?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Ebinur Lake wetland is the most representative temperate arid zone wetland ecosystem in China and it is the centre of the oasis and desertification of the Northern slope of Tianshan conjugate. Soil samples were collected from three sites (Tamarix ramosissima, Halocnemum strobilaceum, and Phragmites australis) and different soil layers (0–5, 5–15,15–25, and 25–35 cm) within this wetland during spring, summer and autumn, and the diversity of cultured/uncultured Azotobacter based on the 16S rDNA and nifH genes was characterized. Polymerase chain reaction denaturing gradient gel electrophoresis (PCR-DGGE) and bivariate correlation analysis were used to evaluate the relationship between the diversity of uncultured Azotobacter and soil environmental factors. A total of 17 cultured Azotobacter strains were isolated, five of which had stronger nitrogen-fixation ability (more than 10 mg/mL). Meanwhile, most of the cultured Azotobacter strain sequences were grouped into Bacillus sphaericus and Bacillus subtilis, and fewer sequences were affiliated with Actinomycetes. Furthermore, PCR-DGGE indicated that the diversity of uncultured Azotobacter was not balanced in some samples and the Shannon diversity index varied from 0.685 to 2.626. The uncultured Azotobacter were grouped into autotrophic genera Azotobacter and Rhizobium. Finally, bivariate correlation analysis showed that the diversity of uncultured Azotobacter was significantly correlated with soil organic carbon, inorganic carbon, NH4+-N, NO3−-N and microbial biomass N in the Ebinur Lake wetland in Xinjiang.
Introduction
Nitrogen (N) is known to be an essential factor for biological survival and, while it is adequate in the environment, animals and plants cannot directly use molecular nitrogen [Citation1]. The process of biological nitrogen fixation overcomes this limitation, whereby some microorganisms can reduce nitrogen from the air to ammonia by nitrogenase and use it directly [Citation2,Citation3]. About 175 million tonnes of nitrogen fixation occurs annually in the world, which represents 437% of the world's industrial nitrogen fertilizer. Among them, nitrogen fixation of soil microorganisms is one of the main sources of this element in the soil [Citation4]. In recent years, the use of industrial nitrogen fertilizers has caused some serious pressure to the economy and environment; however, biological nitrogen fixation plays a positive role in the natural nitrogen cycle, which is efficient and causes no environmental damage [Citation5]. Therefore, the nitrogen fixation ecosystem and nitrogen-fixing bacteria resources are gradually becoming a popular topic globally [Citation6].
In general, nitrogen fixing-bacteria can be divided into three categories: biogenic nitrogen-fixing bacteria, symbiotic nitrogen fixation bacteria and combined nitrogen fixation bacteria [Citation7]. The main genera of biogenic nitrogen-fixing bacteria include the genera Azotobacter, Klebsiella, Clostridium, Nostoc, and Rhodospirillum. Symbiotic nitrogen-fixing bacteria include Rhizobium, Frankia, and Anabaena, and combined nitrogen-fixing bacteria include Azospirillum and Beijerinckia genera [Citation8]. Molecular approaches allow the collection of reliable information on the diversity and community composition of nitrogen-fixing bacteria in various environments and currently, 16S rDNA and nifH genes are universally used in biodiversity research of nitrogen-fixing bacteria [Citation8,Citation9].
The Ebinur Lake Wetland National Nature Reserve is the most representative of temperate arid zone wetland ecosystems in China, and it is the centre of oasis and desertification of the northern slope of Tianshan conjugate [Citation10]. The wetland is effective in regulating climate and maintaining regional ecological balance [Citation11]. Because of its special geographic location, there are all kinds of species inhabiting this wetland. However, research on the diversity of species in the Ebinur Lake wetland is still in the preliminary stage. Since nitrogen-fixing bacteria plays a critical role in the natural nitrogen cycle, it is necessary to study the diversity of the nitrogen-fixing bacteria and their relationship to the soil factors in this wetland. In this study, cultured Azotobacter were isolated and sequencing based on 16S rDNA, and uncultured Azotobacter were analyzed based on the nifH gene. Bivariate correlation analysis was used to analyze the relationship between the diversity of uncultured Azotobacter and soil environmental factors, which will lay the foundation for restoration and reconstruction of the wetland ecological environment.
