Abstract
This study investigated the phenotype and function of natural killer T (NKT) cells infiltrating cervical cancer tissues, in an attempt to understand the regulation of NKT cells by cervical cancer cells. Forty-two patients with cervical cancer were included. Flow cytometry was used to analyze the percentage of NKT cells in tumour tissues and its correlation with clinical staging and lymphatic metastasis. The expression of surface receptor and effector molecules on infiltrating NKT cells was determined. The changes of the phenotype, subtype and cytotoxicity of NKT cells were investigated. The regulation of NKT cells by cervical cancer cells was investigated by co-culture with HeLa or SiHa cells. The effect of TGF-β1 on NKT activity regulated by cervical cancer cells was studied. The results showed that the infiltration of NKT cells in cervical cancer tissues was significantly higher than that in tumour-adjacent tissues, and the degree of infiltration was negatively correlated with the progression of the disease. The activity and killing effect of infiltrating NKT were inhibited in cervical cancer tissues, leading to increase of CD4+NKT cells and decrease of CD8−CD4−NKT cells. The activity of NKT cells was down-regulated by co-culture with cervical cancer cells, but subtypes of NKT cells were not altered. Cervical cancer cells inhibited the function of NKT cells by secreting TGF-β1. The study demonstrates that the infiltration of NKT cells in cervical cancer tissues is increased significantly and negatively correlated with tumour progression.
Introduction
Cervical cancer is a common gynaecological tumour, and its incidence is the second highest among all malignant tumours in the female reproductive system [Citation1,Citation2]. Epidemiological statistics show that more than 500,000 new cases of cervical cancer are discovered worldwide each year, and most of them are in developing countries [Citation3]. In China, more than 130,000 new cases and 60,000 deaths of cervical cancer are reported every year [Citation4]. In recent years, the incidence and prognosis of cervical cancer have improved significantly with the use of HPV vaccine, the development of early screening techniques for cervical cancer and the improvement of the clinical treatment level [Citation5]. However, the potential risk of cervical cancer is still high, and seriously endangers women's health [Citation6]. It is believed that tumour invasion and metastasis are the main causes for poor prognosis of cervical cancer [Citation7]. Therefore, further studies on the molecular mechanism of invasion and metastasis of cervical cancer are of great significance for the development of new therapeutic strategies for the disease.
Immune surveillance is an important prerequisite for distant invasion and metastasis of tumour cells [Citation8]. It is reported that various solid tumours are infiltrated with a large number of lymphocytes, which are positively correlated with the prognosis of patients [Citation9]. Although tumour tissues are infiltrated by a large number of immune cells, it is still difficult to prevent the invasion and metastasis of tumour cells, suggesting that tumour cells can escape surveillance and killing by immune cells [Citation10,Citation11]. It is also discovered that tumour cells can induce differentiation of immune cells [Citation12], for example, TAM2 macrophages and Tregs [Citation13,Citation14]. Therefore, further understanding of the interaction between tumour cells and immune cells provides us with the possibility of screening new targets. Of note, in-depth study on phenotypic and functional changes of immune cells in tumour tissues is of great significance for understanding the metastasis of tumours.
Natural killer T (NKT) cells are a T lymphocyte subgroup expressing both T cell receptors and NK cell receptors [Citation15]. Unlike traditional T lymphocytes and NK cells, NKT cells have the characteristics of both T cells and NK cells [Citation16]. For example, NKT cells can identify lipids on cell surface presented by CD1d molecule, get activated after directly identifying foreign antigens and exert killing effect by releasing granzyme and perforin [Citation17]. In human bodies, NKT cells can be divided into CD4+CD8−, CD4−CD8+ and CD4−CD8− NKT cells according to the expression of CD4 and CD8 molecules [Citation18]. NKT cells play important roles in immune regulation besides direct killing effect, and activated NKT cells can secrete large amounts of cytokines with immune regulation, such as interleukin (IL)-4, IL-13, IL-10 (Th2 type) and interferon (IFN)-γ (Th1 type) to regulate the equilibrium state of Th1/Th2 cells [Citation19,Citation20]. In the meantime, NKT cells also play important roles in enhancing the innate immune response of NK cells and macrophages and the acquired immune response of CD4+ and CD8+ T cells (mainly IFN-γ) [Citation21,Citation22]. Therefore, NKT cells are considered to be a bridge linking innate immune response and acquired immune response. It is discovered that NKT cells play important roles in inhibiting autoimmune diseases (such as type 1 diabetes mellitus) and rheumatic diseases, anti-infective immunity and cancer therapies [Citation23,Citation24]. In the present study, the phenotype and function of CD3+CD56+NKT cells in cervical cancer tissues are preliminarily studied, and the interaction mechanism between CD3+CD56+NKT cells and cervical cancer cells is discussed.
