?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The aim of this study was to investigate the anti-ageing effect of Yisui moxibustion on regulating the klotho protein and insulin (INS)/IGF-1 signalling cascade in d-galactose mice. Sixty male Kunming mice were randomly divided into five groups after the Morries test: the normal sodium, model, moxa cone moxibustion 1 and 2, and sham groups. The treatment and model were given d-galactose injection for 70 days, while mice in the normal sodium group were injected the same dosage of normal sodium. From day 13 after the first injection, the three treatment groups were treated every day for 58 days. The INS and klotho in the cerebral tissues were measured. p-Akt, p-FoxO3a, Klotho, SOD2 expression were observed immunohistochemically. The escape latency of mice in the model and sham were significantly prolonged, and the spanning original platform times were significantly decreased compared with the normal sodium and moxa cone moxibustion 1 and 2 (p < 0.01). The content of INS and klotho in the model was significantly lower than the normal sodium, moxa cone moxibustion 1 and 2 (p < 0.01). Compared with the model and sham, p-Akt and p-FoxO3a expression in moxa cone moxibustion 1 and 2 were markedly decreased (p < 0.05), and the expressions of klotho and SOD2 were significantly increased (p < 0.05) in hippocampal CA1. Yisui moxibustion could exert the antioxidative effect on klotho protein, which negatively regulated the insulin signalling pathway and its downstream elements in cerebral tissue and hippocampal CA1, so as to enhance learning and memory capacities, delay brain ageing.
Introduction
Oxidative stress (OS) is considered one of the important factors in the process of a range of neurodegenerative diseases in the elderly, such as Alzheimer's disease (AD). Growing nerve cell damage caused by OS, especially significant in the hippocampal CA1 area, occurs with increasing age [Citation1]. In recent years, the influence of insulin and its biological dysfunction on the nervous system has already attracted great interest. The insulin signalling transduction that occurs in the disorder, might trigger the characteristic pathological changes of AD [Citation2]. Insulin is considered to be a protective nerve agent, which does not only regulate the energy homoeostasis, but also the glucose metabolism providing nutrition to nerve cells [Citation3]. New research found that insulin may be a potential protective agent for the treatment of oxidative stress-induced diabetic encephalopathy [Citation4]. The insulin/insulin-like growth factor-I (IGF-I) signalling pathway, known as an important signalling pathway in organisms, is closely related to cognitive function, including learning and memory, and also participates in the process of regulating cell proliferation, metabolism and apoptosis [Citation5,Citation6]. However, a recent study demonstrated that long-term suppression of IGF1R signalling alleviates AD progression and promotes neuroprotection, the underlying mechanisms remaining largely elusive [Citation7]. Klotho is a new anti-ageing gene, so its overexpression can inhibit ageing and prolong lifespan. The mechanism might involve inhibiting the insulin signalling and oxidative stress [Citation8].
Moxa cone moxibustion, a non-pharmaceutical therapy, has been widely applied since JIN-TANG dynasty. Recent studies have shown that acupuncture and moxibustion treatment have obtained certain effect on mild cognitive impairment and brain oxidative state [Citation9–11]. Our previous study [Citation12] demonstrated that it can be a free radical scavenger complement, can protect the cerebral cortex and the hippocampal neuron structure and improve the function of learning and memory, so as to delay the process of ageing in the brain. Based on it, we performed this study to observe the effect of Yisui moxibustion (a kind of moxa cone moxibustion) on the insulin and Klotho level in cerebral tissue, and the expression of p-Akt, p-FoxO3a, Klotho, SOD2 in the hippocampal CA1 area in d-galactose ageing mice, then try to investigate the mechanism of delaying senescence with Yisui moxibustion on a molecular level.
Materials and methods
Animals
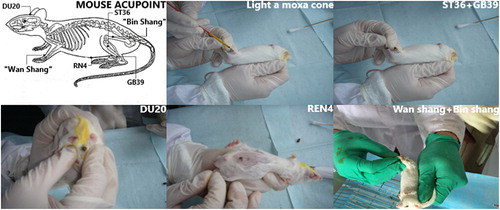
A total of 60 healthy male Kunming mice, 3 months of age, weighing 30 ± 2 g, were purchased from the SPF Laboratory Animal Center of Guangxi University of Chinese Medicine, China (license number: SCXK [Gui] 2014-0002). Mice were neurally developed and had normal learning and memory function. The mice were all able to swim at least 2600 cm/2 minutes in the Morris water maze (MWM), and were randomly divided into five groups with 12 mice per group: normal sodium control, model, sham, moxa cone moxibustion 1 (acupoint Zusanli (ST36) and Xuanzhong (GB39)), moxa cone moxibustion 2 (acupoint Baihui (DU20) and Guanyuan (RN4)), and sham (nonacupoint ‘Wan Shang’ and ‘Bin Shang’) groups. The mice were housed with food and water freely available in the SPF Laboratory Animal Center of Guangxi Traditional Chinese Medical University, China (license number: SYXK [Gui] 2010-0001).