Materials and methods
Ethics statements
In this study, the samples were collected with the permission of the Gao Xiang, Director of the Ebinur Lake Wetland National Nature Reserve Administration of Xinjiang and Xu Wei, Chief of the Ebinur Lake Wetland Bird Island Station in Xinjiang.
Site description, sample collection and physicochemical characteristics analysis
Ebinur Lake Wetland National Nature Reserve is located in the northwestern part of Jinghe county in Xingjiang (82°36'–83°50'E, 44°30'–45°09'N), in the southwest of the Junggar Basin, which is the centre of the lowest depression and salt-water in this area [Citation10]. In this study, Tamarix ramosissima (S1), Halocnemum strobilaceum (S2) and Phragmites australis (S3) were sampled, and five sample plots were selected in each site at different soil layers (0–5, 5–15, 15–25, and 25–35 cm) in October 2011, April 2012, and July 2012. The basic information of the samples from the Ebinur Lake wetlands is shown in . The soil samples were placed into sterile plastic bags and transported to the laboratory on ice. All samples were then immediately kept at −20 °C until use for molecular analysis. Following Lu [Citation12], organic carbon (OC), inorganic carbon (IC), total nitrogen (TN), ammonium (NH4+-N), and nitric nitrogen (NO3−-N) content, as well as microbial biomass C and microbial biomass N were assayed.
Table 1. Basic description of sampling sites in the Ebinur Lake wetland.
Isolation, purification, identification and sequencing of cultured Azotobacter from soil in Ebinur Lake wetland
Collected soil samples were air-dried for 4 h and isolation was performed by serial dilution technique [Citation13]. For preparation of soil suspensions, 5 g soil was added to 45 mL sterile water and mixed well, then 1 mL of soil suspension was transferred using a sterilized pipette to a Petri dish containing selective media. Isolation of cultured Azotobacter was performed in duplicates at the appropriate concentration (10−2) in Ashby's nitrogen-free Agar medium. After incubation at 30 °C for four days, the appearance of colonies was recorded, and individual colonies were selected and maintained as pure cultures for further study. Isolated colonies were sub-cultured and identified by using morphological and biochemical tests. Various tests were performed for Azotobacter, including Gram stain, nitrogenase activity and catalase activity [Citation14].
The 16S rDNA of cultured Azotobacter was analyzed by polymerase chain reaction (PCR). Primers (EuB-338-F: 5′-ACTCCTACGGGAGGCAGC-3′ and RM2-R: 5′-GATCTCTACGCATTTCACCGCTAC-3′) were used for amplification [Citation15]. Each cultured Azotobacter strain isolated from soil was used to prepare a bacterial suspension as a DNA template. PCR reactions were performed in a final volume of 25 μL, containing 1 μL template DNA, 1 μL primer, 12 μL EasyTaq PCR SuperMix and 11 μL ddH2O. Amplifications were done in an Eppendorf 22331 Hamburg PCR machine with a program consisting of initial denaturation of 96 °C for 20 s, followed by 25 cycles each consisting of denaturation at 94 °C for 45 s, a primer extension at 72 °C for 60 s, and a final extension of 72 °C for 10 min. These reactions were repeated to check the reproducibility of the amplification. The banding pattern was visualized on an ultraviolet transilluminator and sequenced by Beijing Liuhe Genomics Technology Co., Ltd. (Beijing, China) for phylogenetic analysis.
The analysis of uncultured Azotobacter from soil in Ebinur Lake wetland
DNA extraction, PCR, DGGE and sequencing
To improve the extraction efficiency, a pre-lysis buffer (TENP buffer 15 mL) washing step was introduced before the extraction procedure [Citation16]. The protocol of Zhang [Citation17] was used for total genomic DNA extraction from 5 g freeze-dried soil. DNA extracts were kept at −20 °C until use. The extracted DNA was checked by 1% agarose gel electrophoresis.
The composition of uncultured Azotobacter communities was assessed by PCR-denaturing gradient gel electrophoresis (PCR-DGGE). The nifH gene was amplified from genomic DNA using a nested PCR approach. The first-round PCR was carried out with specific nifH PCR primers (FGPH19 and PoR) [Citation15]. These first-stage PCR products were then used as templates in the second-round PCR, which was conducted using primers (PoIF: TGCGAYCCSAARGCBGACTC and AQER: GACGATGTAGATYTCCTG-GC). DGGE was performed at 60 °C in 1× TAE (Tris base, acetic acid and ethylene diamine tetraacetic acid) at 200 V for 5 h on a DCode gel system (BioRad, Hercules, CA, USA) using an 8% polyacrylamide gel with 30%–70% (M/V) gradient of urea formamide denaturant. Gels were stained with SRBR Green for 20 min as described previously [Citation18] and visualized in an ultraviolet gel imaging system. Specific or common bands were excised from the gel, re-amplified under the conditions described in the first-round PCR and sequenced by Beijing Liuhe Genomics Technology Co., Ltd.
