Abstract
Tobacco mosaic virus (TMV) causes severe damage and economic losses of tomato in crop yield production and quality worldwide. Tomato plants infected with TMV usually show a mosaic pattern and chlorosis symptoms that result from affected chloroplasts. To elucidate tomato–TMV interactions, the transcriptional profiles of tomato tissues at different time intervals were analysed using a differential display-polymerase chain reaction technique. Compared to mock-inoculated plants, and based on gene expression changes in tomato plants, the representative down- and up-regulated genes were selected for further analysis. The Basic Local Alignment Search Tool at the National Center for Biotechnology Information (NCBI-BLAST) analysis revealed that the three up-regulated gene transcripts at 15 days post-inoculation were coding for chloroplast (MG565980 and MG565979) and kinesin-like proteins (MG565981). Additionally, the down-regulated gene was a chloroplast-related gene (MG565978). Interestingly, the two chloroplast genes, MG565978 and MG565980, shared more than 96% sequence identity, and their transmembrane profiles were nearly identical. On the other hand, many protein kinase C phosphorylation, casein kinase II phosphorylation and N-myristoylation sites were detected within the MG565981 gene. Moreover, the high similarity between the kinesin gene and many Arabidopsis defensin genes indicated that it might play an important role in the plant defence system against TMV infection. It should be stressed that studies of the pathways in which chloroplast and kinesin-like protein genes are involved may elucidate the mechanisms of tomato tolerance to viral infection and can lead us to a more comprehensive understanding of tomato–TMV interactions.
Introduction
Plant diseases, especially plant viruses, cause a variety of problems and are responsible for huge losses of crop production and quality throughout the world [Citation1]. Thus, viral infections must be controlled in order to keep up the standard and abundance of food, fibre and feed produced by farmers around the globe [Citation2]. Tomato (Solanum lycopersicum L.) is the second most important cultivated and consumed vegetable crop after potato, worldwide [Citation3,Citation4]. Due to their suitability for genome manipulation, tomato plants are used as a model plant for scientific research in order to study the metabolic and fundamental biological processes and analyse plant-pathogen interactions [Citation5].
Tobacco mosaic virus (TMV, genus Tobamovirus) is one of the most economically relevant viral threats to many economically important plants such as tobacco, tomato, pepper and potato, worldwide [Citation6]. Many plant virologists consider it the most vital and serious plant virus [Citation7]. Tomato plants are systemically infected, resulting in mosaic and chlorosis symptoms, often associated with plant deformations or altered structure and morphology. Moreover, many characteristics and gene expression level changes similar to stress and defence responses have been reported after viral infection [Citation8].
Chloroplasts are plastids, one of the foremost dynamic organelles of plant cells. Chloroplasts perform photosynthesis, synthesise major phytohormones and are crucial for inter- organelle signalling [Citation9]. Chloroplasts, as the main target for viruses, play a crucial role in the defence response and undergo enormous structural and functional damage during viral infection [Citation9,Citation10]. There is abundant and increasing evidence that chloroplast-associated genes are the most affected genes during viral infection [Citation11].
Kinesins, a superfamily of microtubule-based motor proteins, are present among all eukaryotic organisms [Citation12–14]. In plants, kinesins directly or indirectly contribute to cell division and growth in diverse tissues [Citation15]. In addition to their roles in mitosis, morphogenesis and signal transduction [Citation16], kinesins affect vesicle transport, organelle distribution, cellulose microfibril order and microtubule organisation [Citation13,Citation15,Citation17,Citation18].
The differential display-polymerase chain reaction (DD-PCR) technique provides a robust methodology for the fast identification of differentially expressed genes through scanning the differentially expressed mRNA in pathogen-infected plants [Citation19–22]. Many studies on virus–host interactions use DD-PCR to isolate and characterise genes related to virus resistance [Citation1,Citation23,Citation24]. Consequently, through using the DD-PCR technique, this study determined whether there were differentially expressed up- and/or down-regulated genes during tomato-TMV interactions, which are essential to better understand the tomato immune defence system against TMV infection. Moreover, further study of the function of these genes and the interactions between them will lead to improved tomato resistance against viral infections.
Materials and methods
Viral infections, sample collection and RNA extraction
The Egyptian TMV isolate used in this study (Acc# MG264131) was continuously maintained in our lab on Nicotiana benthamiana plants through a mechanical inoculation process. At 35 days after planting, two true leaves of Heinz 1706 tomato cultivar (Solanum lycopersicum L.) seedlings were dusted with carborundum and gently inoculated with 1 mL of semi-purified TMV [Citation25]. Three inoculated plants were collected at 6, 9 and 15 days post-inoculation (dpi), as well as control plants. Mock-treated plants inoculated with phosphate buffer only were used as control.
