 ?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Depression, a mood disorder characterized by persistently low mood and feeling of sadness, has been a more and more serious problem today. NLRP3 (the NACHT, LRR and PYD domains-containing protein 3) inflammasome-mediated central inflammation is recently considered to contribute to the pathology of depression. Based on previous findings that ginsenoside compound K (CK), a ginsenoside metabolite, exhibited antidepressant effects in rats, here we studied whether the antidepressant effects of CK are associated with inhibiting the oxidative stress, NLRP3 inflammasome or inflammatory cytokine in hippocampus of mice exposed to chronic unpredictable mild stress (CUMS). CK (3, 10 and 30 mg/kg) was intragastrically administered for 4 weeks. The depression-like behaviors, oxidative stress parameters, inflammatory cytokines and NLRP3 expression in the hippocampus were evaluated. The results showed that after 4 weeks of administration, CK (30 mg/kg) alleviated the depressant-like behaviors, including shorter immobility time in both the forced swimming test and the tail suspension test, and increased the sucrose preference. In addition, the levels of IL-1β, IL-18 in the hippocampus were significantly decreased in CK-treated groups. The western blot and immunohistochemistry results revealed that the expression levels of NLRP3 inflammasome components (NLRP3 and cleaved caspase-1) were down-regulated in hippocampus of CK-treated mice. These results demonstrate that the antidepressant effect of CK is, at least partially, associated with suppression of oxidative stress-induced NLRP3 inflammasome activation and inhibition of the release of corresponding inflammatory cytokines in the hippocampus.
Introduction
People are suffering more and more stress and challenges with the competition becoming increasingly fierce in modern society. Sustained stress seriously affects physical and mental health and has become an important cause of depression. There are currently more than 350 million people worldwide suffering from depression [Citation1]. Depression causes a heavy economic and social burden on families and society, and it has been a major factor leading to the global burden of disease.
The pathogenesis of depression has not been thoroughly understood so far. Many scholars have proposed several hypotheses, including the monoamine neurotransmitter imbalance, neural endocrine disorder and neural plasticity [Citation2]. These hypotheses can partly explain the pathogenesis of depression, but they all have their limitations. At present, anti-inflammatory treatment has gradually become a new research interest of depression prevention and treatment [Citation3]. A large amount of evidence indicates that interleukin-1β (IL-1β), an important cytokine mediated by NLRP3 inflammasome activation, plays an important role in the pathogenesis of depression, and that is associated with stress-induced emotional disorders and behavioral manifestations [Citation4].
Ginsenoside compound K (CK, 20-O-β-(glucopyranosyl)-20(S)-protopanaxadiol), the metabolite of panaxadiol ginsenosides, has been gaining considerable attention, because it shows various biological activities such as anticancer, anti-inflammation and hepatoprotective effects [Citation5]. Our previous study demonstrated, for the first time, that ginsenoside CK could improve the depressive-like behavior in rats with CUMS (chronic unpredictable mild stress)-induced depression, which may be related to the regulation of hippocampal and prefrontal cortical neurotransmitters [Citation6]. In addition, our series of studies on ginsenoside CK also found that it could alleviate kidney damage in diabetic nephropathy rats by inhibiting NF-κB and NLRP3 inflammasome signalling pathways [Citation7]. Therefore, given the available experimental evidence, we speculate that ginsenoside CK may attenuate central inflammation by inhibiting NLRP3 inflammasome of the central nervous system, which may be another mechanism of the antidepressant effect of CK. In the present study, the protective effect of CK on depression in mice with CUMS-exposure was investigated as a follow-up study. We established a mouse model with CUMS depression to confirm the effects of CK on depression and to explore the effects on oxidative stress, cytokines release and NLRP3 protein expression in hippocampus.
Materials and methods
Materials
Ginsenoside compound K (CAS: 39262-14-1, B21045) was provided by Shanghai Yuanye Biotechnology Co., Ltd (Shanghai, China). Malondialdehyde (MDA) assay kit, superoxide dismutase (SOD) assay kit and Glutathione peroxidase (GSH-Px) assay kit were from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). Interleukin-1β assay kit and IL-18 assay kit were from eBioscience (San Diego, California, USA). Anti-NLRP3 antibody, anti-cleaved caspase-1 antibody was from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Horseradish peroxidase-conjugated IgG was from Zsbio (Beijing, China). Multifunctional mice locomotion activity recorder (YLS-1A) was provided by Shandong Academy of Medical Sciences equipment station (Shandong, China). Mini-Protean Tetra Electrophoresis System (1658000) was from Bio-Rad Company (USA).