Phylogenetic analysis
The DNA sequences, including from cultured and uncultured Azotobacter, were compared to sequences available in the GenBank database using BLAST software (http://www.ncbi.nlm.nih/gov/blast/). The top-hit 16S rDNA gene and nifH gene sequences from GenBank were retrieved, and all sequences were aligned using the Clustal X program [Citation19]. The phylogenetic tree was constructed using the neighbour-joining criterion [Citation20] of the MEGA 4 software [Citation21] with 1000 bootstrap tests.
Statistical analysis
In Quantity One software (Bio-Rad), DGGE patterns were used for analyzing the Azotobacter band number, mobility and intensity through a digitally quantitative approach. Similarity matrices for this clustering analysis were generated from pairwise comparison of banding patterns (presence/absence of bands) of all samples, using the Dice Coefficient (CD) as follows: CD =2j/(a + b), where j is the number of common bands between lanes A and B, a is the total number of bands in lane A and b is the total number of bands in lane B. Samples generating similar banding patterns were clustered by means of the unweighted pair group method with arithmetic averages (UPGMA), resulting in the construction of dendrograms. The diversity index was used, the Shannon-Wiener, richness, and evenness, to estimate the diversity of Azotobacter strains. The diversity index (Shannon-Wiener, H) and (evenness, E) were calculated as follows:
(1)
(1)
(2)
(2)
where S is the total number of bands in DGGE patterns and Pi is the band of dominance. Bivariate correlation analysis was used to assess the relationship between diversity of the Azotobacter community structure from DGGE patterns with environmental factors (OC, IC, TN, NH4+-N, NO3−-N), microbial biomass C and microbial biomass N by SPSS16.0 software and Excel 2007.
Nucleotide sequence accession numbers
The obtained 16S rRNA gene sequences were deposited to the Gen-Bank database under accession numbers MH656758-MH656771. The accession numbers of the nifH gene were MH550093-MH550104.
Results and discussion
Isolation of cultured Azotobacter from soil in Ebinur Lake wetland
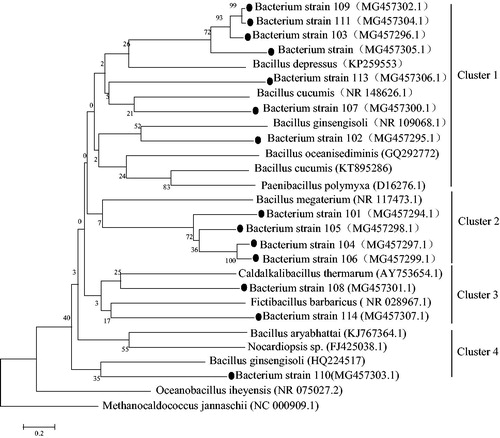
Isolated, cultured Azotobacter were sub-cultured in Ashby's nitrogen-free agar medium, and morphological and biochemical identification tests are tabulated shown in . A total of 17 strains were isolated from the soil and cultured, and the majority of strains were Gram-positive and had positive catalase activity (). The 16S rDNA sequence the similarity between strains No. 1 and No. 7 was 99.7%, and the similarity of strains No. 3, 13, and 15 reached 99.1%. Strains with similarity of over 99% could be considered as being from the same strain genus. Based on this, strains No. 1 and 7, as well as strains No. 3, 13, 15 were independently combined and considered as the same bacteria. Subsequently, 14 sequences were used to build the phylogenetic tree of the Azotobacter 16S rDNA sequences using the neighbour-joining method in the MEGA 6 software.
Table 2. Morphological characteristics of cultured Azotobacter and the result of Gram stain and enzyme activities.
