Abstract
This study prospectively evaluated the effect of 0.1% fluorometholone (FML) combined with meibomian gland expression (MGX) on dry eye disease (DED) and meibomian gland dysfunction (MGD).The study included 48 patients (96 eyes) with moderate to severe DED. The patients were treated with topical FML and MGX. The patients underwent Keratograph 5 M to evaluate quantitatively (THM, BUT, R-scan) and semi-quantitatively the meibomian gland loss (meiboscore). The methods included slit-lamp examination to evaluate meibum quality (meibum score), fluorescein staining and ocular surface disease index (OSDI) questionnaire. All patients (15 patients were also diagnosed with systemic immune disease) completed the study. The Schirmer I test results showed no statistical significance. After treatment, the fluorescein staining score, meibum score and R-Scan were decreased from the baseline values at week 2. The meiboscore was decreased at month 3 and the OSDI questionnaire scores were lower than the baseline at month 1. For subjects with systemic immune disease, the fluorescein staining score and meibum score were lower than the baseline. Tear BUT reached peak at month 1 and dropped to baseline at month 3. R-Scan and OSDI were decreased at months 1 and 3 compared to the baseline. The Schirmer I Test values and the meiboscore were not different from the baseline. The study demonstrated that 0.1% FML combined with MGX improves symptoms of moderate to severe DED.
Introduction
Based on data in the past ten years, the incidence of dry eye disease (DED) in China is the highest in the world, being 20.0–52.4% [Citation1–5]. In addition, the incidence of meibomian gland dysfunction (MGD) in the population over the age of 40 in Asian countries is as high as 38–68% [Citation6,Citation7]. DED is a multifactorial disease caused by inadequate tear production and/or fast evaporation of tears. DED is classified into two primary categories: lacrimal hyposecretion type and excessive tear evaporation type [Citation8]. Hyposecretion of tears is considered to be caused by anomaly of the aqueous layer, whereas excessive tear evaporation is thought to be caused by lipid layer dysfunction. In clinical practice, it is common that these two situations exist simultaneously. Therefore, the aim of treatment for DED is to improve both the aqueous layer and the lipid layer.
Numerous studies have shown that inflammation plays a pivotal role in the pathophysiology of DED [Citation9–12]. External drying factors cause the production of multiple inflammatory factors by ocular surface epithelial cells. Inflammation leads to tissue damage and neuroreceptor dysfunction, further exacerbates the reduction of tear secretion, and leads to a vicious cycle of DED. Therefore, blocking inflammatory pathways is the key to break this vicious cycle. Anti-inflammatory therapy (topical steroids and topical cyclosporine) have been shown to be effective in several clinical trials [Citation13–16]. As for patients with MGD, meibomian gland expression (MGX) is one of the most effective and economic methods [Citation17–19]. By now, combined use of anti-inflammatory therapy and MGX has not been reported for the treatment of DED. In the present study, we combine the two treatments to evaluate their value in short-, medium- and long-term treatment for DED. This study aims to provide useful information for clinical application.
Subjects and methods
Study subjects
Adult patients diagnosed with moderate to severe DED at Aier Eye Hospital (Taiyuan, Shanxi, China) between September 2017 and March 2018 were included in the present study. Topical artificial tears, lubricants or ointment had no effect on these included subjects, despite of long-term use. The exclusion criteria were as follows: ocular surface infection, pterygium, conjunctivochalasis, or history of ocular surgery within 3 months.
Forty-eight patients (96 eyes) were finally enrolled and all of them finished the study. The relevant clinical information about the patients is detailed in . The 48 subjects included 36 women (75%) and 12 men (25%), with a mean age of 60.2 ± 9.1 years. Among the 48 patients, 15 were diagnosed with systemic immune disease (8 RA, 6 SS and 1 Sarcoidosis) and treated with oral prednisone. Among all 96 eyes, 10 eyes had concurrent filamentous keratitis, 4 eyes had concurrent Superior Limbic Keratoconjunctivitis (SLK), and 30 eyes had concurrent refractive error. All 48 subjects had a medication history of artificial tears, which failed to control the symptoms of ocular discomfort. The artificial tears were 0.1% sodium hyaluronate (Hycosan, Eusan GmbH, Germany), polyethylene glycol (Alcon, Fort Worth, TX, USA), Carbomer Eye Gel (Bausch-Lomb, Berlin, Germany) or other unidentified eye drops.
