL. The minimal salt media was sterilized by autoclaving at 121 °C for 15 min at 15/psi. Then, sterilised 2,2-DCP and NaCl were added to the autoclaved media to the desired concentrations. Using pH meter (Consort C3010, Belgium), pH of the media was adjusted to pH 8 ± 0.2 and incubated with shaking at 200 rpm at 30 °C for 9 days. In order to isolate the pure colonies of the halophile, solid medium that consisted of Oxoid bacteriological agar No. 1 (1.5% w/v) was prepared prior to sterilization. The mixed culture of 0.1 mL was spread onto solid minimal media containing 10 mM 2,2-DCP previously filter sterilized through a 0.2 μm nylon-membrane disc and was incubated at 30 °C.
Morphological, physiological and biochemical characteristics of the strain
Characterization of the physiological and biochemical properties of the bacterial strain included Gram-staining, motility test, carbohydrate fermentation and gelatin hydrolysis [Citation36]. All results were systematically analyzed according to the Bergey’s Manual of Determinative for Bacteriology [Citation37]. Following an overnight incubation in LB medium, tests for oxidase and catalase activity was carried out in 1% (w/v) tetramethylene diamine [Citation38], and consequently observed for evolution of gas and bubble formation in a 2.5% (w/v) hydrogen peroxide solution, respectively [Citation39]. Morphological observation was done under a scanning electron microscope, Hitachi SU5020 FES electron microscope (magnification, of X11.0K, accelerating voltage, 2.0K) equipped with an SE(L) detector at a 16.2 mm working distance. The sample was placed onto an aluminum foil disc and the Leica EM CPD300 critical point dryer (Leica Microsystems GmbH, Germany) was used to dry the sample followed by coating with platinum before viewing. Other biochemical tests: citrate utilization, methyl red, indole production, Voges–Proskauer reactions, urease production and nitrate reduction, were also performed according to a previously described method [Citation39].
PCR amplification of 16S rDNA
Bacterial 16S rDNA universal primers 27 F (5′-AGAGA TTGATCCTGGCTCT G-3′) and 1492R (5′-GGTTTCCTTGTTACGAC AT-3′) were synthesized by Soygen Biotechnology Laboratories (Istanbul, Turkey). Genomic DNA was extracted using Promega Bacterial DNA Isolation Kit AMBER (Istanbul, Turkey), as described by the manufacturer. PCR conditions were carried out according to the following: initial denaturation (94 °C, 1 min), denaturation (95 °C, 30 s), annealing (55 °C for 40 s), extension (72 °C, 1 min; 30 cycles) and final extension (72 °C, 10 min). The total volume of the PCR reaction (50 μL) contained 0.5 μL of DNA template (104 ng), 0.1 μL of 27F (25 μmol/L), 1 μL of 1492 R (25 μmol/L), 1 μL of dNTP (10 mmol/L), 4.0 μL of MgCl2 (25 mmol/L), 5.0 μL of 10× PCR buffer and 0.5 μL Taq polymerase. The PCR products were purified and sequenced by Soygen Biotechnology Laboratories. The sequence identity was searched by the BLASTn analysis using NCBI database. The 16S rDNA gene sequence data was analyzed on the MEGA7 software package and the phylogenetic tree was constructed using the neighbour-joining method [Citation40].
Investigation of optimum growth conditions
The effects of 2,2-DCP for parameters viz. concentration, pH, temperature, rotary speed and medium salinity on the growth rate of the halophilic bacterial cultures were examined using minimal salts medium supplemented with 2,2-DCP to final concentrations of 5, 10, 20, 30 and 40 mmol/L, respectively. The initial pH in the growth medium was adjusted to pH 6, 7, 8, 9 and 10, using 1 mol/L HCl or 1 mol/L NaOH. The effect of the speed of shaking was investigated for 150, 175, 200, 225 and 250 rpm, respectively. Several concentrations of NaCl in the liquid media were prepared: 50 (5%), 100 (10%), 150 (15%). 200 (20%), 250 (25%) and 300 (30%) g/L. The optimized conditions were then determined by calculating the bacterial growth rate with incubation over a period of 9 days.