Animals
Male KM mice, weighing 18–22 g, were provided by Jilin University Laboratory Animal Center (the animal license number: SCXK (2011-0004)) and were raised under SPF (specific-pathogen-free) conditions. Animals were kept in 12-hour light/12-hour dark cycle (light, 8:00 a.m.–8:00 p.m.) and room temperature was kept at 22 ± 2 °C.
Ethics statement
The animal experiments were carried out consistent with the provisions of China Animal Welfare Act and the Guide of NIH Experimental Animal Management. The experiment was approved by the Ethical Committee of Experimental Animals of Changchun University of Chinese Medicine.
Experimental designs
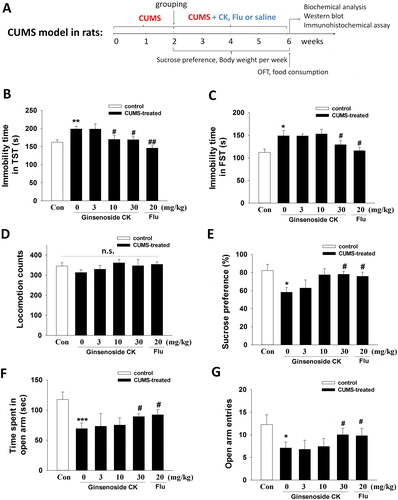
Ninety mice were randomly divided into five groups including CUMS group, CK (3 mg/kg) group, CK (10 mg/kg) group, CK (30 mg/kg) and fluoxetine group (20 mg/kg) group, 15 mice per group. Two weeks after CUMS, saline, CK or fluoxetine was administered once daily for 4 weeks. Depression-related behavioral testing was performed after the end of administration. After all behavioral experiments, mice were decapitated immediately. Nine hippocampal tissue homogenates from each group were used for western blotting and enzyme-linked immunosorbent assay (ELISA), whereas the other six hippocampal tissues were used for immunohistochemistry. The CUMS procedure and drug treatment is shown in .
Figure 1. Schematic representation of the experimental procedure and behavioral test (A). Effects of CK or fluoxetine on immobility time of tail suspension test (B) and forced swimming test (C), locomotor activity (D), sucrose preference test (E), time spent (F) and entries (G) in open arm of high plus maze test in CUMS mice. The values are expressed as means ± SEM (n = 13–15/group). Statistical significance, *p < 0.05, **p < 0.01, ***p < 0.001 compared with the control group; #p < 0.05, ##p < 0.01 compared with the CUMS group; n.s. represents no significance among all groups.
Tail suspension test (TST)
One hour after the administration, the mice were fixed with a strip at a distance of 2 cm from the tip of the tail to make it suspend. After a 2 min adaptation, the immobility time within 4 min was recorded. The criterion is that the animal stops struggling and the body is vertically suspended.
Forced swim test (FST)
One day after TST, the mice were placed in a plexiglass bucket with height of 25 cm, diameter of 12 cm, water depth of 10 cm and water temperature of 23 ± 2 °C. After 2 min of adaptation, the accumulate immobility time within 4 min was recorded (keep the head floating on the water without struggling).
Elevated plus-maze (EPM) test
The mouse High Plus maze consists of two open arms and two closed arms (length × width: 30 cm × 5 cm), with a central square area of 5 cm × 5 cm. At the beginning of the experiment, the mice were placed on the central platform with the head facing an open arm. The exploratory behavior was recorded for 5 min, including the number of times a mouse entered the open and closed arms, and the time spent in each arm. At the end of each experiment, 75% alcohol was used to wipe the maze, and the next mouse was placed.
CUMS procedure
The CUMS model was set up as described previously [Citation6]. The stimulation included: fasting for 24 h, water deprivation for 24 h, illumination for 24 h, 4 °C cold water for 5 min, wet cage (200 mL water/cage, 24 h), titled cage (45°, 24 h), clip tail (1 min), noise (30 min). Mice were stimulated daily by two random stresses for 6 weeks.