Phylogenetic analyses of cultured Azotobacter in Ebinur Lake wetland
The phylogenetic tree showed that all cultured Azotobacter sequences were grouped into four clusters (); among these, Cluster 1 represents a large proportion, including Paenibacillus polymyxa, Bacillus cucumis, Bacillus depressus, Bacillus ginsengisoli, and Bacillus oceanisediminis. The sub strains of Bacillus (Paenibacillus) spp. are mainly in Cluster 1, while those of Bacillus megasporus in the Cluster 2 (NR 117473.1). There were only four sequences in Cluster 3, among which included a new genus of Bacillus found in recent years (such as AY753654.1), suggesting that there may be other nitrogen-fixing bacteria of this genus in the Ebinur Lake wetland. The Nocardiopsis genus (such as FJ425038.1) was found in Cluster 4, supporting that there may be undeclared nitrogen-fixing populations in the Ebinur Lake wetlands. Overall, the analysis of the four clusters showed that the cultured Azotobacter isolates were mainly composed of Bacillus spp. and a small proportion of Actinomycetes.
The community structure of uncultured Azotobacter based on nifH gene in different soil layers and seasons in the Ebinur Lake wetland
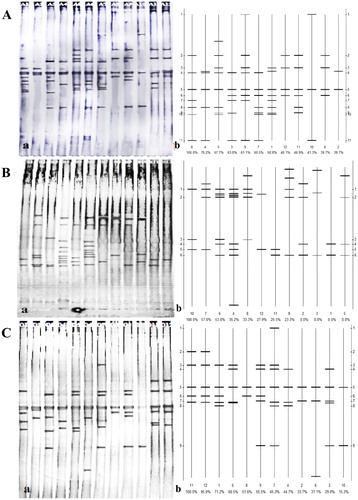
From the DGGE profiling of uncultured Azotobacter (), 36 PCR products derived from different soil layers and different seasons in the three sites, were separated. There was diversity in uncultured Azotobacter during spring (), with some differences in number, mobility and intensity of bands in each lane, although band 5 (b)) was observed in all lanes. The maximum number of bands was found in lanes A1 and A5, and the least number found in lane A2 (b)). Band 1 (b)) was observed only in A9 and A10 lanes (a)), suggesting that these Azotobacter were sensitive to environmental change. The diversity of uncultured Azotobacter in summer is shown in ; some diversity in the community structure of uncultured Azotobacter emerged in summer, with no bands common among any lane (b)). Band 3 was observed only in lanes S6, S8, and S10 (a)), and maximum diversity observed in lane S4, indicative of the differences in these sample soil sites in summer. From the diversity of uncultured Azotobacter in autumn shown in , there are some differences in the number, mobility and intensity of every band in each lane, but band 5 (b)) was observed in all lanes (samples) and band 7 was in almost all lanes. In addition, lanes O2, O8, and O10 had the minimum number of bands (a)).
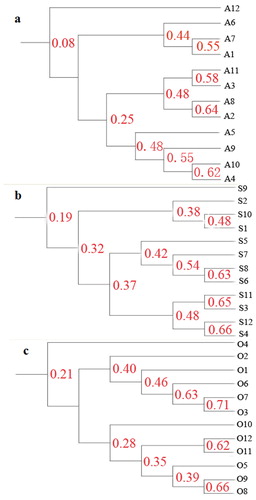
Clustering analysis across sites indicated that banding patterns were distributed into two main clusters (). The Dice similarity coefficient showed that the similarity of all spring soil samples was between 7.4% and 63.6%. In spring soil samples, there were two main clusters, one was comprised of A12 and the other was composed of the lanes A1–A11 (; among them, the similarity of A2 and A8 reached 63.6% similarity. This supports that the community structure of uncultured Azotobacter in different soil layers and different sites had diversity in the Ebinur Lake wetland during spring. For summer soil samples, the Dice similarity coefficient showed that similarity of all samples was between 12.8% and 65.8%; S3, S4, S5, S6, S7, S8, S10, and S12 clearly grouped together (. For autumn soil samples, the Dice similarity coefficient showed 11%–71.3% similarity between all the samples. Clustering analysis of uncultured Azotobacter in autumn indicated that there were three main clusters (. This suggests that there are large differences in the community structure of uncultured Azotobacter in different soil layers and different sites during autumn.