Results and discussion
Characterization of strain CTBmeg1
Minimal media containing 20% (w/v) of NaCl supplemented with 10 mmol/L of 2,2-DCP was used to screen for new halotolerant microorganisms capable of using this substance as an only source of carbon. The halophilic microorganisms were indeed present in the water samples collected from the hypersaline TuzGölü Lake, appearing as faint yellow colonies (2–4 mm in diameter) after 9 days of incubation at 30 °C (). Such colonies were not observed on the negative control medium (without 2,2-DCP) (). This indicated that the isolate growing on the media supplemented with 2,2-DCP might possess a unique potential in metabolizing 2,2-DCP in the presence of high salt concentration. Development of dark blue colour following Gram staining affirmed that the bacteria were gram-positive ().When viewed under the scanning electron microscope (SEM), strain CTBmeg1 appeared to have a bacilli-like shape with width and length between 745 ± 69.5 nm and 1.96 ± 32.7 μm, respectively ().The biochemical properties of strain CTBmeg1 were closely affiliated with those of Bacillus megaterium strains YJB3 [Citation41], WS24 [Citation42], G3 [Citation43] and BJC3.1 [Citation44]. The overall properties of strain CTBmeg1 bacterium is tabulated in .
Figure 2. Morphological properties of strain CTBmeg1. (a) Pure colony of strain CTBmeg1 on 10 mmol/L 2,2-DCP minimal media agar supplemented with 10% NaCl at 30 °C after 9 days of incubation; (b) negative control (plate not supplemented with 2,2-DCP); (c) Gram-staining (observed under 100× oil immersion magnification); (d) SEM micrograph of isolate visualized at 11,000× magnification, 2.0 kV accelerating voltage. Note: Bar: 5μm. Bacterial size was measured using Hitachi SU5020 SEM software.
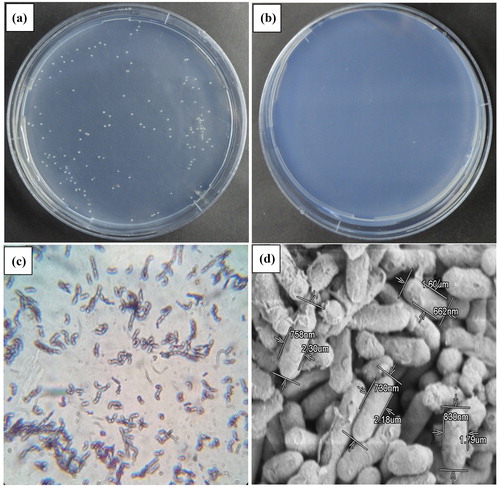
Table 1. comparison of morphological and biochemical characteristics of CTBmeg1 and other B. megaterium strains.
Identification of strain CTBmeg1
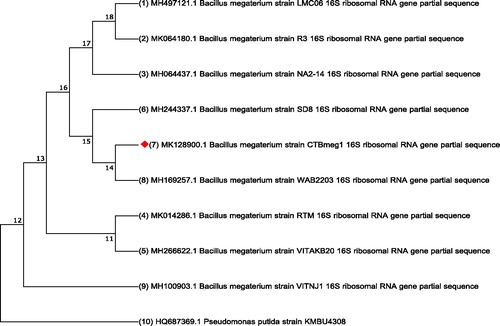
The partial 16S rDNA gene sequence (GenBank accession No. MK128900) of strain CTBmeg1 was obtained as a continuous stretch of 860 bp that was highly similar to that of B. megaterium (97%). The constructed phylogenetic tree from the 16S rDNA sequences revealed that strain CTBmeg1 is closely related to B. megaterium strain WAB2203 (MH169257.1) and other B. megaterium strains (). Based on the finding, the bacterial isolate was identified as B. megaterium CTBmeg1.