Sucrose preference test
Mice in each group were fasted for 24 h, and then the sugar consumption experiment was carried out. During the experiment, the animals were given one bottle of 1% sucrose water (100 mL) and another bottle of water (100 mL). After 3 h drinking, the consumption of sucrose water and distilled water was measured. The sucrose preference was calculated by the following formula:
Oxidative stress and antioxidant activity assays
The SOD and GSH-Px activities and MDA content in hippocampus tissues were measured by ELISA. The homogenate was centrifuged at 3000 r/min for 10 min to separate the supernatant and the supernatant was preserved at 4 °C for use. The MDA, SOD and GSH-Px were measured by using commercially available kits according to the manufacturer’s instructions (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Western-blot assay
Mice were sacrificed by cervical dislocation. The hippocampal protein was extracted with RIPA lysate and tolyl fluoride (BI YUN TIAN Biotechnology Institute P0013B). Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE; 10%) was employed with a 20 μg sample volume and 80/120 V constant voltage for 150 min, which was followed by electrotransfer to a PVDF (polyvinylidene difluoride) membrane (0.45 μm). The membrane was blocked by 5% skim milk under 37 °C for 1 h. After washing three times, the membrane was incubated with β-actin antibody (1:2000), cleaved caspase-1 (1:500), NLRP3 (1:500) in TBST (tris-buffered saline with Tween 20) plus 5% milk, followed by incubation with horseradish peroxidase-conjugated IgG as the secondary antibody. The membrane was then washed with TBST buffer (3 × 10 min) before enhanced chemiluminescence (ECL) developing reagent was added. Finally, the relative expression of cleaved caspase-1 and NLRP3 protein was calculated by using β-actin as an internal reference. The level of staining density was quantified by Image-Pro Plus 6.0.
Inflammatory cytokines measurements in hippocampus
The levels of IL-1β and IL-18 proteins were determined by an enzyme-linked immunosorbent assay (ELISA) kit (eBioscience, San Diego, California, USA) according to the manufacturer’s protocol. Nine mice in each group were used to determine the level of inflammatory cytokines.
Immunohistochemistry of hippocampus
Main steps: (1) Mice hippocampus tissue sections were placed at room temperature for 30 min, and then washed with TBST (Shanghai Boyi Biotechnology Co., Ltd.) of pH 7.4. (2) Endogenous peroxidase was blocked with 3% hydrogen peroxide and incubated with non-immune rabbit serum for 10 min at room temperature. (3) Rabbit anti-mice NLRP3 polyclonal antibody (1:100), rabbit anti-mice cleaved caspase-1 polyclonal antibody (1:200) were added to the section, 4 °C overnight. (4) Subsequently they were allowed to react with the biotinylated secondary antibody at 37 °C for 2 h, then cover slipped. The hippocampal CA1 regions and the prefrontal cortex were examined. We used six mice in each group for immunohistochemistry, and we assessed the expression of the target protein with six slides per mice.
Statistical analysis
Statistical analysis was carried out by SPSS 13.0 software (SPSS Inc., Chicago, IL, USA). All values were expressed as means with standard error of the means (± SEM). One-way analysis of variance (ANOVA) was used to compare the difference among three or more groups, with post-hoc Tukey’s HSD test or Dunnett’s t test for individual group comparisons. The level of significance was set at p < 0.05.
Results and discussion
CK attenuates depressive-like behavior of CUMS mice
Our previous study [Citation6] found that ginsenoside CK could improve the depressive-like behavior in rats with CUMS-induced depression. In the present study, we revalidated the antidepressant effect of ginsenoside CK in a CUMS model in mice, and evaluated the central inflammation mechanism of action of CK by examining the expression of NLRP3 inflammasome components (NLRP3 and caspase-1) and the inflammatory cytokines IL-1β and IL-18 in the hippocampus. As shown in , after the 6-week CUMS process, the immobility time of TST and FST () in the model group was significantly increased (p < 0.01, p < 0.05), while the sucrose preference () was significantly decreased (p < 0.05), indicating that the depression model was established (one-way ANOVA, TST: F(5, 84) = 3.19, p < 0.05; FST: F(5, 84) = 1.33, p < 0.05; sucrose preference: F(5, 81) = 1.40, p < 0.05). In addition, the time spent and the entries () in open arm were significantly reduced in CUMS mice in the high plus maze test (one-way ANOVA, time spent: F(5, 82) = 2.46, p < 0.05; entries: F(5, 82) = 2.70, p < 0.05), indicating that anxiety also occurred simultaneously. After 4 weeks of CK administration, compared with the model group, ginsenoside CK (30 mg/kg) significantly increased the immobility time of both of TST and FST (p < 0.01, p < 0.05), increased the sucrose preference ratio (p < 0.05), and significantly reduced the time spent and the entries in open arm in the high plus maze test (p < 0.05). However, there was no significant difference in locomotion activity among these groups (, one-way ANOVA, F(5, 84) = 0.71, p > 0.05). These data showed that administration of CK prevented CUMS-induced depressive behaviors and anxiety-like behaviors in mice exposed to CUMS. These findings are consistent with our previous study in rats [Citation6].