The index of Shannon-Wiener represents the richness and stability of the community structure of soils in the entire ecosystem. For researching diversity in this study, the Shannon-Wiener index and evenness of the species was used to estimate the diversity of samples. shows the Shannon-Wiener richness and evenness at different layers, sites and seasons. The Shannon Wiener index was between 0.685 and 2.358 in spring, with a peak in soil layer 25–35 cm from the T. ramosissima community area (index of richness from 1 to 11); there were also obvious changes in the dynamic of the Shannon-Wiener evenness, which ranged from 0.3759 to 0.9998. In summer soil samples, there were no obvious changes in the dynamic of the Shannon Wiener evenness, which ranged from 1.911 to 2.626 (). In addition, the highest richness point (15) appeared in the 25–35 cm soil layer of the T. ramosissima community area, and there were no obvious changes in the dynamic of the Shannon-Wiener evenness. In autumn, the Shannon-Wiener index was between 1.766 and 2.442, with the peak occurring in the 25–35 cm soil layer of the P. australis community area. However, the lowest index occurred in the 25–35 cm soil layer of the H. strobilaceum community area. The index of richness in autumn ranged from 4 to 10, with the highest point in the 0–5 cm layer of the H. strobilaceum and P. australis community areas, and the lowest the 5–15 cm layer of the T. ramosissima community area (index of evenness between 0.757 and 0.985).
Table 3. Shannon-Wiener index (H), richness (S), and evenness (E) of AOB communities in spring, summer, and autumn soil samples.
Phylogenetic analyses of uncultured Azotobacter from the Ebinur Lake wetland based on the nifH gene
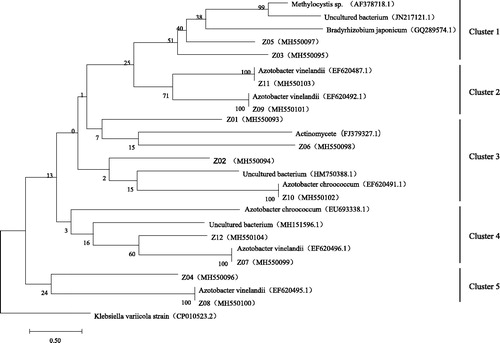
Twelve bands were excised from the DGGE gel profiles, re-amplified and sequenced by Beijing Liuhe Genomics Technology Co., Ltd. The phylogenetic trees based on the nifH gene were inferred by the neighbour-joining method (). Phylogenetic analysis showed that nifH sequences from different soil layers and seasons in the Ebinur Lake wetland were grouped into five clusters. Among them, Clusters 2, 3, 4, and 5 were affiliated with Azotobacter spp., including Azotobacter chroococcum and Azotobacter vinelandii, while the actinomycetes (such as FJ379327.1) were found in Cluster 3. Cluster 1 was mainly comprised of Rhizobium spp., with sequences homologous to Methylocystis spp. and Bradyrhizobium japonicum. In addition, most of the nifH gene sequences were grouped with uncultured Azotobacter based on sequence analysis.
Relationships of uncultured Azotobacter community structure and environmental factors
The details of collected samples and their physiochemical parameters are summarized in . The content of OC, IC, TN, NH4+-N, NO3−-N, microbial biomass C and microbial biomass N were 0.566–152.381 g/kg, 0.007–0.022 g/kg, 2.910–0.407 mg/kg, 7.04–9.65 mg/kg, 1.52–3.34 mg/kg, 0.001–0.03 mg/kg, and 1.76–4.76 mg/kg, respectively. According to China's nutrient grading table (NY/T391-2000), the content of OC falls in the third level, and total nitrogen in the fifth level of nutrition; the results of environmental factors showed that most of the soil quality indicators in summer were significantly higher than those in spring and autumn in the Ebinur Lake wetland. Meanwhile, the quality of soil in different layers was middle soil layer (5–25 cm)>deep soil (25–35 cm)>surface soil (0–5 cm).
Table 4. Physical and chemical properties of soil sampled in different seasons and from different sites of Ebinur Lake wetland.