Ethics statement
All experimental protocols were performed in strict accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals and animal ethical and welfare were approved by the First Affiliated Hospital of Guangxi University of Chinese Medicine Institutional Review Board (Lot number: [2015] 002). During the experiment, two mice died in each of the model and sham group, suffering from anorexia before death, whereas one mouse died in the normal sodium and moxa cone moxibustion 2 groups, suffering from eating too much. Finally, there were 11 mice in the normal sodium control group and moxa cone moxibustion 2 groups, 10 mice in the model and sham groups and 12 mice in the moxa cone moxibustion 1 group.
Establishment of subacute ageing models
A subacute ageing model was established by cervical subcutaneous injection of d-galactose solution 1000 mg/kg per day [Citation13], at a d-galactose: normal sodium ratio of 10(g): 100 (mL) 100 mg/mL, for 70 successive days. The same dosage of normal sodium was injected into the mice in the normal sodium control group at the same time.
Intervention
From the 13th day after subcutaneous injection of d-galactose (Sinopharm Chemical Reagent Co., Ltd. Shanghai, China), three treatment groups were treated every day from 9:00 a.m. until 11:30 a.m. for 58 days. The moxa cones (3 mm × 5 mm, 12–14 mg, 6 mm high) were used at each acupoint [Citation14,Citation15] in the moxa cone moxibustion 1 group via Zusanli (ST36, at muscle groove of 3 mm inferior to the head of the fibula), Xuanzhong (GB39, 5 mm superior to the lateral tip of the malleolus behind the fibula) and moxa cone moxibustion 2 group via Baihui (DU 20, the middle of the parietal bone), Guanyuan (RN4, 1 cm inferior to the umbilicus). The sham point was at 1 mm superior to the dorsal carpal joint (named ‘Wan Shang’) and 2 mm superior to the median knee patella joint (named ‘Bin Shang’). The moxa cones made of moca (Eastern Moxa Plant, Suzhou, China), shaped like kernel, lasted 10–20 s each time and were replaced if the mice began to struggle. Each acupoint required three moxa cones and a total of six moxa cones for each treatment. Treatment was performed every day, for a total of 58 days. At the same time, the model and normal sodium control groups underwent no therapeutic intervention but were handled to the same extent as the treatment groups ().
Morris water maze test
The water maze test was performed by a TM-Vision experimental behaviour Morris water maze system with a video-tracking system software (Institute of Physiology, China Academy of Sciences, Beijing, China), used to collect information about mouse movement (latency, swim path, distance and speed) [Citation16]. Animals were trained to locate an escape platform in a circular pool (120 cm in diameter. Water was made opaque with a temperature of 25 ± 1 °C) and a platform (11 cm in diameter) was placed in the centre of the middle of the second quadrant of the pool and submerged by 1 cm. Performance was evaluated as the time taken to reach and climb onto the platform (escape latency). Mice were trained and tested for a total of 7 days; on day 1, mice were allowed to swim freely in the pool for 2 minutes to acclimatize to the conditions, and so that we could confirm their ability to swim over 2600 cm and at a speed of more than 28 cm/s.
Place navigation test
From the 2nd day to the 6th day, mice underwent eight trials of place navigation training per day (10- to 15-minute inter-trial rest interval). There were four trials in the morning and four trials in the afternoon. The animals were released from the different quadrants with their heads facing the pool wall. When a mouse found the platform, it was allowed to stay on it for 15 seconds. If mice did not find the platform within the allocated 60 seconds, they were trained to locate a visible (cued) platform, by being guided gently to the platform (i.e. ‘rescue’) and allowed to stay for 15 seconds. After completing each trial, the mice were removed from the pool, dried with a soft towel and returned to their home cage.
Spatial probe test
On the 7th day, each animal was given a 120-second spatial probe test to evaluate the memory retention of the task. During the probe test, the platform was removed, and searching behaviour in the target quadrant (where the platform was located during place navigation training) was measured. The animals were released from the centre of the quadrant opposite the target (i.e. the second) quadrant. Spanning (original) frequency during the probe test (i.e. the frequency of passes through the former position of the platform) was used to evaluate the performance in this task. The shorter escape latency and the larger spanning (original) frequency indicated a better learning and memory function.