CK alleviates CUMS-induced oxidative stress
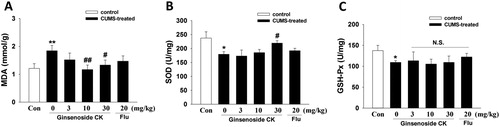
Mounting evidence suggests that the pathophysiology of depression is associated with inflammatory processes in the central nervous system characterized by microglial activation, cytokine release, increased oxidative stress and changes in glutathione system regulation [Citation8–10]. The aggravation of the inflammatory process can lead to a significant increase in pro-inflammatory cytokines such as IL-1β, reactive oxygen species (ROS) and nitric oxide. There is evidence that excess ROS may be associated with immune activation [Citation11], increased oxidation of monoaminergic neurotransmitters and lipid peroxidation in patients with depression [Citation12]. Another recent study found that in depression associated with posttraumatic stress disorder or not, the cytokines and oxidative stress were significantly different in depression patients [Citation13], suggesting that oxidative stress contributes to the pathogenesis of depression. In addition, the levels of anti-oxidant substances are reduced in patients with depression [Citation14]. Therefore, the level of oxidative stress in the hippocampus was examined in the present study (). Compared with the control group, the hippocampal MDA level of model group was significantly increased, while the activity of the antioxidant enzymes was decreased (one-way ANOVA, MDA: F(5, 47) = 9.81, p < 0.01; SOD: F(5, 48) = 4.06, p < 0.05; GSH-Px: F(5, 47) = 0.34, p > 0.05). The oxidative stress-related indices, including the content of MDA () and the activity of SOD (), were significantly improved in mice treated with CK (30 mg/kg), indicating that CK could alleviate the oxidative stress and enhance the antioxidant ability in hippocampus. However, ginsenoside CK had no significant effect on the GSH-Px level (, p > 0.05). Our previous study found that CK inhibits the renal oxidative stress possibly by down-regulating the expression of renal NADPH oxidase in diabetic nephropathy mice [Citation7]. In addition to our observation, the antioxidant activity of CK has also been reported by other scholars. Park et al. [Citation15] found that CK could inhibit the production of ROS and the NF-κB signalling pathway in LPS-activated BV-2 microglia.
Figure 2. Effects of CK or fluoxetine (Flu) on MDA levels (A), SOD activity (B) and GSH-Px content (C) in hippocampus tissues measured by ELISA. All data are expressed as means ± SEM (n = 8–9/group). Significant differences: *p < 0.05, **p < 0.01 compared with the control group; #p < 0.05, ##p < 0.01 compared with the CUMS group. N.S., non-significant.
CK decreases IL-1β and IL-18 in the hippocampus of CUNS mice
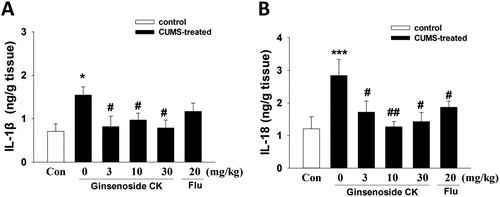
Oxidative stress can activate immune and inflammatory processes, which contribute to the development of depression. Inflammatory cytokines IL-1β in the prefrontal cortex of depressed rats were involved in the pathophysiological process of depression [Citation16]. Lu et al. found microglia activation and elevated levels of IL-6, IL-1β and TNF-α in the hippocampus of CUMS-exposed mice [Citation17]. Therefore, we focused on examining the hippocampus in the present study. Here, the levels of IL-1β and IL-18 in the hippocampus were detected by ELISA. As shown in , the content of IL-1β and IL-18 in the hippocampus of mice in the model group was significantly higher than that in the control group. CK (3, 10 and 30 mg/kg) could reduce the level of IL-1β and IL-18 (one-way ANOVA, IL-1β: F(5, 48) = 13.30, p < 0.001; IL-18: F(5, 48) = 16.25, p < 0.001). Further, the high dose CK (30 mg/kg) could decrease the level of these two inflammatory cytokines even close to the level in the control group. The data that CUMS exposure induced elevated levels of IL-1β protein in hippocampus are consistent with our previous diabetic nephropathy study in renal tissue [Citation7]. The anti-inflammatory and neuroprotective effects of CK have also been supported by other studies. CK (oral, 30 mg/kg) inhibited lipopolysaccharide (LPS)-induced microglial activation in ischemic brain tissue in mice by inhibition of inflammatory factors by regulating ROS/MAPK signalling pathways [Citation18].