The relationships between diversity of uncultured Azotobacter and environmental factors were analyzed by SPSS software (). With the change of seasons, there were significant positive correlations between Shannon-Wiener diversity index of uncultured Azotobacter and organic carbon (r = 0.732, p < 0.01 in spring; r = 0.483, p < 0.05 in autumn), inorganic carbon (r = 0.691, p < 0.05 in spring; r = 0.664, p < 0.05 in summer; r = 0.538, p < 0.05 in autumn), NH4+-N (r = 0.479, p < 0.05 in spring; r = 0.588, p < 0.05 in summer; r = 0.648, p < 0.05 in autumn) and microbial biomass N (r = 0.499, p < 0.05 in spring; r = 0.683, p < 0.05 in autumn). In contrast, there was a significant negative correlation between NO3−–N (r= −0.483, p < 0.05 in spring; r= −0.689, p < 0.05 in summer; r= −0.538, p < 0.05 in autumn) and the diversity of uncultured Azotobacter. Meanwhile, with the change of soil layers, there were significant positive correlations between Shannon-Wiener diversity index of uncultured Azotobacter and NH4+-N (r = 0.383, p < 0.05, in 15–25 cm soil layer), NO3−–N (r = 0.052, p < 0.05, in 0–5 cm soil layer), as well as microbial biomass C and N.
Table 5. Correlation analysis between Shannon-Wiener diversity index of Azotobacter and environmental factors.
Correlation analysis between the cultured/uncultured Azotobacter and soil environmental factors
Nitrogen-fixing microorganisms convert N2 in the air into nitrogen that can be directly used by plants. This function of nitrogen fixation in microorganisms determines its essential position in living and life and thus nitrogen fixation of microbial resources, functional analysis and resource exploration have been popular among many researchers [Citation22]. Based on traditional microbial culture methods, 17 strains cultured Azotobacter were isolated from the soil of the Ebinur Lake wetlands. The nitrogen fixation capacity of 12 strains was 1–10 mg/mL and the nitrogen fixation capacity of the five other strains was more than 10 mg/mL based on previous reports, a strain was evaluated as having potent nitrogen fixation ability when nitrogen content was greater than 10 mg/mL [Citation23,Citation24]. Sequence analysis showed that most sequences grouped with Bacillus sphaericus and Bacillus subtilis, while fewer sequences belonged to Actinomycetes. This result differs from a previous study by Xu et al. [Citation25]. The differences could be attributed to the large evaporation of wetland soil water, and the rise and fall of lake water resulting in saline-alkali seepage. Soil salinization is very strong that may result in a habitat more suitable for Bacillus spp. than Actinomycetes [Citation26,Citation27]. When considering evolutionary relationships, the homology of some strains was quite distant, indicating that there may be some nitrogen-fixing functional flora yet to be discovered in the Ebinur Lake wetland.
However, due to the limitations of in vitro experiments, laboratory culture of microorganisms can only cultivate <1% of the microbe species and many microorganisms cannot be cultured. In recent years, studies have used molecular methods to analyze environmental microbes and overcome the shortcomings of the traditional approaches. Therefore, the population structure and diversity of nitrogen-fixing bacteria was examined by non-culture methods in this study [Citation28]. The DGGE profiles of spring, summer and autumn samples showed different bands banding patterns, and every lane contained a special band in different sites and layers. Moreover, there were differences between the communities based on DGGE patterns: in the T. ramosissima community area, the highest diversity of uncultured Azotobacter was found during summer in the middle soil layer; in the H. strobilaceum community area, the highest diversity of uncultured Azotobacter was found during summer in the surface soil layer; and in the P. australis community area, the highest diversity of uncultured Azotobacter was found during autumn in the deep layer soil, possibly due to abundant nitrogen content in autumn soil. The results showed that there were fluctuations in the community of nitrogen-fixing bacteria in the wetland, and the overall bacterial diversity was rich. The main functional uncultured Azotobacter was autotrophic Azotobacter and Rhizobium. As evident from the phylogenetic tree (), the sequence similarity of uncultured Azotobacter was not high, ranging between 67% and 92%, which suggests that the species of uncultured Azotobacter were different based on high salt and alkali environment in the Ebinur Lake wetland. In this study, DGGE showed that there was an important difference between the nifH gene and cultured Azotobacter in the soil, which might be attributed to the DGGE approach only detecting dominant populations. In addition, many of the nifH functional genes isolated from non-culturable microorganisms were similar, which limits the identification of specific populations. Moreover, knowledge about the enrichment of nitrogen-fixing bacteria is still expanding, some nitrogen-fixing bacteria might be undiscovered. The structure of the nitrogen-fixing bacteria populations in the Ebinur Lake wetland has also made a significant contribution to improving the nitrogen-fixing bacteria resource library [Citation29,Citation30].