Subjects and methods
Patients
A total of 42 patients with cervical cancer who received surgical treatments at Maternal and Child Health Hospital of Jiangxi Province between May 2016 and December 2017 were included in the present study. Both tumour tissues and tumour-adjacent tissues were obtained from all patients. The patients were aged between 32 and 57 years (mean, 45.8 years). None of the patients had previous history of malignant tumours, chemotherapy, radiotherapy, autoimmune diseases or long-term intake of drugs. In addition, 25 patients had lymph node metastasis (N1 group) and 17 patients had no lymph node metastasis (N0 group). According to the staging and grading criteria published by The Union for International Cancer Control (UICC) in 2003 [Citation25], 14 patients were at stage I, 13 patients were at stage II, 10 patients were at stage III and five patients were at stage IV. Peripheral blood from 10 healthy subjects was used for flow cytometry sorting of NKT cells.
Ethics statement
All procedures performed in this study were approved by the Ethics Committee of Maternal and Child Health Hospital of Jiangxi Province. Written informed consent was obtained from all patients or their families.
Cells
Cervical cancer HeLa and SiHa cell lines were purchased from Cell Bank, Chinese Academy of Sciences (Shanghai, China), and cultured in RPMI-1640 medium supplemented with 10% fetal bovine serum (FBS), 100 IU/mL penicillin and 100 IU/mL streptomycin at 37 °C, 5% CO2 and 70% humidity. The cells were passaged every three days, and those in logarithmic growth were collected for experiments.
Using Ficoll lymphocyte separation fluid (Sigma-Aldrich, St. Louis, MO, USA), peripheral blood mononuclear cells (PBMCs) were separated according to the manufacturer’s manual. Briefly, 3 mL peripheral blood was gently added on top of 3 mL Ficoll lymphocyte separation fluid before centrifugation at 400–650g and 4 °C for 20 min. Then, the middle mist-like layer was aspirated and resuspended with 10 mL phosphate-buffered saline (PBS). After centrifugation at 250g for 10 min, the supernatant was discarded and the cell pellet was resuspended in 5 mL PBS, followed by additional centrifugation at 250g for 10 min. After discarding supernatant, the cells were resuspended in 200 μL PBS. Afterwards, the 200 μL cell suspension was mixed with 20 μL FITC-CD3 antibodies and APC-CD56 antibodies before incubation at room temperature for 15 min. Then, NKT cells were purified using a flow cytometric sorter. The resulting NKT cells were cultured in RPMI-1640 medium supplemented with 500 IU IL-2, 100 IU IL-15 and 10% FBS at 37 °C and 5% CO2 for 5 days before use.
Detection of surface receptors and effector molecules of NKT cells by flow cytometry
Tumour tissues and tumour-adjacent tissues were ground and prepared into single-cell suspension, and infiltrating monocyte lymphocytes were separated using lymphocyte separation solution. Peripheral blood was subjected to separation of lymphocytes, which were adjusted to 1 × 106 per well. NKT cells (1 × 105) were added into 1.5-mL Eppendorf tubes and the total volume was adjusted to 100 μL. Then, 20 μL fluorescent-labelled antibodies (activated receptors: NKp30, NKp44, NKp46, CD16 and NKG2D; inhibitory receptors: 158b and G2A; proliferation marker: Ki67; typing markers: CD4 and CD8; intracellular effector molecules: GranB, Perforin and IL-10) () were added before incubation at room temperature for 30 min. After washing with cold PBS three times, NKT cells were centrifuged at 800g for 5 min and collected. Finally, 200 μL cold PBS was added to resuspend the labelled cells, followed by flow cytometry (FACSCanto II; BD Biosciences, Franklin Lakes, NJ, USA).
Table 1. Fluorescent-labelled antibodies.
Co-culture of cervical cancer cells and NKT cells
For the co-culture experiments, 1 × 105 cervical cancer cells were seeded into 24-well plates and cultured at 37 °C and 5% CO2. Purified NKT cells were added into transwell chambers with 0.3 μm pore diameter, and the chambers were placed into the 24-well plates containing cervical cancer cells. The volume of the upper chamber was 200 μL and that of the lower chamber was 500 μL. After 48 h, NKT cells in the upper chamber were used for killing experiments and analysis of phenotype.
Detection of NKT cell killing effect by flow cytometry
HeLa or SiHa cells (2 × 104) were mixed with respective NKT cells at a ratio of 1:3 before incubation in 24-well plates for 24 h. Then, the supernatant was collected and centrifuged at 12,000g and 4 °C for 10 min. The resulted supernatant was aliquoted and stored at −80 °C before use. These cells were subsequently analyzed using flow cytometry.