Figure 3. Effects of CK or fluoxetine (Flu) on IL-1β (A) and IL-18 (B) in the hippocampus of CUNS mice. The data were measured by ELISA. All data are expressed as means ± SEM (n = 9/group). Significant differences: *p < 0.05, ***p < 0.001 compared with the control group; #p < 0.05, ##p < 0.01 compared with the CUMS group.
CK inhibits the expression of NLRP3 and cleaved caspase-1 in hippocampus of CUMS mice
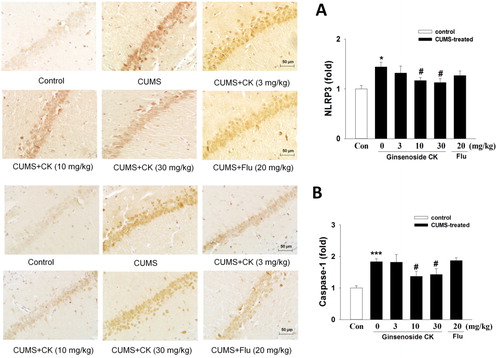
Pro-IL-1β was induced by priming signals such as NLRP3 inflammasome, which activation requires a second stimulation such as ROS [Citation19]. Pro-IL-1β is then processed into mature active forms through NLRP3 in cells. Based on different evidence, it is assumed that the NLRP3 inflammasome is a bridge between oxidative stress and depression. On the one hand, stress leads to the production of ROS and adenosine triphosphate, which are key activators of NLRP3 inflammasome. On the other hand, IL-1β exposure causes depressive behaviors, including pleasure deficiency and inhibition of social exploration. As shown in , the western blot results showed that NLRP3 and cleaved caspase-1 expression levels in the hippocampus tissue of CUMS mice were increased significantly (p < 0.05). Compared with the model group, CK could significantly down-regulate the expression of NLRP3 and cleaved caspase-1. As shown in , the inhibitory effect of CK on the expression of NLRP3 protein was also verified in the immunohistochemical experiment of the hippocampus CA1 region. These data suggest that the administration of CK could suppress the activation of the NLRP3 signalling pathway in the hippocampus tissue of CUMS mice. Our previous study found that the components (NLRP3, ASC and caspase-1) of NLRP3 inflammasome were excessively expressed in the kidney tissue of diabetic nephropathy mice induced by high-fat diet/streptozotocin, and the secretion of IL-1β and IL-18 was also increased, and these could be inhibited by the administration of CK [Citation7]. Thus, we speculated that inhibiting the activation of NLRP3 inflammasome and subsequently blocking the secretion of inflammatory cytokines might be, at least, one of the mechanisms underlying the protective effect of CK in CUMS-induced depression.
Figure 4. Effects of CK or fluoxetine (Flu) on NLRP3 (A) and cleaved caspase-1 (B) expression in the hippocampus region of CUMS mice determined by western blot. The relative optical densities normalized to β-actin are shown below the bands. Data are represented as means ± SEM (n = 4/group). Significant differences: *p < 0.05 compared with the control group; #p < 0.05 compared with the CUMS group.
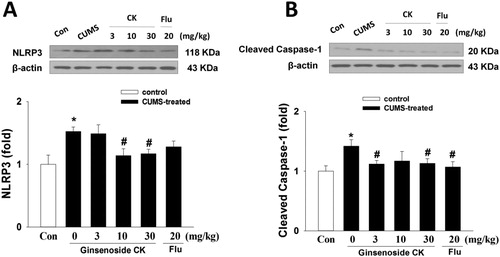
Figure 5. Effects of CK or fluoxetine (Flu) on the NLRP3 (A) and caspase-1 (B) expression in the hippocampus CA1 region of CUMS mice by immunohistochemistry. Positive cells are represented as brown spots. Bar = 50 µm. The level of staining density was quantified by Image-Pro Plus 6.0 and presented as means ± SEM (n = 4/group). Significant differences:*p < 0.05, ***p < 0.001 compared with the control group; #p < 0.05 compared with the CUMS group.