Based on correlation analyses, the diversity and community structure of uncultured Azotobacter correlated with organic carbon, inorganic carbon, NH4+-N, NO3—N, and microbial biomass N, indicating that a complex combination of environmental factors might shape the overall level of Azotobacter diversity in the Ebinur Lake Wetland. NH4+-N and NO3−-N might have an influence on the diversity of Azotobacter. In addition, studies have also shown that the species diversity of Azotobacter was significantly related to soil pH, manganese, zinc, and freeze-thaw action [Citation24,Citation31]. In recent years, the content of ammonia and nitrogen has increased dramatically because of the amount of fertilizers and pesticides used on farmlands upstream of the Ebinur Lake wetland [Citation32]. The moderate rise in ammonia and nitrogen content might be better suited for the growth of Azotobacter [Citation33]. However, the higher concentrations of ammonia could also hinder the diversity of Azotobacter, and it has an impact on other organisms. In order to protect wetland biodiversity and promote wetland ecological restoration, it is necessary to supervise the rational fertilization of upstream farmland and reduce the destruction of wetlands by human beings [Citation34].
Conclusions
By analyzing the diversity of Azotobacter in the rhizosphere soil of different plants in different seasons, the succession process of Azotobacter diversity in the rhizosphere soil of the same plant in Ebinur Lake Wetland and the differences in the diversity of Azotobacter in the rhizosphere soil of different plants can be revealed more comprehensively. The diversity of Azotobacter in Ebinur Lake Wetland was monitored in real time, which can provide scientific basis for wetland environmental restoration. This study utilized culture-based and non-culture based techniques to study nitrogen-fixing bacteria from the Ebinur Lake wetland. We provided evidence of the presence of Azotobacter, and a total of 17 cultured Azotobacter strains were isolated from soil by the culture method; most of the strain sequences were grouped into B. sphaericus and B. subtilis, and fewer sequences were grouped with actinomycetes. The uncultured Azotobacter, based on nifH gene analysis, were grouped into autotrophic Azotobacter spp. and Rhizobium spp. Furthermore, the diversity of uncultured Azotobacter might be strongly influenced by soil organic carbon, inorganic carbon, NH4+-N, NO3—N, and microbial biomass N in the Ebinur Lake wetland in Xinjiang.
Disclosure statement
This work was supported by the National Natural Science Foundation of China (grants 31560040 and 31160026).
References
- Butet A. Nitrogen content of tissues and trophic choices of the wood mouse, Apodemus sylvaticus, in an oligotrophic ecosystem. Crop Sci. 1990;65:124–131.
- Knight JD. Frequency of field pea in rotations impacts biological nitrogen fixation. Can J Plant Sci. 2012;92:1005–1011.
- Rubio EJ, Montecchia MS, Tosi M, et al. Genotypic characterization of Azotobacteria isolated from Argentinean soils and plant-growth-promoting traits of selected strains with prospects for biofertilizer production. Sci World J. 2013;2013:1–603.
- Chen G, Zhu H, Zhang Y. Soil microbial activities and carbon and nitrogen fixation. Res Microbiol. 2003;154:393.
- Weisany W, Raei Y, Allahverdipoor KH. Role of some of mineral nutrients in biological nitrogen fixation. Bull Eng Geol Environ. 2013;2:77–84.
- Fong AA. Mesoscale variability in nitrogen-fixing bacteria and rates of nitrogen fixation in the North Pacific Ocean [dissertation]. University of Hawaii at Manoa. 2006;6:32–37.
- Pühler A, Aguilar MO, Hynes M. Advances in the genetics of free-living and symbiotic nitrogen fixing bacteria. Adv Nitrogen Fixation Res. 1984;8:609–619.
- Kizilova AK. Evaluation of the diversity of nitrogen-fixing bacteria in soybean rhizosphere by nifH gene analysis. Microbiology. 2012;81:672–681.
- Dadok M, Beglarian M, Mehrabian S, et al. Phylogenetic identification of nitrogen-fixing bacteria isolated from the rhizosphere of asparagus plants using 16s rRNA and the effect of zinc on isolated strains. J Ilam Univ Med Sci. 2013;20:112–120.
- Ren J, Jin H, Mao YE, et al. Analysis and evaluation of water quality of Aibihu Lake Wetland Natural Reserve. J Arid Land Resour Environ. 2011;25:154–157.