Plant total RNA extraction and cDNA synthesis
Total RNA was extracted from the collected plants using the RNeasy Mini Kit according to the manufacturer’s instructions (QIAGEN). After checking the purity and concentration of extracted RNA, the first strand of cDNA synthesis in a total reaction volume of 25 μL was performed. The reaction mixture contained 2.5 μL of 5X buffer with MgCl2, 2.5 μL of 2.5 mmol/L deoxynucleoside triphosphates (dNTPs), 1 μL of oligo(dT) primer (20 pmol/μL), 4 μL of random hexamer primers, 1 μg of purified RNA and 200 U of reverse transcriptase (RT) enzyme (M-MLV; Fermentas). In a thermal cycler (Eppendorf), the reverse transcriptase reaction was performed at 42 °C for one hour and deactivated at 72 °C for 10 min. After holding at 4 °C, the reaction mixture was stored at -20 °C until used.
Differential display (DD)-PCR and detection of up/down-regulated genes
The five different arbitrary primers () were used to scan the mRNA transcripted genes of infected plants as well as mock-treated plants. For DD-PCR, 1 µL of cDNA was added to 2.5 µL Taq polymerase buffer 10x (Promega, USA) containing a final concentration of 1 mmol/L MgCl2, 0.2 mmol/L dNTPs, 0.4 mmol/L of primer () and 0.2 µL Taq polymerase (5 U/µL) in a final reaction volume of 25 µL. The PCR reaction program started with an initial denaturation at 95 °C for two minutes followed by forty cycles. Each cycle was programmed with 95 °C for one minute, 30 °C for one minute and 72 °C for one minute. At the end of the last cycle, a final extension step at 72 °C for 10 min was added. The RT-PCR amplification products were electrophoresed in 0.5X TBE (Tris–borate–ethylenediaminetetraacetic acid buffer) with 1.5% agarose, stained with ethidium bromide and visually analysed by a gel documentation system.
Table 1. List of primers used in DD-PCR and total number of up/down-regulated bands.
Sequencing analysis and phylogenetic construction
The selected PCR products (up/down-regulated genes) were sequenced directly after being excised and purified from the gel with a PCR clean-up column kit (QIAGEN, Germany). Sanger sequencing of selected genes was performed using BigDye® Terminator v3.1 Cycle Sequencing kit and a 3130xl Genetic Analyzer system (Applied Biosystems). The obtained DNA nucleotide sequences were analysed using the Basic Local Alignment Search Tool at the National Center for Biotechnology Information (NCBI-BLAST; https://blast.ncbi.nlm.nih.gov/Blast.cgi) to confirm the identity of the obtained sequences. The phylogenetic tree was analysed and generated by MEGA5 software program using UPGMA (unweighted pair group method with arithmetic mean) statistical analysis method with 2000 bootstrap replicates. The transmembrane domain profiles were predicted by the TMpred program (https://embnet.vital-it.ch/software/TMPRED.form.html) [Citation26].
Results and discussion
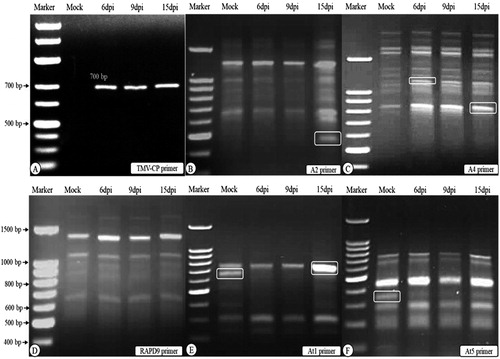
Plant viruses are some of the most important problems for food security, where they play significant roles in crop losses all over the world [Citation1]. Among the viruses, the Tobacco mosaic virus (TMV) is one of the most studied. It is used as a model plant virus in fundamental biological research towards understanding of host–virus interactions [Citation27]. TMV infects many plant species, including tomato, causing light or dark green mosaic symptoms and distortions of the leaves [Citation28,Citation29]. In the present study, the TMV- inoculated tomato plants at 15 dpi showed identical mosaic symptoms to those reported previously [Citation29,Citation30]. By using RT-PCR with specific TMV-coat protein (CP) gene primers, a DNA fragment of 700 bp was detected in the infected tissues at 6, 9 and 15 dpi (). No virus was detected in mock-inoculated samples.
Figure 1. Agarose gel electrophoresis of RT-PCR detection (A) of Tobacco mosaic virus-coat protein (TMV-CP) and DD-PCR (B to F) using primers A2, A4, RAPD 9, At1 and At5. Lane Marker: DNA marker 100 bp ladder, lane “Mock” control (mock-inoculated plant tissues), lanes 6 dpi, 9 dpi and 15 dpi: plant tissues collected at 6, 9 and 15 dpi of TMV. White round rectangle indicates the bands that were selected for sequencing. Note: Agarose gel electrophoresis (1.5%) in TBE buffer.
To better clarify the molecular basis of tomato-TMV interactions with rapid identification of the differentially expressed tomato genes during viral infection, the DD-PCR technique was performed using five arbitrary primers (). Today, DD-PCR is increasingly effective in isolating and characterising genes differentially expressed amongst cells, tissues or individuals [Citation1,Citation22,Citation24]. Many plants defend themselves against pathogens, such as viruses, bacteria and fungi, through altering their gene expressions [Citation31].