Optimum growth conditions of Bacillus megaterium CTBmeg1
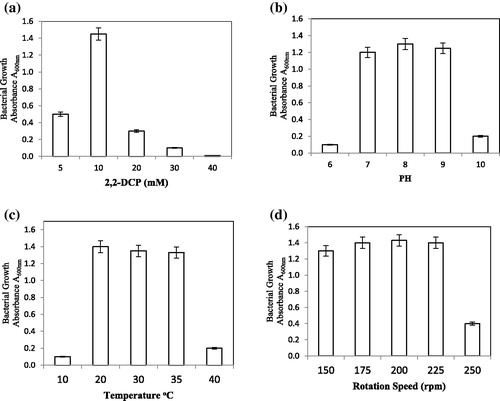
Considering that high multiple significant factors may influence the growth conditions and the consequent bioremediation efficacy of B. megaterium CTBmeg1, such factors were examined in this study. illustrates that the bacterial strain CTBmeg1 grew optimally in media containing up to 30 mmol/L of 2,2-DCP. However, concentrations of 2,2-DCP that exceeded 30 mmol/L inhibited the growth of CTBmeg1, presumably due to increased toxicity of the 2,2-DCP [Citation45]. As shown in , bacterial growth occurred within a medium pH range of pH 7.0–9.0, notably, similar to the natural pH of TuzGölü Lake. According to Oren [Citation46] and Litchfield and Gillevet [Citation47], the high concentration of NaCl in thalassohaline brines has resulted in these lakes having slightly alkaline pHs. Still, neutral pH values are generally favourable to support the growth of most halophililic microorganisms [Citation48]. The best growth temperature was observed to occur in a narrower range, between 20 and 35 °C (), while growth was not observed at 10 °C. The halophilic B. megaterium DSM 32T, isolated from hypersaline Lake in Spain, grew over a wider temperature range between 10 and 55 °C [Citation49]. The variable growth temperature range seen here between the two bacterial strains might be due to evolutionary adaptation to the different habitats from which they were isolated. shows that strain CTBmeg1 was less affected by the change in the rotation speed. Rotation speeds between 150 to 225 rpm are important for sustaining the required amount of dissolved oxygen in the media.
Figure 4. Growth of B. megaterium CTBmeg1 at varying growth conditions. (a) 2,2-DCP concentrations; (b) pH values; (c) temperature degrees; (d) rotation speed.
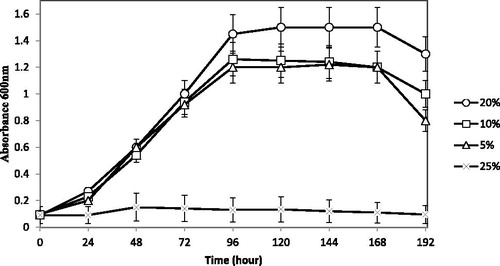
Using the above optimum growth conditions, we aimed the second part of the analysis towards obtaining an efficient bioremediation process by optimizing the NaCl concentration in the growth media. shows the growth curve of B. megaterium CTBmeg1 at different concentrations of NaCl. As seen in the figure, the bacteria showed tolerance towards increasing concentrations of NaCl, reaching as high as 20% (20 g/L or 3.5 mol/L) and exhibited a cell-doubling time of 26.41 h.
It is well known that strains of B. megaterium can tolerate high salt concentrations, for instance, B. megaterium DSM 32 T tolerates up to 25% (250 g/l or 4.5 M) [Citation49]. However, despite strain CTBmeg1 being able to grow in highly saline media, the bacterium species was not highly reliant on salt for growth; thus, in low concentration of salt (5%) the isolate still survived and grew. Halophiles usually necessitate the presence of NaCl for growth, unlike halotolerant organisms, which do not require NaCl but can grow under saline conditions. A possible explanation for this phenomenon is the heterogeneity of the hypersaline habitats, where the salinity can change markedly in space and time. Consequently, highly halophilic microorganisms are periodically eliminated and the euryhaline types are consistently favoured.