Specimen preparation
After the intervention finished and after completion of the Morris Water Maze test, the mice were sacrificed by cervical dislocation under light ether anaesthesia 24 h after the end of MWM. The left and right hemispheres were separated on ice cubes after sacrifice. About 150 mg tissue homogenates were prepared with a ratio (w/v = 1: 9) of 0.15 g wet cerebral cortex of the right hemisphere added to nine times as much 0.9% NaCl (about 1.35 mL) by using a homogenizer. These were centrifuged at 2 000 r/min for 10 minutes, then stored at −20 °C until use. The left hemisphere was fixed in 4% paraformaldehyde (Boster Biological Engineering Co., Ltd. Wuhan China) and embedded in paraffin.
Measurement of homogenate insulin and klotho level with enzyme-linked immunosorbent assay (ELISA)
The insulin and klotho levels in the cerebral tissue were measured by using enzyme-linked immunosorbent assay (ELISA) kits (Shanghai Lianshuo Biological Co., Ltd. Shanghai China and Wuhan Huamei (CUSABIO) Biological Engineering Co., Ltd., Wuhan, China, respectively).
Immunohistochemistry staining
The fixed hippocampal tissues were cut into 24 pieces of continuous coronal sections after dehydration, clearing, wax immersion and paraffin embedding. SP immunohistochemical method was used to detect the expression of p-Akt (ser473, Santa Cruz Bio, USA), p-FoxO3a (Ser253, Abcam, USA), Klotho (Abcam, USA) and SOD2 (Abcam, USA). The detection procedure was as follows: Streptavidin-perosidase (SP) immunohistochemical method was used to detect the expression of p-Akt, p-FoxO3a, Klotho and SOD2. After dewaxing and hydration, sections were bathed in phosphate buffered saline (PBS) (pH 7.4) with 3 × 5 min, then high pressure repaired in 0.01 mol/L citric acid buffer (pH 6.0) with whiffing in 2 min, embathed in PBS (pH 7.4) with 3 × 5 min again, followed by adding 3% H2O2 solution to each slice for incubation at room temperature for 10 min. The samples were then bathed in PBS (pH 7.4) with 3 × 5 min again; PBS was discarded and 50 μL rabbit anti-mouse p-Akt antibody (diluted in 1:200), p-FoxO3a antibody (diluted in 1:50), Klotho antibody (diluted in 1:50) and SOD2 antibody (diluted in 1:100) were added to each slice for incubation at 4 °C for 15 hours. This was followed by bathing in PBS (pH 7.4) with 3 × 5min again, discarding PBS and adding 50 μL streptavidin-perosidase labelled goat anti-rabbit antibody (PV-8000 test kits, Zsbio Co Ltd, china) to each slice for incubation at room temperature for 30 min. Next, the samples were bathed in PBS (pH 7.4) with 3 × 5 min again and PBS was discarded. Then, 100 μL of freshly formulated 3,3’- diaminsobenzidine (DAB) as a colour developing reagent was added to each slice for developing for 1-3 min, followed by bathing with distilled water for 3 min, comparative staining with haematoxylin solution for 3 min and bathing with distilled water for 5 min. Then, 1% HCl aqueous solution was added for differentiation in 30 s–1 min and the samples were bathed with distilled water for 5 min. After gradient ethanol dehydration and xylene clearing, tissue sections were sealed with neutral gum. The cerebral cortex and hippocampal CA1 region were screened under a light microscope (Olympus, Tokyo, Japan) according to the stereotaxic atlas of the rat brain [Citation17]. Six non-overlapping areas were selected for measuring neuronal density, using Image-Pro Plus 6.0 (IPP) software for analysis. Analysis method: firstly, the IPP program system was used for optical density correction and measurement of the integrated optical density (IOD) and the area of the yellow region (area of interest, AOI) of the nerve cell immunohistochemistry reaction in the picture to calculate the average optical density (AOD) as follows:
Statistical analysis
The values of all variables are presented as means with standard deviation (±SD). All analyses were conducted using SPSS software version 23.0 (SPSS Inc, Chicago, IL, USA). After one-way analysis of variance (ANOVA) and repeated-measure analyses of variance were performed, LSD-t tests were used to compare individual pairs of groups. Kruskal–Wallis test was used for continuous variables with heterogeneity of variance. The level of statistical significance was set at p < 0.05 or p < 0.01.