Table 1. Clinical data of dry eye patients.
Ethical approval and consent to participate
All procedures performed in this study were approved by the Ethics Committee of Capital Medical University. The entire study adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all patients or their families before any study examinations were performed.
Consent for publication
Written informed consent forms for publication of any associated data and accompanying images were obtained from all patients or their parents, guardians or next of kin.
Diagnosis
The diagnosis of DED was according to the consensus of DED by the Asia Dry Eye Society (2017) [Citation20]. DED was diagnosed if two out of the following three symptoms were present: tear film breakup time ≤5 s, Schirmer tear test value ≤5 mm/5 min, and corneal surface damage (fluorescein staining score ≥3, and maximum score = 9). MGD was diagnosed if two out of the following symptoms were present: eyelid margin irregularity, telangiectasias, obstruction of meibomian gland opening and eyelid mucocutaneous displacement.
Treatment
All patients were topically given 0.1% fluorometholone (FLM) (Santen Pharmaceutical Co., Ltd., Osaka, Japan) four times a day for 2 weeks. Before therapeutic MGX, patients were treated with eyelid-warming patch for 30 min and received topical anaesthesia. MGX was operated by experienced nurses, who used a stainless-steel paddle (Asicon Medical Instrument Co., Ltd., Jiangsu, China) to consecutively squeeze the superior and interior eyelids. This treatment was conducted once a week in 2 weeks. All patients were instructed to continue warm compresses with eyelid massage every night by themselves. All 48 patients received intraocular pressure (IOP) examination. After the use of 0.1% FML for 2 weeks, 2 out of 48 patients had elevated IOP above 21 mmHg (23.5 and 25.0 mmHg, respectively). We did not cancel steroid eye drops or prescribe anti-glaucoma drugs, and kept detecting daily IOP until the IOP was below 21 mmHg, unless IOP elevation could affect the optic nerve fiber or cause possible glaucoma.
Ocular surface disease index (OSDI)
All patients were asked to complete the OSDI© survey before examination. This questionnaire gives a range from 0 (no symptoms) to 100 (severe symptoms).
Corneal fluorescein staining
Corneal staining was evaluated under a slit-lamp 2 min after instillation of Fluorescein Sodium Opthalmic Strips (Jingming New Technological Development Corp., Tianjin, China) into the lower eyelid conjunctival sac. When no staining was observed, the score was 0; when scattered dots of stains were observed, the score was 1; when moderate staining was observed, the score was 2; when severe staining was observed, the score was 3 [Citation21]. The corneal staining scores were divided into three groups: F1 group, score ≤3 points; F2 group, 3 < score ≤ 6; F3 group: 6 < score ≤ 9.
Tear function assessment by Schirmer I test without anaesthesia
SIT was used to assess the tear secretion capacity [Citation22]. A standard 5 × 40 mm Schirmer test strip (Jingming New Technological Development Corp., Tianjin, China) was placed in the conjunctival sac situated at 2/3 of the outer edge of the lower eyelid. The length of wetting of the test strip was measured after 5 min with eyes closed. According to the amount of tear secretion, the Schirmer I test scores were divided into three groups: S1 group, Schirmer I test ≤ 3 mm; S2 group, 3 mm < Schirmer I test ≤ 5 mm; S3 group, 5 mm < Schirmer I test ≤ 10 mm.
Tear film assessment by Keratograph 5M
All subjects underwent examination by Keratograph 5M (Oculaus GmbH, Wetzlar, Germany). Tear meniscus height (TMH), non-invasive break-up time (NIBUT), R-scan and meibomian gland image were recorded. The degree of meibomian gland loss (meiboscore) was evaluated as follows: Grade 0, no loss of meibomian gland; Grade 1, loss of meibomian gland was less than 1/3 of the whole gland area; Grade 2, loss was between 1/3 and 2/3 of the whole gland area; Grade 3, loss was greater than 2/3 of the whole gland area. The total maximal meiboscore was 6 for both upper and lower eyelids.