Despite the fact that the average salinity level of TuzGölü Lake consists of approximately 35% NaCl, this study found that bacterial growth did not occur at concentrations of NaCl higher than 25%. A probable reason for the reduced bacterial growth at 35% NaCl in this study, may be related to the bacteria growing in nutrient poor media that lacked any carbon source other than 2,2-DCP. However, when cultivated in nutrient rich media, notable bacterial growth was observed even at high concentrations of NaCl reaching up to 35%. The outcome seen here corroborates a report by Sorokin et al. [Citation50] that multiplication and survival of microorganisms in saline water is impacted by the availability of nutrients in the environment. Since strain CTBmeg1 can grow in a relatively broad salinity range, this indicated the prospective use of the bacterium as an agent for cleaning up environments of varying salinity, including seawater (3.5% NaCl) polluted by halogenated hydrocarbons.
Dehalogenase gene sequencing and analysis
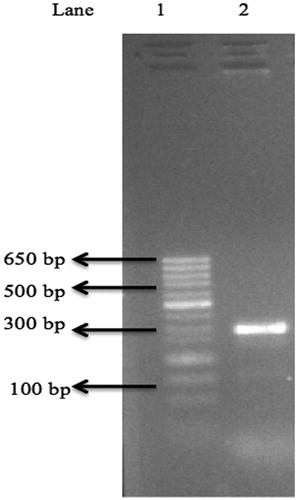
To further confirm the presence of the dehalogenase gene in B. megaterium CTBmeg1, amplification of the dehalogenase gene was carried out. depicts the produced PCR bands showing sizes similar to that reported by Hill et al. [Citation51]. Therefore, the partial sequence of putative dehalogenase gene (503 bp) was designated as dehCTBmeg1.
Conclusions
A new halogenated hydrocarbon-degrading bacterium CTBmeg1 identified as B. megaterium was isolated from the hypersaline environment of TuzGölü Lake, Turkey. The phenotypic and phylogenetic characteristics indicated that the B. megaterium CTBmeg1 showed promising capacity for degradation of halogenated compounds and was adapted to high salinity and pH conditions. Strain CTBmeg1 identified in this study is one of the few haloalkanoic-degrading bacteria belonging to the genus B. megaterium and is the first ever to be isolated from a hypersaline environment. Notably, the findings in this study conveyed the possibility of CTBmeg1 as a cost-effective bioremediation agent for cleaning up marine environments polluted by halogenated compounds.
Disclosure Statement
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Funding
The authors would like to thank Ondokuz Mayis University for the joint financial support of the project (PYO.ZRT.1904.17.059).
References
- Oren A, Gurevich P, Azachi M, et al. Microbial degradation of pollutants at high salt concentrations. Biodegradation. 1992;3:387–398.
- Le Borgne S, Paniagua D, Vazquez-Duhalt R. Biodegradation of organic pollutants by halophilic bacteria and archaea. J Mol Microbiol Biotechnol. 2008;15:74–92.
- Duarte CM, Holmer M, Olsen Y, et al. Will the oceans help feed humanity?. Bioscience. 2009;59:967–976.
- Besseling E, Wegner A, Foekema EM, et al. Effects of microplastic on fitness and PCB bioaccumulation by the lugworm Arenicola marina (L.). Environ Sci Technol. 2013;47:593–600.
- Lin M, Hu X, Chen W, et al. Biodegradation of phenanthrene by Pseudomonas sp. BZ-3, isolated from crude oil contaminated soil. Int Biodeterior Biodegrad. 2014;94:176–181.
- Anyasi RO, Atagana HI. Biological Remediation of polychlorinated biphenyls (PCB) in the environment by microorganisms and plants. Afr J Biotechnol. 2011;10:18916–18938.
- Wei X, Jiang Y, Chen X, et al. Amycolatopsis flava sp. nov., a halophilic actinomycete isolated from dead sea. Antonie Van Leeuwenhoek. 2015;108:879–885.
- Mehrshad M, Amoozegar MA, Makhdoumi A, et al. Halovarius luteus gen. nov., sp. nov., a novel extremely halophilic archaeon from Urmia salt lake, Iran. Int J Syst Evol Microbiol. 2015;65:2420–2425.
- Baati H, Amdouni R, Gharsallah N, et al. Isolation and characterization of moderately halophilic bacteria from Tunisian solar saltern. Curr Microbiol. 2010;60:157–161.