Results and discussion
Learning and memory functions of mice before and after treatment
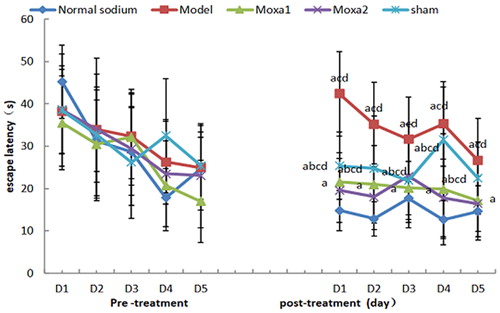
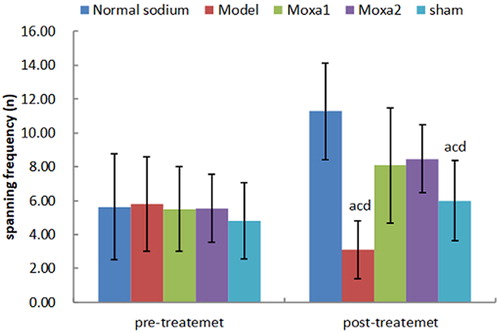
First, we evaluated the learning and memory functions of the animals ( and ). There were no significant differences among the five groups in the place navigation and the spatial probe test performance prior to modelling (p > 0.05). After modelling and treatment, the escape latency of mice in the model group and the sham group were significantly prolonged, and the spanning original platform frequency was significantly decreased compared with the normal sodium control group and moxa cone moxibustion groups 1 and 2 (p < 0.01). The escape latency in the normal sodium control group was significantly shorter than that in the moxa cone moxibustion groups 1 and 2 (p < 0.01), whereas there was no significant difference between the moxa cone moxibustion groups 1 and 2 (p > 0.05). The spanning original platform frequency in the normal sodium control group was significantly higher than that in the moxa cone moxibustion groups 1 and 2 (p < 0.01), whereas there was no significant difference between the moxa cone moxibustion groups 1 and 2 (p > 0.05). It indicated that Yisui moxibustion could improve the learning and memory functions of d-galactose mice.
Effect of Yisui moxibustion on the level of INS and klotho in brain tissue of d-galactose-induced ageing mice
The content of INS and klotho in the brain tissue of model group was significantly lower than that in the normal sodium control group, moxa cone moxibustion1 and 2 group (p < 0.01), whereas compared with the sham group, there was no significant difference (). Compared with the normal sodium control group, there was no significant difference in the moxa cone moxibustion 1 and 2 groups (p > 0.05), whereas there was significant difference in the sham group (p < 0.05). Compared with the sham group, there was significant difference in the moxa cone moxibustion 1 and 2 groups (p < 0.05), but there was no significant difference between the moxa cone moxibustion 1 and 2 groups (p > 0.05). This suggests that Yisui moxibustion could increase the level of INS and klotho in the brain tissue of d-galactose mice, which is beneficial to an anti-ageing function.
Table 1. Effect of Yisui moxibustion on the level of INS and klotho in brain tissue of d-galactose-induced ageing mice.
Effect of Yisui moxibustion on the expression of P-Akt, p-FoxO3a, klotho, SOD2 in the hippocampal CA1 area of d-galactose-induced ageing mice
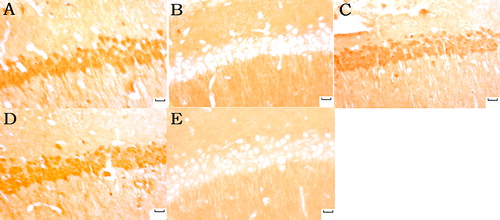
p-Akt, p-FoxO3a, klotho, and SOD2-positive neurons were stained brown. p-Akt and p-FoxO3a expression increased in the model and the sham groups with dark brown, p-Akt and p-FoxO3a expression decreased in the normal sodium group, as well as in the moxa cone moxibustion 1 and 2 groups ( and ). The staining of the normal sodium group was most light with the minimum p-Akt and p-FoxO3a.
Figure 4. p-Akt (Phospho-Akt) expression in hippocampal CA1 under light microscope (immunohistochemical staning ×400). Normal sodium group (A), Model group (B), Moxa cone moxibustion 1 group (C), Moxa cone moxibustion 2 group (D), Sham group (E). Scale bars in Panel A, B, C, D and E represent 20 μm.
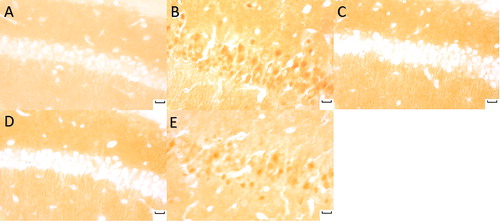
Figure 5. p-FoxO3a (Phospho-FoxO3a) expression in hippocampal CA1 under light microscope (immunohistochemicalstaning × 400). Normal sodium group (A), Model group (B), Moxa cone moxibustion 1 group (C), Moxa cone moxibustion 2 group (D), Sham group (E). Scale bars in Panel A, B, C, D and E represent 20 μm.