Detection of apoptosis by flow cytometry
Tumour cells (1 × 106) in each group were washed with cold PBS twice, and subjected to flow cytometry using ANXN V FITC APOPTOSIS DTEC KIT I (BD Biosciences, Franklin Lakes, NJ, USA) following the manufacturer’s manual to detect cell apoptosis. Cells with ANNEXIN V-positive values were early apoptotic cells, those with PI-positive values were necrotic cells and those with double positive values were late apoptotic cells.
Immunohistochemistry
Cervical cancer cells (0.5 × 105) were seeded onto slides in 24-well plates. On the next day, the cells were washed with cold PBS and fixed with cold 4% paraformaldehyde for 10 min. After washing twice with cold PBS for 5 min, the cells were lysed with 0.5% Triton X-100 for 3 min, followed by washing with PBS twice. Then, IL6 and TGF-β1 primary antibodies (1:50 dilution) were added for incubation at 4 °C overnight. On the next day, inactivation of endogenous oxidase, incubation with secondary antibody and DAB (3,3-diaminobenzidine) staining were performed. The samples were observed under a light microscope.
Statistical analysis
The results were analyzed using SPSS 17.0 statistical software (IBM, Armonk, NY, USA). Measurement data were expressed as mean values with standard deviations (±SD). Data were tested for normality. Multigroup measurement data were analyzed using one-way analysis of variance (ANOVA). In case of homogeneity of variance, Least Significant Difference and Student–Newman–Keuls methods were used; in case of heterogeneity of variance, Tamhane’s T2 or Dunnett’s T3 method was used. Comparison between two groups was carried out using Student’s t-test. p < .05 indicated statistically significant differences.
Results and discussion
Infiltration of CD3+CD56+ NKT cells in cervical cancer tissues is significantly higher than that in tumour-adjacent tissues, and the degree of infiltration is negatively correlated with the progression of cervical cancer
Postoperative recurrence and distant metastasis are the main causes of poor prognosis in patients with cervical cancer, and this process is closely related to the maintenance of cancer stem cells, microenvironment, gene mutation and so on [Citation26,Citation27]. It is discovered that a large number of monocyte lymphocytes infiltrate cervical cancer tissues, but they are still unable to prevent the metastasis of cancer cells, indicating that cancer cells have the ability to escape immunological surveillance [Citation28]. CD3+CD56+NKT cells are a kind of natural immune cells with strong heterogeneity [Citation29]. They directly recognize and kill tumour cells, play a role of immune regulation by secreting Th1 or Th2 cytokines and have potential clinical application value in cancer treatment [Citation29]. In the present study, we find that NKT infiltration is increased in cervical cancer tissues and positively correlated with tumour development. In the meantime, the function of CD3+CD56+NKT cells is significantly inhibited. Further studies show that cervical cancer cells can inhibit the function of CD3+CD56+NKT cells by secreting TGF-β1.
Surgery combined with radiotherapy and chemotherapy is an important means of clinical treatment for cervical cancer. Unfortunately, patients often die of recurrence and metastasis after operation [Citation30]. Immunotherapy is a new tumour treatment method that has arisen in recent years. The development of PD1/PDL1, monoclonal antibodies, Car-T, Car-NK and other technologies provides a new direction to improve the prognosis of patients with cervical cancer [Citation31,Citation32]. A large number of infiltrating mononuclear lymphocytes inhibit the metastasis and growth of tumours, but the low activity or immature differentiation of infiltrating mononuclear lymphocytes limit their functions [Citation33,Citation34]. It is important to study the regulation mechanism of infiltrating immune cells and to interfere with their key targets for restoring their immune activity. NKT cells are innate immune cells that can directly kill target cells or secrete cytokines to regulate the activity of immune cells after activation by T cell receptor [Citation35]. It is reported that NKT cells have anti-tumour immunity. For example, co-culture of NKT cells from glioma patients with a mixture of mature dendritic cells and α-galactosylceramide leads to proliferation of NKT cells, which secrete cytokines and activate NK cells and T cells to kill tumour cells [Citation36]. In addition, modification of NKT cells by CAR technology can enhance the killing effect NKT cells on malignant melanoma [Citation37]. At present, NKT cells in cervical cancer are less reported, and their distribution and functional changes in tumour tissues are still unclear.