- Birbir M, Ogan A, Calli B, et al. Enzyme characteristics of extremely halophilic archaeal community in tuzkoy salt mine, Turkey. World J Microbiol Biotechnol. 2004;20:613–621.
- Sanchez-Porro C, Martin S, Mellado E, et al. Diversity of moderately halophilic bacteria producing extracellular hydrolytic enzymes. J Appl Microbiol. 2003;94:295–300.
- Tazi L, Breakwell DP, Harker AR, et al. Life in extreme environments: microbial diversity in Great Salt Lake, Utah. Extremophiles. 2014;18:525–535.
- Birbir M, Kalli N, Johannson C. Examination of salt quality of Sereflikochisar lake used in the Turkish leather industry. J Soc Leath Tech Chem. 2002;86:112–117.
- Birbir M, Sesal C. Extremely halophilic bacterial communities in Şereflikoçhisar salt lake in Turkey. Turk J Biol. 2003;27:7–22.
- Edbeib MF, Wahab RA, Huyop F. Halophiles: biology, adaptation, and their role in decontamination of hypersaline environments. World J Microbiol Biotechnol. 2016;32:1–23.
- Mongodin EF, Nelson KE, Daugherty S, et al. The genome of Salinibacter ruber: convergence and gene exchange among hyperhalophilic bacteria and archaea. Proc Natl Acad Sci USA. 2005;102:18147–18152.
- Eisenberg H. Life in unusual environments: progress in understanding the structure and function of enzymes from extreme halophilic bacteria. Arch Biochem Biophys. 1995;318:1–5.
- Karan R, Khare SK. Purification and characterization of a solvent-stable protease from Geomicrobium sp. Emb2. Environ Technol. 2010;31:1061–1072.
- Gomes J, Steiner W. The biocatalytic potential of extremophiles and extremozymes. Food Technol Biotechnol. 2004;42:223–235.
- Edbeib MF, Wahab RA, Kaya Y, et al. In silico characterization of a novel dehalogenase (DehHX) from the halophile Pseudomonas halophila HX isolated from Tuz Gölü lake, Turkey: insights into a hypersaline-adapted dehalogenase. Ann Microbiol. 2017;67:371–382.
- Häggblom MM, Knight VK, Kerkhof LJ. Anaerobic decomposition of halogenated aromatic compounds. Environ Pollut. 2000;107:199–207.
- Van Pée KH, Unversucht S. Biological dehalogenation and halogenation reactions. Chemosphere. 2003;52:299–312.
- Shahidul Islam M, Tanaka M. Impacts of pollution on coastal and marine ecosystems including coastal and marine fisheries and approach for management: a review and synthesis. Mar Pollut Bull. 2004;48:624–649.
- Birnbaum LS, Fenton SE. Cancer and developmental exposure to endocrine disruptors. Environ Health Perspect. 2003;111:389–394.
- Qing Li Q, Loganath A, Seng Chong Y, et al. Persistent organic pollutants and adverse health effects in humans. Part A. J Toxicol Environ Health. 2006;69:1987–2005.
- Hayes TB, Case P, Chui S, et al. Pesticide mixtures, endocrine disruption, and amphibian declines: are we underestimating the impact?. Environ Health Perspect. 2006;114:40–50.
- Chiba Y, Yoshida T, Ito N, et al. Isolation of a bacterium possessing a haloacid dehalogenase from a marine sediment core. Microbes Environ. 2009;24:276–279.
- Zhang J, Xin Y, Cao X, et al. Purification and characterization of 2-haloacid dehalogenase from marine bacterium Paracoccus sp. Deh99, isolated from marine sponge Hymeniacidon perlevis. J Ocean Univ China. 2014;13:91–96.
- Novak HR, Sayer C, Isupov MN, et al. Biochemical and structural characterisation of a haloalkane dehalogenase from a marine Rhodobacteraceae. FEBS Lett. 2014;588:1616–1622.
- Rye CA, Isupov MN, Lebedev AA, et al. Biochemical and structural studies of a l-haloacid dehalogenase from the thermophilic archaeon Sulfolobus tokodaii. Extremophiles. 2009;13:179–190.