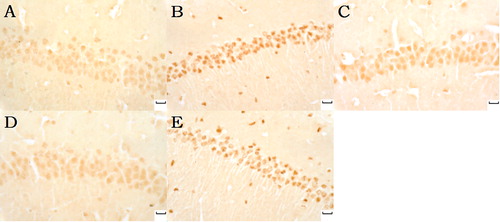
Klotho and SOD2-positive neurons were most lightly stained with the minimum klotho and SOD2 expression in the model group and the sham group. Klotho and SOD2 expression increased in the normal sodium and in the moxa cone moxibustion 1 and 2 groups with dark brown ( and ).
Figure 6. Klotho expression in hippocampal CA1 under light microscope (immunohistochemicalstaning ×400). Normal sodium group (A), Model group (B), Moxa cone moxibustion 1 group (C), Moxa cone moxibustion 2 group (D), Sham group (E). Scale bars in panel A, B, C, D and E represent 20 μm.
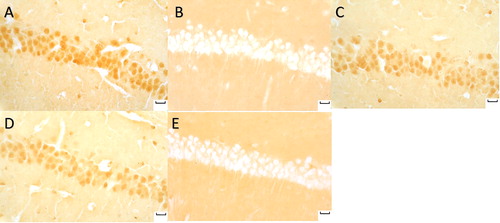
Figure 7. MnSOD (SOD2) expression in hippocampal CA1 under light microscope (immunohistochemicalstaning ×400). Normal sodium group (A), Model group (B), Moxa cone moxibustion 1 group (C), Moxa cone moxibustion 2 group (D), Sham group (E). Scale bars in Panel A, B, C, D and E represent 20 μm.
As shown in , compared with the normal sodium control group, the AOD of p-Akt and p-FoxO3a expressions in the hippocampal CA1 area of the model group and the sham group were markedly increased (p < 0.05), and the AOD of klotho and SOD2 expressions were significantly decreased (p < 0.05). Compared with the model group and sham group, the AOD of p-Akt and p-FoxO3a expressions in the hippocampal CA1 area of the moxa cone moxibustion 1 and 2 groups were markedly decreased (p < 0.05), and the AOD of klotho and SOD2 expressions were significantly increased (p < 0.05) .
Table 2. Effect of Yisui moxibustion on the expression of p-Akt, p-FoxO3a, klotho, SOD2 in hippocampal CA1 area of d-galactose-induced ageing mice.
These results indicate that Yisui moxibustion could decrease the expression of p-FoxO3a, p-Akt and increase the level of klotho and SOD2 in the hippocampal CA1 area of d-galactose mice. This suggests that Yisui moxibustion could enhance learning and memory capacities and delay brain aging by koltho protein, which inhibits the insulin signal transduction on the one hand, and on the other, promotes antioxidation by negatively regulating the insulin/IGF-1 signalling pathway, decreasing the expression of p-FoxO3a and p-Akt, and increasing the expression of SOD2.
Klotho is an anti-ageing gene, and the deficiency of this gene in mice would cause similar aging phenomenons as in humans [Citation18,Citation19]. The over-expression of klotho in mice can extend the lifespan, as well as increase the antioxidant activity [Citation20]. Recent studies indicated that the activation of ROS signaling and increased oxidative stress in the brain with ageing are involved in the development of AD. The cognitive disorder caused by oxidative stress could be recognized as brain ageing [Citation21]. Recent research found that the incidence of AD is closely related to brain insulin deficiency and resistance, so that AD is also considered as type-3 diabetes [Citation22]. From a clinical perspective, compensatory hyperinsulinaemia to overcome systemic insulin resistance is thought to be a healthy goal, because it circumvents the immediate catastrophic consequences of hyperglycaemia; however, studies found that reduced insulin/IGF1 signalling in CNS extends life span and delays neurodegeneration processes [Citation23]. It was already pointed out that moderate inhibition of the insulin/IGF-1 signalling pathway is a conserved evolutionary mechanism of inhibiting aging [Citation24]. A similar mechanism was found in centenarians [Citation25]. There have been two opinions about the relationship between the effect of the insulin signalling pathway and ageing: one is that insulin has good for neuroprotective effects [Citation26], the other is that reducing insulin sensitivity plays an important role in delaying ageing and protecting the nervous system [Citation27]. Age-related astrocytic dysfunction caused by diminished IGF-1 signalling may contribute to the pathogenesis of Alzheimer's disease and other age-associated cognitive pathologies [Citation28]. Among the genes that regulate aging, the insulin-FOXO signalling pathway plays a central role, as this pathway regulates the lifespan in multiple species, and has been associated with exceptional longevity – being a centenarian [Citation29]. Recent evidence indicates klotho protein can negatively regulate the PI3k/Akt signalling pathway, thus increasing the expression of MnSOD (SOD2) mediated by FoxO3a, and enhancing the effect of antioxidant stress on the body [Citation30]. Upregulated klotho expression, which decreased Akt and Forkhead box class O1 phosphorylation, moreover, inhibited the insulin-like growth factor 1 pathway and induced Forkhead box class O1 activation, possibly contributing to the neuroprotective effect against age-related AD [Citation31].