Evaluation of meibomian gland secretion and blockage by slit-lamp
Moderate pressure was applied to the edge of the middle upper eyelid using a thumb. Meibum flew out from the meibomian gland. Meibum was scored as follows: Grade 0, clear fluid; Grade 1, cloudy fluid; Grade 2, particulate fluid; Grade 3, toothpaste-like fluid. The scores were given by two individual ophthalmologists and averaged.
Statistical analyses
Descriptive statistics (means, standard deviations, and percentages) were computed for the demographic and clinical variables, VA and VF defects. The test was performed to compare variances, and one-way analysis of variance (ANOVA) Kruskal–Wallis test was used for group comparison.
Results and discussion
Outcome of patients without systemic immune diseases (SID)
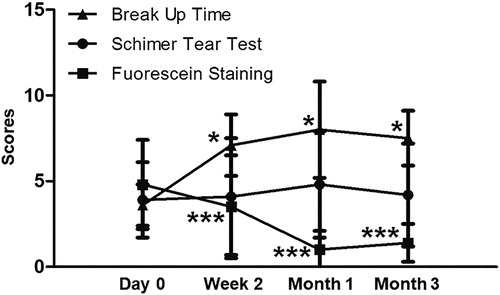
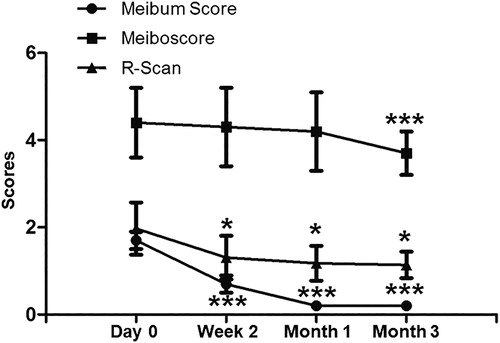
To evaluate the effects of the combination treatment, ocular parameters were measured. The Schirmer I test scores of each group at 2 weeks, 1 month and 3 months after treatment were not significantly different from those before treatment. Compared with the baseline level, the fluorescein staining scores of F1 and F2 groups were significantly decreased at week 2 after treatment, whereas that of F3 group was significantly decreased one month after treatment. TMH began to rise one month after treatment (P = 0.023), but dropped back to the baseline level 3 months after treatment. Tear BUT began to increase significantly two weeks after treatment, reached a peak at one month, and dropped to the baseline level at the third month. OSDI began to decrease significantly two weeks after treatment, and reached significantly different levels compared with the baseline one and the one three months after treatment (). Meibum score and R-Scan began to decrease two weeks after treatment compared with baseline, reached the lowest level one month after treatment, and maintained stable three months after treatment. Meanwhile, the meiboscore three months after treatment was significantly decreased compared with the baseline value (). The results suggest that combined treatment with 0.1% FML and MGX alters the ocular parameters in patients without SID.
Outcome of patients with SID
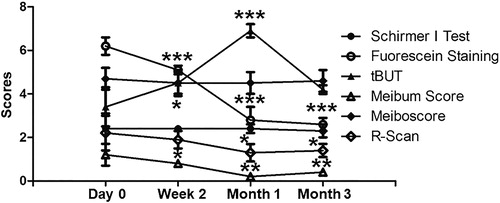
Compared with the baseline level, no difference was found in Schirmer I test and meiboscore at week 2, month 1 and month 3 after treatment. The fluorescein staining score and the meibum score were significantly decreased at all time points compared with the baseline level. Tear BUT was elevated at week 2 (P = 0.0418), reached a peak at month 1 (P = 0.0005), and finally dropped back to the baseline level at month 3 (P = 0.0799). R-Scan and OSDI were significantly decreased at months 1 and 3 compared with baseline (). The results indicate that combined treatment with 0.1% FML and MGX changes the ocular parameters in patients with SID.