- Novak HR, Sayer C, Panning J, et al. Characterisation of an l-haloacid dehalogenase from the marine psychrophile Psychromonas ingrahamii with potential industrial application. Mar Biotechnol. 2013;15:695–705.
- Drienovska I, Chovancova E, Koudelakova T, et al. Biochemical characterization of a novel haloalkane dehalogenase from a cold-adapted bacterium. Appl Environ Microbiol. 2012;78:4995–4998.
- Oren A. Industrial and environmental applications of halophilic microorganisms. Environ Technol. 2010;31:825–834.
- Yildiz M, Soğanci AS. Evaluation of geotechnical properties of the salt layers on the lake tuz. Sci Res Essays. 2010;5:2656–2663.
- Hareland WA, Crawford RL, Chapman PJ, et al. Metabolic function and properties of 4-hydroxyphenylacetic acid 1-hydroxylase from Pseudomonas acidovorans. J Bacteriol. 1975;121:272–285.
- Kloos WE, Tornabene TG, Schleifer KH. Isolation and characterization of micrococci from human skin, including two new species: Micrococcus lylae and Micrococcus kristinae. Int J Syst Bacteriol. 1974;24:79–101.
- Garrity G, Boone DR, Castenholz RW. Bergey's manual of systematic bacteriology: Volume one: the Archaea and the deeply branching and phototrophic bacteria. New York: Springer; 2012.
- Faller A, Schleifer KH. Modified oxidase and benzidine tests for separation of staphylococci from micrococci. J Clin Microbiol. 1981;13:1031–1035.
- Colwell RR, Grigorova R. Current methods for classification and identification of microorganisms. Methods Microbiol. 1987;19:405–458.
- Kumar S, Stecher G, Tamura K. Mega7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–1874.
- Feng N-X, Yu J, Mo C-H, et al. Biodegradation of di-n-butyl phthalate (DBP) by a novel endophytic Bacillus megaterium strain YJB3. Sci Total Environ. 2018;616:117–127.
- Zhang J, Liu J, Meng L, et al. Isolation and characterization of plant growth-promoting rhizobacteria from wheat roots by wheat germ agglutinin labeled with fluorescein isothiocyanate. J Microbiol. 2012;50:191–198.
- Liu M, Luo K, Wang Y, et al. Isolation, identification and characteristics of an endophytic quinclorac degrading bacterium Bacillus megaterium Q3. PLoS One. 2014;9:e108012.
- Li W, Xie X, Shi Q, et al. A study of the microbes and efficiency of the preservative system of a sauce. China Condiment. 2009;9:43–46.
- Edbeib MF, Wahab RA, Huyop F. Characterization of an α-haloalkanoic acid–degrading Pseudomonas aeruginosa MX1isolated from contaminated seawater. Bioremediat J. 2016;20:89–97.
- Oren A. Microbial life at high salt concentrations: phylogenetic and metabolic diversity. Saline Syst. 2008;4:2–14.
- Litchfield CD, Gillevet PM. Microbial diversity and complexity in hypersaline environments: a preliminary assessment. J Ind Microbiol Biotechnol. 2002;28:48–55.
- Oren A. Molecular ecology of extremely halophilic archaea and bacteria. FEMS Microbiol Ecol. 2002;39:1–7.
- Garabito M, Márquez M, Ventosa A. Halotolerant bacillus diversity in hypersaline environments. Can J Microbiol. 1998;44:95–102.
- Sorokin DY, Tourova TP, Galinski EA, et al. Extremely halophilic denitrifying bacteria from hypersaline inland lakes, Halovibrio denitrificans sp. nov. and Halospina denitrificans gen. nov., sp. nov., and evidence that the genus name Halovibrio fendrich 1989 with the type species Halovibrio variabi. Int J Syst Evol Microbiol. 2006;56:379–388.
- Hill KE, Marchesi JR, Weightman AJ. Investigation of two evolutionarily unrelated halocarboxylic acid dehalogenase gene families. J Bacteriol. 1999;181:2535–2547.