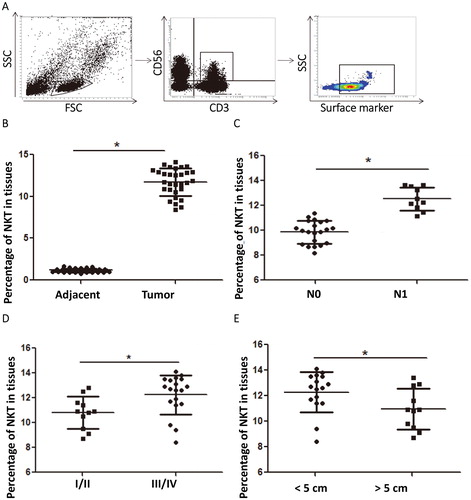
To examine the distribution and clinical relevance of CD3+CD56+ NKT cells in cervical cancer tissues, we performed flow cytometry. The data showed that the percentage of NKT cells in cervical cancer tissues was significantly higher than that in tumour-adjacent tissues (p < .05) (). The percentage of NKT cells in cervical cancer tissues from patients with lymphatic metastasis was significantly higher than that from patients without lymphatic metastasis (p < .05) (). The percentage of NKT cells in tumour tissues form patients at stages III/IV was significantly higher than that from patients at stages I/II (p < .05) (). The percentage of NKT cells in tumour tissues with a diameter over 5 cm was significantly lower than that in tumour tissues with a diameter smaller than 5 cm (p < .05) (). The results suggest that the infiltration of NKT cells in cervical cancer tissues is significantly higher than that in tumour-adjacent tissues, and the degree of infiltration is negatively correlated with the progression of cervical cancer.
Figure 1. Distribution and clinical relevance of CD3+CD56+ NKT cells in cervical cancer tissues. (A) Flow cytometric analysis of NKT cells. (B–E) Percentages of NKT cells in (B) tumour-adjacent tissues and cervical cancer tissues, (C) patients without (N0 group) or with (N1 group) lymphatic metastasis, (D) patients at stages I/II and III/IV and (E) tumour tissues with a diameter less than and larger than 5 cm. *, p < .05.
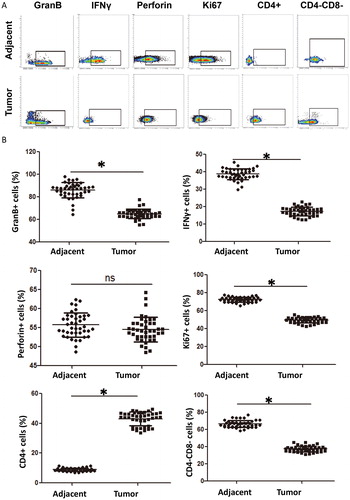
Similar to NK cells, NKT cell activation is regulated by activated receptors and inhibitory receptors, and can exert cytotoxic effects by releasing GranB, perforin and IFNγ [Citation22]. Our study shows that the expression of activated receptors NKp30, NKG2D and CD16 is significantly down-regulated, the expression of inhibitory receptor 158b is elevated, the expression of effector molecules GranB and IFNγ is inhibited and the expression of proliferation marker Ki67 is down-regulated in NKT cells from cervical cancer tissues, suggesting that the function of infiltrating NKT cells is weakened. It is reported that CD4+CD8− NKT cells mainly secrete Th2 cytokines, while CD4−CD8− NKT cells secrete Th1 cytokines, suggesting that infiltrating NKT cells may be related to the regulation of Th2/Th1 balance in tumour microenvironment [Citation38]. Our study shows that CD4+CD8− NKT cells are increased significantly in cervical cancer tissues, while CD4−CD8− NKT cells are decreased, suggesting that NKT cells tend to differentiate into CD4+ subtype that has cancer promoting effect after entering tumour tissue microenvironment.
Activity and killing effect of infiltrating NKT are inhibited in cervical cancer tissues, leading to increase of CD4+NKT cells that promote tumour progression and decrease of CD8−CD4−NKT cells that inhibit tumours
Most tumour tissues have immunosuppressive microenvironment in which the activity of infiltrating immune cells is inhibited [Citation39]. Tumour cells are a major component in the tissue microenvironment, and are involved in regulating the remodelling of the microenvironment [Citation40]. For example, tumour cells secrete matrix metalloproteinases to decompose extracellular matrix, thereby promoting their own metastasis [Citation41]. Tumour cells can recruit Treg cells into tissue microenvironment, inhibit the activity of immune cells and promote tumour metastasis [Citation42]. It is also shown that tumour cells inhibit immune cell activity through PD-1/PDL-1 signalling pathway, thereby evading immune surveillance [Citation43].
To determine the changes in the phenotype and function of infiltrating NKT cells in tumour tissues in our study, flow cytometry was carried out. The data showed that the percentages of CD3+CD56+ NKT cells expressing activated receptors NKp30, CD16 or NKG2D in tumour tissues were significantly lower than those in tumour-adjacent tissues (p < .05) (), and the percentage of NKT cells expressing inhibitory receptor 158b in tumour tissues was significantly higher than that in tumour-adjacent tissues (p < .05) (). The percentages of NKT cells expressing effector molecules GranB and IFNγ in tumour tissues were significantly lower than those in tumour-adjacent tissues (p < .05) (). The percentage of NKT cells expressing proliferation marker Ki67 in tumour tissues was significantly lower than that in tumour-adjacent tissues (p < .05) (). The percentage of CD4+NKT cells in tumour tissues was significantly higher than that in tumour-adjacent tissues (p < .05), whereas that of CD4−CD8−NKT cells in tumour tissues was significantly lower than that in tumour-adjacent tissues (p < .05) (). These results indicate that the activity and killing effect of infiltrating NKT are inhibited in cervical cancer tissues, leading to increase of CD4+NKT cells that promote tumour progression and decrease of CD8−CD4−NKT cells that inhibit tumours.