Combination treatment with 0.1% FML and MGX gives better improvement in patients without SID than in patients with SID
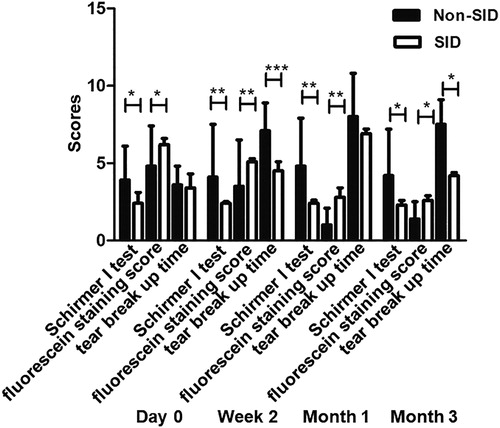
To test whether SID affects the effect of the combination treatment, we compared the ocular parameters in patients with or without SID. The Schirmer I test score in patients without SID was higher than that in patients with SID at week 2 (P = 0.0048), month 1 (P = 0.0080) and month 3 (P = 0.02). The fluorescein staining score in patients without SID was lower than that in patients with SID at baseline (P = 0.024), week 2 (P = 0.0041), month 1 (P = 0.0055) and month 3 (P = 0.0140). The tear BUT in patients without SID was higher than that in patients with SID at week 2 (P = 0.0011) and month 3 (P = 0.0204) (). The results suggest that the combination treatment with 0.1% FML and MGX has more improvement in patients without SID than in patients with SID.
Figure 4. Comparison of Schirmer I test results, fluorescein staining scores and tear break up time (tBUT) between SID patients and non-SID patients. *P < 0.05; **P < 0.01; ***P < 0.001.
This present clinical retrospective study evaluated the efficacy of topical 0.1% FML combined with MGX in preventing the exacerbation of moderate or severe DED signs. In this study, we gave patients appropriate treatment according to TFOS DEWS II recommendation [Citation23,Citation24], and completed short-term, medium-term and long-term follow-up. We found that patients treated with 0.1% FML and MGX achieved better status of ocular surface. Considering the efficacy of topical corticosteroids as a short-term DED treatment [Citation25,Citation26], combined MGX therapy with our treatment strategy, which is more suitable for the characteristics of Chinese DED patients and long-term maintenance. Our study showed that the percentage of plugged meibomian gland occlusion and meibum score were significantly decreased after 2 weeks of treatment. In addition, the meiboscore at three months after treatment was significantly lower than baseline. The OSDI questionnaire score was also lower than baseline at month 1. These results suggest that FLM and MGX improve MGD ocular surface changes and symptoms. Our data showed that this combination therapy is more effective than single drug [Citation27,Citation28] and other treatments [Citation19]. These findings confirm our concept to treat DED at an early stage to break the vicious cycle of immune response. Of note, long-term ocular surface maintenance mainly depends on meibomian gland function.
The Schirmer I test results were divided into 3 grades according to severity, in order to assess the effect accurately, and this result showed no change in each group from the baseline. The fluorescein staining score was significantly lower than baseline at week 2. However, the fluorescein staining score in the severe group (fluorescein staining score at 6–9) was lower than baseline at month 1. These data are in agreement with a previous report on Schirmer I test and fluorescein score [Citation24]. Moreover, tear BUT is significantly increased than baseline at week 2. Increased tear BUT in the present study is the main result of improved meibomian gland function.
We have also analyzed data of 15 patients (6 SS) with SID. The results are in concordance with previous studies [Citation29,Citation30]. Although the patients with DED had improvements in their ocular surface signs (fluorescein staining score and R-Scan), meibomian gland function (meibum score and tear BUT) and symptoms (OSDI questionnaire score), these improvements were still less than those in patients without SID. In patients without SID, tear BUT was decreased (P = 0.0078) and meibum score was increased (P = 0.4237) compared to those in patients with SID at month 3. This suggests that the meibomian gland function cannot be maintained without an anti-inflammatory drug. In addition, patients with SID had a higher meiboscore than those without SID (P = 0.0244), suggesting that meibomian gland dysfunction is more severe in patients with SID than in those without SID, being consistent with previous publications [Citation31–33]. However, further studies are still needed to elucidate how meibomian gland cells are affected by inflammatory cells and to develop an effective method to protect the meibomian gland function.