Being easy to operate and seldom giving side effects, moxibustion has the unique advantages in the prevention and treatment of ageing. Moxibustion has the effect of warming and nourishing, strengthening the kidneys and stabilizing the organism. Brain ageing is located in the cerebral, as what is mentioned in Miraculous Pivot (33rd chapter of the seas theory), insufficient marrow leads to dizziness and tinnitus, fatigue [Citation32]. Zusanli (ST36), which is the He-sea point of the stomach channel, can invigorate Qi and strengthen the spleen. Xuanzhong (GB39), which is the influential point of marrow, stemmed from The Classic on Difficulty (Nan Jing) - 45th chapter, can replenish vital essence, and nourish the marrow. The Compendium of Acupuncture and Moxibustion (Zhenjiu Dacheng) states that the combination of two points is capable of preventing prior stroke [Citation33]. Baihui (DU20), the crossing point of the channels of Foot-Shaoyang, Hand-Shaoyang, Foot-Taiyang, Foot-Jueyin and Du, can not only benefit the marrow but also activate the brain and help regain consciousness. Guanyuan (RN 4), the crossing point of the channels of three foot-yin and the Ren, is the key point to warm the kidney and strengthen Yang. The combination of two points is the experiential prescription from Professor Si Tuling and Zhang Jiawei to treat brain disease and prevent ageing [Citation12]. Based on it, moxibustion group 1 selecting distal acupoints, pays attention to the role of the spleen and the stomach in anti-ageing, which is the source of qi and blood; and moxibustion group 2 with the combination of distant–local acupoints focuses on tonifying the kidney for brain marrow. Four acupoints in this study, nourishing brain marrow by spleen and kidney, especially ST36 and DU20, have been shown to improve learning and memory. According to the clinical effect of acupoints in our study, we chose acupoint prescriptions in Yisui Moxibustion, intended to compare two pair acupoint prescriptions to determine which one was better in the anti-aging process. Moxibustion group 1 (ST36 + GB39) selecting distal acupoints, focuses on nourishing the spleen and stomach in the prevention of ageing, being the source of Qi and blood. Moxibustion group 2 (DU20 + RN4), with the combination of distant–local acupoints focuses on tonifying the kidney for brain marrow. Four acupoints in this study, nourishing brain marrow by spleen and kidney, especially ST36 and DU20, have been shown to improve learning and memory [Citation10,Citation11]. Correlative researches [Citation34,Citation35] showed that oxidative stress-induced peripheral hyperinsulinaemia and insulin resistance in d-galactose senile mice and rats, which is a better model of AD. In a study of the expression of MnSOD in the hippocampus of AD patients, the CA1 region in AD was the most severely affected by the disease, and the normal compensatory mechanisms may be insufficient to protect this region from free radical oxidative damage [Citation36]. Moxibustion plays a key role against brain aging via improving the state of oxidative stress, activities of neuroprotection and antiapoptosis [Citation37,Citation38]. Meanwhile, the effect of moxibustion on the insulin signalling pathway caused by oxidative stress in the brain is seldom reported so far. This study revealed that Yisui moxibustion was associated with increased insulin level in the brain tissue. Similarly, compared to the normal sodium control, the klotho protein level was higher than that in the model group, indicating that klotho protein possibly lowered the insulin sensitivity in the brain. Meanwhile, down-regulated expression of p-Akt and p-FoxO3a were found in the hippocampal CA1 area in the treatment groups, which could suggest that klotho protein had inhibitory influence on the downstream insulin signalling pathways, which was related to improved learning and memory in oxidative stress mice induced by d-galactose. The rising insulin levels in the treatment groups may be a positive feedback of the inhibitory influence on the downstream insulin signalling pathways by klotho protein [Citation39]. As demonstrated [Citation30], klotho protected Tac-induced oxidative stress by negatively regulating the PI3K/AKT pathway and subsequently enhancing FoxO3a-mediated MnSOD expression. Some scholars [Citation40,Citation41] also pointed out AD could be treated by the up-regulated expression of klotho inhibiting the IGF-1 pathway, which induces the transactivation activity of FOXO1, and then improves the oxidative stress status in the brain. Therefore, upregulation of endogenous klotho expression is of high clinical relevance as a possible anti-ageing strategy.