Figure 2. Phenotype of NKT cells in cervical cancer tissues. (A) Analysis of surface receptors on NKT cells in tumour-adjacent and tumour tissues by flow cytometry. (B) Percentages of NKT cells with positive expression of surface receptors in tumour-adjacent and tumour tissues. *, p < .05; ns, not significant.
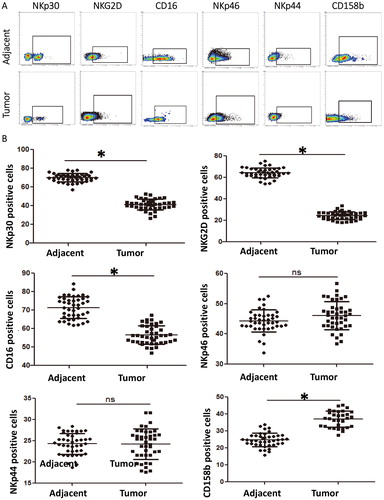
Figure 3. Expression of effector molecules and subtypes of NKT cells in cervical cancer tissues. (A) Analysis of effector molecules and subtypes of NKT cells in tumour-adjacent and tumour tissues by flow cytometry. (B) Percentages of NKT cells with positive expression of effector molecules and subtypes in tumour-adjacent and tumour tissues. *, p < .05; ns, not significant.
Activity of NKT cells is down-regulated by co-culture with cervical cancer cells, but the subtypes of NKT cells are not altered
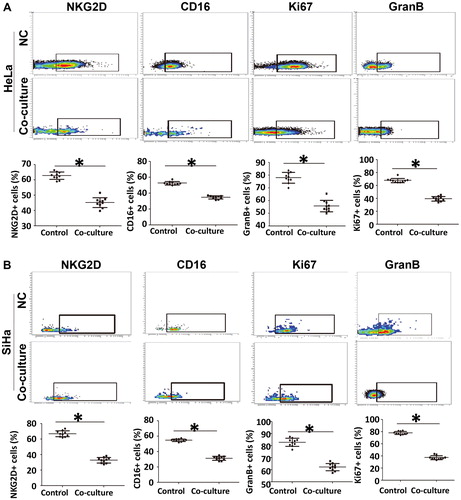
To test whether cervical cancer cells regulate the activity of NKT cells, we co-cultured NKT cells with HeLa cells or SiHa cells. The data showed that the percentages of NKT cells with expression of activated receptors NKG2D and CD16 after co-culture with HeLa cells or SiHa cells were decreased (p < .05) compared with those of NKT cells cultured alone (). The percentages of NKT cells with expression of Ki67 after co-culture with HeLa cells or SiHa cells were decreased (p < .05) compared with those of NKT cells cultured alone (). Moreover, the percentages of NKT cells with expression of GranB after co-culture with HeLa cells or SiHa cells were decreased (p < .05) compared with those of NKT cells cultured alone (). These results suggest that the activity of NKT cells is down-regulated by co-culture with cervical cancer cells, but the subtypes of NKT cells are not altered.
Figure 4. Regulation of phenotype and function of NKT cells by cervical cancer cell lines. (A–B) Percentages of NKT cells with positive expression of NKG2D, CD16, Ki67 and GranB after co-culture with (A) HeLa or (B) SiHa cells. Flow cytometry was used to analyze the phenotype and function of NKT cells. NC, negative control. *, p < .05.
Cervical cancer cells inhibit the function of NKT cells by secreting TGF-β1
Cytokines are a class of active proteins which play important roles in the regulation of immune cell activity. Tumour cells can secrete a variety of cytokines, such as IL-6 and TGF-β, to chemotaxis or regulate immune cells. IL-6 and TGF-β can also inhibit the activity of immune cells in addition to acting on tumour cells. For example, tumour-associated fibroblasts induce PDL1+ neutrophils via the IL-6/STAT3 pathway, which leads to the formation of immunosuppressive environment in hepatocellular carcinoma [Citation44]. Moreover, M2 macrophages inhibit the activity of NK cells by secreting TGF-β1 [Citation45].