There are also some limitations in the study. First, it was an uncontrolled study. Second, the number of SS patients was small, so patients with SID were enrolled, and the actual data in SS patients may have possibly been underestimated. Future studies are needed to compare the value of the combination therapy with other treatments in a randomized, placebo-controlled way.
Conclusions
The present study demonstrated that combined use of 0.1% FML and MGX can improve the symptoms of moderate and severe DED, even in patients with SID. Moreover, MGX could improve meibomian gland function for at least 3 months, but not in patients with SID. This is likely a reason why poor clinical results and unsatisfaction are observed in SID patients.
Author contributions
The final version of the manuscript has been read and approved by all authors, and each author believes that the manuscript represents honest work. AL designed the study. YF and YY were responsible for ophthalmology experiments. WG analyzed the data. All authors collaborated to interpret the results and develop the manuscript.
Consent for publication
Written informed consents for publication of any associated data and accompanying images were obtained from all patients or their parents, guardians or next of kin.
Ethical approval and consent to participate
All procedures performed in this study were approved by the Ethics Committee of Capital Medical University. Written informed consent was obtained from all patients or their families.
Acknowledgments
We would like to thank Dr. Hui Zhao from Shanxi Medical University for her kind assistance in data collection and analysis.
Availability of data and materials
The datasets used and/or analyzed in the present study are available from the corresponding author upon reasonable request.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Lu P, Chen X, Liu X, et al. Dry eye syndrome in elderly Tibetans at high altitude: a population-based study in China. Cornea. 2008;27:545–551.
- Jie Y, Xu L, Wu YY, et al. Prevalence of dry eye among adult Chinese in the Beijing Eye Study. Eye. 2009;23:688–693.
- Tian YJ, Liu Y, Zou HD, et al. [Epidemiologic study of dry eye in populations equal or over 20 years old in Jiangning District of Shanghai]. [Zhonghua yan ke za zhi] Chin J Ophthalmol. 2009;45:486–491.
- Guo B, Lu P, Chen X, et al. Prevalence of dry eye disease in Mongolians at high altitude in China: the Henan eye study. Ophthalmic Epidemiol. 2010;17:234–241.
- Han SB, Hyon JY, Woo SJ, et al. Prevalence of dry eye disease in an elderly Korean population. Arch Ophthalmol. 2011;129:633–638.
- Uchino M, Dogru M, Yagi Y, et al. The features of dry eye disease in a Japanese elderly population. Optom Vis Sci. 2006;83:797–802.
- Siak JJ, Tong L, Wong WL, et al. Prevalence and risk factors of meibomian gland dysfunction: the Singapore Malay eye study. Cornea. 2012;31:1223–1228.
- Lemp MA. Report of the National Eye Institute/Industry workshop on clinical trials in dry eyes. CLAO J. 1995;21:221–232.
- Kunert KS, Tisdale AS, Stern ME, et al. Analysis of topical cyclosporine treatment of patients with dry eye syndrome: effect on conjunctival lymphocytes. Arch Ophthalmol. 2000;118:1489–1496.
- Siebelmann S, Gehlsen U, Huttmann G, et al. Development, alteration and real time dynamics of conjunctiva-associated lymphoid tissue. PLoS One. 2013;8:e82355.
- Niederkorn JY, Stern ME, Pflugfelder SC, et al. Desiccating stress induces T cell-mediated Sjogren's syndrome-like lacrimal keratoconjunctivitis. J Immunol. 2006;176:3950–3957.
- Stern ME, Gao J, Schwalb TA, et al. Conjunctival T-cell subpopulations in Sjogren's and non-Sjogren's patients with dry eye. Investig Ophthalmol Vis Sci. 2002;43:2609–2614.
- Avunduk AM, Avunduk MC, Varnell ED, et al. The comparison of efficacies of topical corticosteroids and nonsteroidal anti-inflammatory drops on dry eye patients: a clinical and immunocytochemical study. Am J Ophthalmol. 2003;136:593–602.