- Liu Y. Protect wetland and improve eco-environment of Ebinur Wetland Lake. EST. 2004;2004:3–5.
- Lu RK. Methods of soil agricultural chemistry analyses. Agricult Sci Technol. 1999;11:22–34.
- Boone DR, Castenholz RW, Garrity GM. Bergey’s manual of systematic bacteriology. New York (NY): Springer; 2011.
- Parte A, Krieg NR, Ludwig W. Bergey’s manual of systematic bacteriology. New York (NY): Springer; 2009;38:443–491.
- Ri-Le W, Gang LI, Dian-Lin Y, et al. nifH gene diversity and community structure of soil nitrogen-fixing bacteria in Hulunbeier grassland. Inner Mongolia Chin J Ecol. 2011;30:790–797.
- Yong Z, Zhihua Z, Wu L, et al. DNA extraction from soil for molecular microbial community analysis. JAES. 2005;12:24–30.
- GuiLin Z. Study of inoculated moderately Halobacterium salinarium salination cotton field’s microorganisms population constructions [master’s thesis], Shihezi University, Xinjiang; 2010.
- Bassam BJ, Gresshoff PM. Silver staining DNA in polyacrylamide gels. Nat Protoc. 2007;2:2649–2654.
- Thompson JD, Higgins DG, et al. The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 1997;25:48–82.
- Saitou N. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol. 1987;1987:425.
- Tamura K, Dudley J, Nei M, et al. MEGA4, a molecular evolutionary genetic analysis MEGA software version 4.0. Mol Biol Evol. 2007;24:596–1599.
- Zou Y, Zhang J, Yang D, et al. Effects of different land use patterns on nifH genetic diversity of soil nitrogen-fixing microbial communities in Leymus chinensis steppe. Acta Ecol Sin. 2011;31:150–156.
- Vacca G, Wand H, Nikolausz M, et al. Effect of plants and filter materials on bacteria removal in pilot-scale constructed wetlands. Water Res. 2005;39:1361–1373.
- Chen SL, Tsai MK, Huang YM, et al. Diversity and characterization of Azotobacter isolates obtained from rice rhizosphere soils in Taiwan. Ann Microbiol. 2018;68:17–26.
- Xu Y, Wang T, Li H, et al. Variations of soil nitrogen-fixing microorganism communities and nitrogen fractions in a Robinia pseudoacacia chronosequence on the Loess Plateau of China. Catena. 2019;174:316–323.
- Li YH, Zhao ML, Li FD. Soil respiration in typical plant communities in the wetland surrounding the high-salinity Ebinur Lake. Front Earth Sci. 2018;12:611–624.
- Wang J, Ding J, Abulimiti A, et al. Quantitative estimation of soil salinity by means of different modeling methods and visible-near infrared (VIS-NIR) spectroscopy, Ebinur Lake Wetland, Northwest China. Peer J. 2018;6:11–22.
- Marques E, Gross E, Teixeira Dias JC, et al. Ammonia oxidation (amoA) and nitrogen fixation (nifH) genes along metasandstone and limestone caves of Brazil. Geomicrobiol J. 2018;35:869–878.
- Zhao X, Zhou ZG, Guo LV, et al. The characteristics of soil microbe in Panjin reed wetland. J Meteor Environ. 2006;22:n64–67.
- Zhongqiong W, Weidong W, Guibing Z. A comparative study on the diversity of rhizospheric bacteria community structure in constructed wetland and natural wetland with reed domination. Acta Ecol Sin. 2011;31:4489–4498.
- Ma XF, Chu XZ, Ma Q. Effects of freeze-thaw on soil enzyme activity and microbial quantity of Haloxylon ammodendron community in Ebinur Lake area. Arid Reg Geogr. 2015;38:1190–1201.
- Wu J. Climatic change record from stable isotopes in Lake Aibi, Xinjiang during the past 1500 years. Quaternary Sci Rev. 2004;24:585–590.
- Ishac YZ, Selim AA, Ramadan EM, et al. Effect of some insecticides on the biological activity of Azotobacter chroococcum A36. I. Ammonia and amino nitrogen contents and nitrogen-fixing efficiency. Soil Biol Conserv Biosphere. 1984;12:60–89.
- Yori G, Yang ST, Zibibra S. Impact of land use change on ecosystem service value in Aibi Lake Basin, Xinjiang. J Agricult Eng. 2019;35:260–269.