To understand the mechanism by which cervical cancer cells regulate the function of NKT cells, we tested whether IL-6 or TGF-β1 inhibits NKT cell activity. Immunohistochemistry showed that expression of IL-6 and TGF-β1 in cervical cancer cells was positive (. Then, TGF-β1 antibody was added into the co-culture system as a rescue group. The data showed that the percentages of NKT cells with positive expression of NKG2D, CD16 or Ki67 in the rescue group were significantly higher (p < .05) than those in the co-culture group (. Moreover, addition of TGF-β1 recombinant protein into the medium reduced (p < .05) the percentages of NKT cells with positive expression of NKG2D, CD16 and Ki67 compared with those in the co-culture group (. Flow cytometry showed that NKT cells treated with TGF-β1 had lower killing effect on cervical cancer cells (p < .05) compared with the control group (. The results indicate that cervical cancer cells inhibit the function of NKT cells by secreting TGF-β1.
Figure 5. Mechanism by which cervical cancer cells regulate the phenotype and function of NKT cells. (A) Expression of IL-6 and TGF-β1 in cervical cancer cells determined by immunohistochemistry. (B) Effect of TGF-β1 antibody (rescue group) on the inhibition of NKT cells by cervical cancer cells. NC, negative control. *, p < .05.
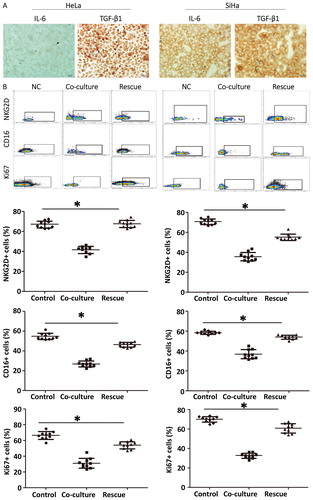
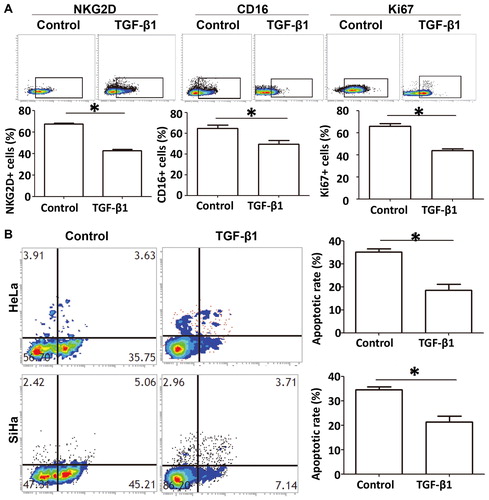
Figure 6. Inhibitory effect of TGF-β1 on the phenotype and function of NKT cells. (A) Percentage of NKT cells with positive expression of NKG2D, CD16 and Ki67 after treatment with TGF-β1, as determined by flow cytometry. *, p < .05. (B) Apoptosis of cervical cancer cells after treatment with TGF-β1. *, p < .05.
Conclusions
The present study demonstrates that NKT infiltration is increased in cervical cancer and negatively correlated with tumour progression. The activity and function of NKT cells infiltrating cancer tissues are significantly inhibited. Cervical cancer cells inhibit the expression of NKG2D, CD16 and Ki67 by secreting TGF-β1, reduce immune surveillance of NKT cells and promote the recurrence and metastasis of the tumour. Inhibition of NKT activity in cervical cancer tissues leads to the decrease of its tumour killing effect. Discovery of the key mechanism to inhibit NKT cell activity and to restore its activity can be of help in killing the tumour.
Author contributions
The final version of the manuscript has been read and approved by all authors, and each author believes that the manuscript represents honest work. YT, MP, SS and LL collaborated to design the study. YT, MP, SS, JH and RL were responsible for performing experiments. YT, MP, SS and LL analyzed the data. All authors collaborated to interpret results and develop the manuscript.
Ethical approval and consent to participate
All procedures performed in the current study were approved by the Ethics Committee of Maternal and Child Health Hospital of Jiangxi Province. Written informed consent was obtained from all patients or their families.
Consent for publication
Written informed consents for publication of any associated data and accompanying images were obtained from all patients or their parents, guardians or next of kin.
Acknowledgements
The authors wish to thank Dr. Baorui Liu of Institute of Oncology, Nanjing Drum Tower Hospital for his help and instructions.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Xu YM, Gong ZJ, Wu HJ. Papillary thyroid carcinoma incidentally found in cervical lymph nodes during neck dissection for patients with tongue squamous cell carcinoma: a 3-case report and literature review. J Oral Maxillofac Surg. 2018;76(11):2454.e1–2454.e6.
- Liang Y, Lu B, Zhou C. Cervical adenoid basal carcinoma: clinicopathologic features of 9 cases with reference to CK17 and Ki-67 expression. J Low Genit Tract Dis. 2019;23(1):77–81.