- Lee HK, Ryu IH, Seo KY, et al. Topical 0.1% prednisolone lowers nerve growth factor expression in keratoconjunctivitis sicca patients. Ophthalmology. 2006;113:198–205.
- Aragona P, Spinella R, Rania L, et al. Safety and efficacy of 0.1% clobetasone butyrate eyedrops in the treatment of dry eye in Sjogren syndrome. Eur J Ophthalmol. 2013;23:368–376.
- Pinto-Fraga J, Enriquez-de-Salamanca A, Calonge M, et al. Severity, therapeutic, and activity tear biomarkers in dry eye disease: an analysis from a phase III clinical trial. Ocul Surf. 2018;16:368–376.
- Finis D, Hayajneh J, Konig C, et al. Evaluation of an automated thermodynamic treatment (LipiFlow®) system for meibomian gland dysfunction: a prospective, randomized, observer-masked trial. Ocul Surf. 2014;12:146–154.
- Thode AR, Latkany RA. Current and emerging therapeutic strategies for the treatment of meibomian gland dysfunction (MGD). Drugs. 2015;75:1177–1185.
- Albietz JM, Schmid KL. Intense pulsed light treatment and meibomian gland expression for moderate to advanced meibomian gland dysfunction. Clin Exp Optom. 2018;101:23–33.
- Tsubota K, Yokoi N, Shimazaki J, et al. New perspectives on dry eye definition and diagnosis: a consensus report by the Asia Dry Eye Society. Ocul Surf. 2017;15:65–76.
- Yokoi N, Uchino M, Uchino Y, et al. Importance of tear film instability in dry eye disease in office workers using visual display terminals: the Osaka study. Am J Ophthalmol. 2015;159:748–754.
- Cho P, Yap M. Schirmer test. I. A review. Optom Vis Sci. 1993;70:152–156.
- Wolffsohn JS, Arita R, Chalmers R, et al. TFOS DEWS II diagnostic methodology report. Ocul Surf. 2017;15:539–574.
- Jones L, Downie LE, Korb D, et al. TFOS DEWS II management and therapy report. Ocul Surf. 2017;15:575–628.
- Hong S, Kim T, Chung SH, et al. Recurrence after topical nonpreserved methylprednisolone therapy for keratoconjunctivitis sicca in Sjogren's syndrome. J Ocul Pharmacol Ther. 2007;23:78–82.
- Nattinen J, Jylha A, Aapola U, et al. Topical fluorometholone treatment and desiccating stress change inflammatory protein expression in tears. Ocul Surf. 2018;16:84–92.
- Pinto-Fraga J, Lopez-Miguel A, Gonzalez GM, et al. Topical fluorometholone protects the ocular surface of dry eye patients from desiccating stress: a randomized controlled clinical trial. Ophthalmology. 2016;123:141–153.
- Amano S, Inoue K. Effect of topical 3% diquafosol sodium on eyes with dry eye disease and meibomian gland dysfunction. Clin Ophthalmol. 2017;11:1677–1682.
- Lim SA, Nam S, Kwok SK, et al. Serologic markers are associated with ocular staining score in primary Sjogren syndrome. Cornea. 2015;34:1466–1470.
- Li J, Zhang X, Zheng Q, et al. Comparative evaluation of silicone hydrogel contact lenses and autologous serum for management of Sjogren syndrome-associated dry eye. Cornea. 2015;34:1072–1078.
- Zang S, Cui Y, Cui Y, et al. Meibomian gland dropout in Sjogren's syndrome and non-Sjogren's dry eye patients. Eye. 2018;32:1681–1687.
- Kang YS, Lee HS, Li Y, et al. Manifestation of meibomian gland dysfunction in patients with Sjogren's syndrome, non-Sjogren's dry eye, and non-dry eye controls. Int Ophthalmol. 2018;38:1161–1167.
- Menzies KL, Srinivasan S, Prokopich CL, et al. Infrared imaging of meibomian glands and evaluation of the lipid layer in Sjogren's syndrome patients and nondry eye controls. Investig Ophthalmol Vis Sci. 2015;56:836–841.