Conclusions
The obtained results suggest that Yisui moxibustion could enhance learning and memory capacities and delay brain ageing by koltho protein, which inhibits the insulin signal transduction on the one hand, and on the other, promotes antioxidation by negatively regulating the insulin/IGF-1 signalling pathway, decreasing the expression of p-FoxO3a and p-Akt, and increasing the expression of SOD2. This study presented evidence of changes in the insulin/IGF-1 signalling pathway caused by oxidative stress and suggested that klotho protein is one of the targets of Yisui moxibustion on the brain insulin signalling system. Meanwhile, it provided evidence for integrated control on clinical brain ageing, which will play a positive role in easing social and economic pressure from ageing caused by neural degenerative diseases.
Acknowledgements
We would express our sincere gratitude to Dr. Jinsheng Wang (Department of Pathology, Guangxi University of Chinese Medicine, Nanning, China) and Dr Zhihong Luo (Acupuncture and Massage College, Guangxi University of Chinese Medicine, Nanning, China) for their help with the experimental research.
Disclosure statement
The authors declare no conflicts of interest.
Funding
This study was financially sponsored by The National Natural Science Foundation of China, No. 81360561; The Science and Technology plan of Guangxi(Gui), No 14124004-1-27; The Science and Technology plan of Guangdong, No. 2016A020215202.
References
- Tian JS, Zhai QJ, Zhao Y, et al. 2-(2-benzofuranyl)-2-imidazoline (2-BFI) improved the impairments in AD rat models by inhibiting oxidative stress, inflammation and apoptosis. JIN. 2018;16(4):385–400.
- Ma Y, Wu D, Zhang W, et al. Investigation of PI3K/PKB/mTOR/S6K1 signaling pathway in relationship of type 2 diabetes and Alzheimer’s disease. Int J Clin Exp Med. 2015;8(10):18581–18590.
- Lee S-H, Zabolotny JM, Huang H, et al. Insulin in the nervous system and the mind: Functions in metabolism, memory, and mood. Mol Metab. 2016;5(8):589–601.
- Song Y, Ding W, Bei Y, et al. Insulin is a potential antioxidant for diabetes-associated cognitive decline via regulating Nrf2 dependent antioxidant enzymes. Biomed Pharmacother. 2018;104:474–484.
- Pristerà A, Blomeley C, Lopes E, et al. Dopamine neuron-derived IGF-1 controls dopamine neuron firing, skill learning, and exploration. Proc Natl Acad Sci USA. 2019;116(9):3817–3826.
- Pardo J, Uriarte M, Cónsole GM, et al. Insulin-like growth factor-I gene therapy increases hippocampal neurogenesis, astrocyte branching and improves spatial memory in female aging rats. Eur J Neurosci. . 2016;44(4):2120–2128.
- George C, Gontier G, Lacube P, et al. The Alzheimer's disease transcriptome mimics the neuroprotective signature of IGF-1 receptor-deficient neurons. Brain. 2017;140(7):2012–2027.
- Abraham CR, Mullen PC, Tucker-Zhou T, et al. Klotho is a neuroprotective and cognition-enhancing protein. Vitam Horm. 2016; 101:215–238.
- Wang S, Yang H, Zhang J, et al. Efficacy and safety assessment of acupuncture and nimodipine to treat mild cognitive impairment after cerebral infarction: a randomized controlled trial. BMC Complement Altern Med. 2016;16(1):361–368.
- Huang KY, Liang S, Yu ML, et al. A systematic review and meta-analysis of acupuncture for improving learning and memory ability in animals. BMC Complement Altern Med. 2016;16(1):297–314.
- Zhang M, Xv GH, Wang WX, et al. Electroacupuncture improves cognitive deficits and activates PPAR-γ in a rat model of Alzheimer’s disease. Acupunct Med. 2017;35(1):44–51.
- Zhao LH, Chen SHJ, Chen H, et al. Anti-aging effects of moxa cone moxibustion/As a free radical scavenger complement. Neural Regen Res. 2011; 6:919–924.
- Xu SHY, Bian RL, Xiu C. Experimental pharmacological methodology. 3rd ed. Beijing: People's Medical Publishing House Co. LTD; 2001.
- Lin WZ, Wang P. Experimental acupuncture science. 1st ed. Shanghai: Shanghai Scientific & Technical Publishers; 1999.
- Hu YL. Animal acupuncture practical handbook. 1st ed. Beijing: China Agriculture Press; 2003.
- Morris RG, Garrud P, Rawlins JN, et al. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297(5868):681–683.
- Bao XM, Shu SY. The stereotaxic atlas of the rat brain. Beijing: People's Medical Publishing House Co. LTD; 1991.
- Shiraki-Iida T, Iida A, Nabeshima Y, et al. Improvement of multiple pathophysiological phenotypes of klotho (kl/kl) mice by adenovirus-mediated expression of the klotho gene. J Gene Med. 2000;2(4):233–242.