- Dekker SE, Ostergard TA, Glenn CA, et al. Posterior cervical laminoplasty for resection intradural extramedullary spinal meningioma: 2-dimensional operative video. Oper Neurosurg. 2019;16(3):392.
- Palhares DMF, Marconi DG, Azevedo TL, et al. Predicting the necessity of adding catheters to intracavitary brachytherapy for women undergoing definitive chemoradiation for locally advanced cervical cancer. Brachytherapy. 2018;17(6):935–943.
- Shin JY, Choi KS, Suh M, et al. Comparison of cervical cancer screening among women with and without hysterectomies: a nationwide population-based study in Korea. BMC Cancer. 2018;18(1):810.
- Thapa N, Maharjan M, Xiong Y, et al. Impact of cervical cancer on quality of life of women in Hubei, China. Sci Rep.. 2018;8(1):11993.
- Su K, Zhao Q, Bian A, et al. A novel positive feedback regulation between long noncoding RNA UICC and IL-6/STAT3 signaling promotes cervical cancer progression. Am J Cancer Res. 2018;8(7):1176–1189.
- Chen G, Huang AC, Zhang W, et al. Exosomal PD-L1 contributes to immunosuppression and is associated with anti-PD-1 response. Nature. 2018;560(7718):382–386.
- Lam TS, van de Meent M, Marijt EWA, et al. Immune surveillance by autoreactive CD4-positive helper T cells is a common phenomenon in patients with acute myeloid leukemia. Eur J Haematol. 2018;101(5):665.
- Fridman WH. From cancer immune surveillance to cancer immunoediting: birth of modern immuno-oncology. J Immunol. 2018;201(3):825–826.
- Liu W, Wei X, Li L, et al. CCR4 mediated chemotaxis of regulatory T cells suppress the activation of T cells and NK cells via TGF-beta pathway in human non-small cell lung cancer. Biochem Biophys Res Commun. 2017;488(1):196–203.
- Shabaneh TB, Molodtsov AK, Steinberg SM, et al. Oncogenic BRAF(V600E) governs regulatory T-cell recruitment during melanoma tumorigenesis. Cancer Res. 2018;78(17):5038–5049.
- Chen X, Takemoto Y, Deng H, et al. Histidine decarboxylase (HDC)-expressing granulocytic myeloid cells induce and recruit Foxp3(+) regulatory T cells in murine colon cancer. Oncoimmunology. 2017;6(3):e1290034.
- Lu Y, Li S, Ma L, et al. Type conversion of secretomes in a 3D TAM2 and HCC cell co-culture system and functional importance of CXCL2 in HCC. Sci Rep. 2016;6(1):24558.
- Lezmi G, Leite-de-Moraes M. Invariant natural killer T and mucosal-associated invariant T cells in asthmatic patients. Front Immunol. 2018;9:1766.
- Smyk DS, Mavropoulos A, Mieli-Vergani G, et al. The role of invariant NKT in autoimmune liver disease: can vitamin D act as an immunomodulator? Can J Gastroenterol Hepatol. 2018;2018:8197937.
- Zhu L, Xie X, Zhang L, et al. TBK-binding protein 1 regulates IL-15-induced autophagy and NKT cell survival. Nat Commun. 2018;9(1):2812.
- Wang XZ, Xue RF, Zhang SY, et al. Activation of natural killer T cells contributes to triptolide-induced liver injury in mice. Acta Pharmacol Sin. 2018;39(12):1847–1854.
- Bae EA, Seo H, Kim BS, et al. Activation of NKT cells in an anti-PD-1-resistant tumor model enhances antitumor immunity by reinvigorating exhausted CD8 T cells. Cancer Res. 2018;78(18):5315–5326.
- Zhao M, Svensson MND, Venken K, et al. Altered thymic differentiation and modulation of arthritis by invariant NKT cells expressing mutant ZAP70. Nat Commun. 2018;9(1):2627.
- Wang HX, Li WJ, Hou CL, et al. CD1d-dependent natural killer T cells attenuate angiotensin II-induced cardiac remodeling via IL-10 signaling in mice. Cardiovasc Res. 2018.
- Fujii SI, Yamasaki S, Sato Y, et al. Vaccine designs utilizing invariant NKT-licensed antigen-presenting cells provide NKT or T cell help for B cell responses. Front Immunol. 2018;9:1267.
- Hoshino A, Tanita K, Kanda K, et al. High frequencies of asymptomatic Epstein-Barr virus viremia in affected and unaffected individuals with CTLA4 mutations. Clin Immunol. 2018;195:45–48.
- Guo H, Xu B, Gao L, et al. High frequency of activated natural killer and natural killer T-cells in patients with new onset of type 2 diabetes mellitus. Exp Biol Med. 2012;237(5):556–562.