Conclusions
The obtained results suggest that ginsenoside compound K exhibits antidepressant effects in mice exposed to chronic unpredictable mild stress. The protective effects of CK may be associated with its antioxidant activity and modulation of NLRP3 inflammasome in the hippocampus.
Disclosure statement
There are no conflicts of interest.
Funding
This study was supported by the Research Program of Jilin Provincial Administration of Traditional Chinese Medicine (grant number: 2019178), National College Students' Innovation and Entrepreneurship Training Program (grant number: 201910199002) and Changchun University of Traditional Chinese Medicine Cultivation Fund Project (grant number: 2018KJ19).
References
- Nielsen B, Cejvanovic V, Wortwein G, et al. Increased oxidation of RNA despite reduced mitochondrial respiration after chronic electroconvulsive stimulation of rat brain tissue. Neurosci Lett. 2019;690:1–5.
- Postal M, Appenzeller S. The importance of cytokines and autoantibodies in depression. Autoimmun Rev. 2015;14(1):30–35.
- Brundin L, Achtyes E. Has the time come to treat depression with anti-inflammatory medication? Acta Psychiatr Scand. 2019;139(5):401–403.
- Morrison FG, Miller MW, Wolf EJ, et al. Reduced interleukin 1A gene expression in the dorsolateral prefrontal cortex of individuals with PTSD and depression. Neurosci Lett. 2019;692:204–209.
- Yang XD, Yang YY, Ouyang DS, et al. A review of biotransformation and pharmacology of ginsenoside compound K. Fitoterapia. 2015;100:208–220.
- Song W, Guo Y, Jiang S, et al. Antidepressant effects of the ginsenoside metabolite compound K, assessed by behavioral despair test and chronic unpredictable mild stress model. Neurochem Res. 2018;43(7):1371–1382.
- Song W, Wei L, Du Y, et al. Protective effect of ginsenoside metabolite compound K against diabetic nephropathy by inhibiting NLRP3 inflammasome activation and NF-kappaB/p38 signaling pathway in high-fat diet/streptozotocin-induced diabetic mice. Int Immunopharmacol. 2018;63:227–238.
- Gonzalez-Parra S, Dauden E. Psoriasis and depression: the role of inflammation. Actas Dermosifiliogr. 2019;110(1):12–19.
- Felger JC. Role of inflammation in depression and treatment implications. Handb Exp Pharmacol. 2019;250:255–286.
- Irwin MR, Piber D. Insomnia and inflammation: a two hit model of depression risk and prevention. World Psychiatry. 2018;17(3):359–361.
- Palta P, Samuel LJ, Miller ER, 3rd, et al. Depression and oxidative stress: results from a meta-analysis of observational studies. Psychosom Med. 2014;76(1):12–19.
- Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41(12 Pt 2):1819–1828.
- Ogłodek EA, Just MJ. The association between inflammatory markers (iNOS, HO-1, IL-33, MIP-1β) and depression with and without posttraumatic stress disorder. Pharmacol Rep. 2018;70(6):1065–1072.
- Maes M, De Vos N, Pioli R, et al. Lower serum vitamin E concentrations in major depression. Another marker of lowered antioxidant defenses in that illness. J Affect Disord. 2000;58(3):241–246.
- Park JS, Park EM, Kim DH, et al. Anti-inflammatory mechanism of ginseng saponins in activated microglia. J Neuroimmunol. 2009;209(1–2):40–49.
- Kupfer DJ, Frank E, Phillips ML. Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Lancet. 2012;379(9820):1045–1055.
- Lu M, Yang JZ, Geng F, et al. Iptakalim confers an antidepressant effect in a chronic mild stress model of depression through regulating neuro-inflammation and neurogenesis. Int J Neuropsychopharm. 2014;17(09):1501–1510.
- Park JS, Shin JA, Jung JS, et al. Anti-inflammatory mechanism of compound K in activated microglia and its neuroprotective effect on experimental stroke in mice. J Pharmacol Exp Ther. 2012;341(1):59–67.
- Xiao Y, Xu W, Su W. NLRP3 inflammasome: a likely target for the treatment of allergic diseases. Clin Exp Allergy. 2018;48(9):1080–1091.