- Uchida A, Komiya Y, Tashiro T, et al. Neurofilaments of Klotho, the mutant mouse prematurely displaying symptoms resembling human aging. J Neurosci Res. 2001;64(4):364–370.
- Rubinek T, Wolf I, Modan-Moses D. The longevity hormone klotho is a new player in the interacion of the growth hormone/insulin-like growth factor 1 axis. Pediatr Endocrinol Rev. 2016;14(1):9–18.
- Chiurchiù V, Orlacchio A, Maccarrone M. Is modulation of oxidative stress an answer? the state of the art of redox therapeutic actions in neurodegenerative diseases. Oxid Med Cell Longev. 2016; 2016:7909380–7909390.
- Kandimalla R, Thirumala V, Reddy PH. Is Alzheimer's disease a type 3 diabetes? A critical appraisal. Biochim Biophys Acta. 2016; 1863(5):1078–1089.
- Sadagurski M, White MF. Integrating metabolism and longevity through insulin and IGF1 signaling. Endocrinol Metab Clin North Am. 2013;42(1):127–148.
- Augustin H, McGourty K, Allen MJ, et al. Impact of insulin signaling and proteasomal activity on physiological output of a neuronal circuit in aging Drosophila melanogaster. Neurobiol Aging. 2018; 66:149–157.
- Vitale G, Barbieri M, Kamenetskaya M, et al. GH/IGF-I/insulin system in centenarians. Mech Ageing Dev. 2017;165(Pt B):107–114.
- Hölscher C. Insulin, incretins and other growth factors as potential novel treatments for Alzheimer's and Parkinson's diseases. Biochm Soc Trans. 2014;42(2):593–599.
- Ratajczak MZ, Bartke A, Darzynkiewicz Z. Prolonged growth hormone/insulin/insulin-like growth factor nutrient response signaling pathway as a silent killer of stem cells and a culprit in aging. Stem Cell Rev and Rep. 2017; 13(4):443–453.
- Logan S, Pharaoh GA, Marlin MC, et al. Insulin-like growth factor receptor signaling regulates working memory, mitochondrial metabolism, and amyloid-β uptake in astrocytes. Mol Metab. 2018; 9:141–155.
- Brunet A. [Aging and the control of the insulin-FOXO signaling pathway]. Med Sci (Paris). 2012;28(3):316–320.
- Lim SW, Jin L, Luo K, et al. Klotho enhances FOXO3-mediated manganese superoxide dismutase expression by negatively regulating PI3K/AKT pathway during tacrolimus-induced oxidative stress. Cell Death Dis. 2017;8(8):e2972–e2984.
- Kuang X, Chen YS, Wang LF, et al. Klotho upregulation contributes to the neuroprotection of ligustilide in an Alzheimer's disease mouse model. Neurobiol Aging. 2014;35(1):169–178.
- Hebei Medical College. Commentary on miraculous pivot. 2nd ed. Beijing: People's Medical Publishing House; 2013.
- Zhang J. Commentary on the compendium of acupuncture and moxibustion. 2nd ed. Beijing: People's Medical Publishing House; 2013.
- Kenawy S, Hegazy R, Hassan A, et al. Involvement of insulin resistance in D-galactose-induced age-related dementia in rats: Protective role of metformin and saxagliptin. PLoS One. 2017;12(8):e0183565–e0183585.
- Gong YS, Guo J, Hu K, et al. Ameliorative effect of lotus seedpod proanthocyanidins on cognitive impairment and brain aging induced by D-galactose. Exp Gerontol. 2016; 74:21–28.
- Marcus DL, Strafaci JA, Freedman ML. Differential neuronal expression of manganese superoxide dismutase in Alzheimer's disease. Med Sci Monit. 2006;12(1):BR8–14.
- Xu H, Zhao B, Cui Y, et al. Effects of Moxa Smoke on Monoamine Neurotransmitters in SAMP8 Mice. Evid Based Complement Alternat Med. 2013;2013:1–178072.
- Liu J, Zhao B, Cui Y, et al. Effects of Shenque Moxibustion on Behavioral Changes and Brain Oxidative State in Apolipoprotein E-Deficient Mice. Evid Based Complement Alternat Med. 2015;2015:804804–804811.
- Cohen E, Dillin A. The insulin paradox: aging, proteotoxicity and neurodegeneration. Nat Rev Neurosci. 2008;9(10):759–767.
- Papaconstantinou J. Insulin/IGF-1 and ROS signaling pathway cross-talk in aging and longevity determination. Mol Cell Endocrinol. 2009;299(1):89–100.
- Bian A, Neyra JA, Zhan M, et al. Klotho, stem cells, and aging. Clin Interv Aging. 2015; 10:1233–1243.