- van Gysen K, Stevens M, Guo L, et al. Validation of the 8th edition UICC/AJCC TNM staging system for HPV associated oropharyngeal cancer patients managed with contemporary chemo-radiotherapy. BMC Cancer. 2019;19(1):674.
- Joo JH, Kim YS, Nam JH. Prognostic significance of lymph node ratio in node-positive cervical cancer patients. Medicine. 2018;97(30):e11711.
- Lopes J, Horta M, Cunha TM. Endometrial cancer after radiation therapy for cervical carcinoma: a radiological approach. Eur J Radiol. 2018;105:283–288.
- Dorta-Estremera S, Colbert LE, Nookala SS, et al. Kinetics of intratumoral immune cell activation during chemoradiation for cervical cancer. Int J Radiat Oncol Biol Phys. 2018;102(3):593–600.
- Kawachi A, Yoshida H, Kitano S, et al. Tumor-associated CD204(+) M2 macrophages are unfavorable prognostic indicators in uterine cervical adenocarcinoma. Cancer Sci. 2018;109(3):863–870.
- Tang X, Tan L, Shi K, et al. Gold nanorods together with HSP inhibitor-VER-155008 micelles for colon cancer mild-temperature photothermal therapy. Acta Pharm Sin B. 2018;8(4):587–601.
- Narvaez J, Juarez-Lopez P, J LL, et al. Rheumatic immune-related adverse events in patients on anti-PD-1 inhibitors: fasciitis with myositis syndrome as a new complication of immunotherapy. Autoimmun Rev. 2018;17(10):1040–1045.
- Tang X, Yang L, Li Z, et al. First-in-man clinical trial of CAR NK-92 cells: safety test of CD33-CAR NK-92 cells in patients with relapsed and refractory acute myeloid leukemia. Am J Cancer Res. 2018;8(6):1083–1089.
- Lin J, Long J, Wan X, et al. Classification of gallbladder cancer by assessment of CD8(+) TIL and PD-L1 expression. BMC Cancer. 2018;18(1):766.
- Lee M, Tayyari F, Pinnaduwage D, et al. Tumoral BRD4 expression in lymph node-negative breast cancer: association with T-bet + tumor-infiltrating lymphocytes and disease-free survival. BMC Cancer. 2018;18(1):750.
- Vuletic A, Jovanic I, Jurisic V, et al. IL-2 And IL-15 induced NKG2D, CD158a and CD158b expression on T, NKT- like and NK cell lymphocyte subsets from regional lymph nodes of melanoma patients. Pathol Oncol Res. 2018.
- Tang B, Wu W, Wei X, et al. Activation of glioma cells generates immune tolerant NKT cells. J Biol Chem. 2014;289(50):34595–34600.
- Simon B, Wiesinger M, Marz J, et al. The generation of CAR-transfected natural killer T cells for the immunotherapy of melanoma. Int J Mol Sci. 2018;19(8):2365.
- Hoya M, Nagamatsu T, Fujii T, et al. Impact of Th1/Th2 cytokine polarity induced by invariant NKT cells on the incidence of pregnancy loss in mice. Am J Reprod Immunol. 2018;79(3):e12813.
- Nwani NG, Sima LE, Nieves-Neira W, et al. Targeting the microenvironment in high grade serous ovarian cancer. Cancers. 2018;10(8):266.
- Saleem J, Wang L, Chen C. Carbon-based nanomaterials for cancer therapy via targeting tumor microenvironment. Adv Healthcare Mater. 2018;7(20):1800525.
- Sun L, Xie S, Ji X, et al. MMP-2-responsive fluorescent nanoprobes for enhanced selectivity of tumor cell uptake and imaging. Biomater Sci. 2018;6(10):2619–2626.
- Adeegbe DO, Liu S, Hattersley MM, et al. BET bromodomain inhibition cooperates with PD-1 blockade to facilitate antitumor response in Kras-mutant non-small cell lung cancer. Cancer Immunol Res. 2018;6(10):1234–1245.
- Pan Z, Di S, Shi B, et al. Increased antitumor activities of glypican-3-specific chimeric antigen receptor-modified T cells by coexpression of a soluble PD1-CH3 fusion protein. Cancer Immunol Immunother. 2018;67(10):1621–1634.
- Cheng Y, Li H, Deng Y, et al. Cancer-associated fibroblasts induce PDL1+ neutrophils through the IL6-STAT3 pathway that foster immune suppression in hepatocellular carcinoma. Cell Death Dis. 2018;9(4):422.
- Nunez SY, Ziblat A, Secchiari F, et al. Human M2 macrophages limit NK cell effector functions through secretion of TGF-beta and engagement of CD85j. J Immunol. 2018;200(3):1008–1015.