Differential display PCR (DD-PCR) and up/down regulated genes
In the present study, the completely differentially expressed genes were carefully investigated through the comparison among the amplified fragments in mock-treated as well as in infected samples at different time points following TMV inoculation. The five primers yielded more than 134 bands with different molecular sizes ranging between 190 and 2500 bp (). The amplified patterns obtained with the RAPD9 primer were identical mimics, and none of the differentiated bands was detected at any time interval (). There were 128 PCR bands held in common between the mock-inoculated and infected samples (monomorphic), whereas six PCR bands were differentially expressed in all the treatments (polymorphic) ().
The results revealed that TMV induced a variation in gene expression within tomato tissues at all intervals. These results agree with previous observations [Citation1,Citation24,Citation32,Citation33]. All polymorphic bands were selected, excised from the agarose gel, purified and submitted for sequencing. Some genes may have been missed and others had low-quality sequence values during the sequencing process. Three up-regulated bands and one down-regulated band were successfully sequenced and characterised. Sequence analysis revealed that the up-regulated transcript genes code for a kinesin-like protein (Acc# MG565981) and chloroplast-associated genes (Acc# MG565980 and MG565979). The down-regulated transcripts coded for another chloroplast-associated gene (Acc# MG565978).
Isolation and sequencing of chloroplast-associated genes
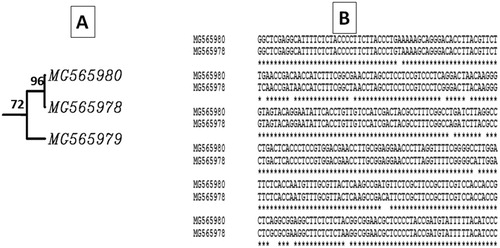
The two up-regulated genes coding for chloroplast proteins were induced and detected in the infected tissues at 15 dpi with the A2 primer and at 6 dpi with the A4 primer (). The first chloroplast-associated gene (Acc# MG565979) was up-regulated and expressed only at 15 dpi with about 450 bp. The second one (Acc# MG565980) had a size about 1100 bp, and was slightly induced in mock-inoculated plants (the band was very faint) compared to that induced at 6 dpi. Intriguingly, the down-regulated gene was found to code for a chloroplast protein. Moreover, it was noticed that the chloroplast genes MG565980 (up-regulated) and MG565978 (down-regulated) are closely related to each other with about 96% similarity, and these results confirm that most likely there are many copies of that gene (). Based on their DNA sequence alignment, there was some A-T transition reflecting the 4% difference between the two genes.
Figure 2. Phylogenetic tree (A) constructed based on the obtained DNA sequences of the chloroplast isolated genes in this study and the sequence alignment (B) between the two closely related chloroplast-associated genes (MG565980 and MG565978).
It is well known that the chloroplast is a common target of plant viruses [Citation34,Citation35]. In addition, it has important roles in plant–virus interactions and is involved in virus replication, movement, symptoms and the systemic defence response [Citation35]. As Manfre et al. [Citation37] report, the production of TMV-like symptoms at 12 dpi with severe systemic mosaic and chlorosis symptoms at 15 dpi were associated with TMV infection and disturbance of chloroplast components. The chloroplasts and photosynthesis-related genes (CPRGs) that associate with chloroplast membranes or locate in chloroplasts have been reported as the majority of significantly changed proteins in the plants susceptible to virus infection [Citation38–41]. Most of these genes are down-regulated in association with the degree of chlorosis severity [Citation36,Citation42–44]. The up-regulation of the chloroplast-related genes at 6 and 15 dpi suggests that these genes play a major role in the tomato defence system against TMV infection.
Our results agree with those of Bhattacharyya et al. [Citation45] and Reuveni et al. [Citation46], who postulated that the mosaic symptoms were typically related to an altered quantity, shape, variety and structure of chloroplasts. In addition to the reduction of chloroplast ribosomal RNA level [Citation47], the inhibition of the nuclear-encoded CPRGs transcription through feedback signalling upon TMV infection has been reported [Citation48]. Similarly, the coat protein of Cucumber mosaic virus (CP) was most likely to repress the CPRGs transcription via the retrograde signalling from the chloroplast into the nucleus [Citation41]. Additionally, Caplan et al. [Citation10,Citation34] reported that chloroplasts are part of the mechanism of N immune receptor-mediated defence against TMV and are involved in infection by DNA viruses [Citation49].
The down-regulated chloroplast gene (MG565978) was amplified with the two different primers (At1 and At5) at 15 dpi in the mock-inoculated plants but was completely shut down in the infected plants at all dpi. This is evidence that there is a direct relationship between this gene and the TMV viral infection. The gene was inhibited in 15 dpi, which indicates that this kind of inhibition was accompanied by appearance of viral symptoms. The disappearance of the band corresponding to this gene in the infected plant tissues along the examined intervals suggested that the virus had been able to control the cell organelles and began to affect the chloroplasts structurally and functionally once the virus entered the plant cell and started propagation. Additionally, the viral load reached the maximum level for cell control at day 15, with symptoms emerging. Clearly, this gene was found in the cells in different copies with different molecular sizes, two copies were observed in the mock-treated plants (15 dpi) at molecular sizes about 650 (At1) and at 380 bp (At5). Moreover, when the transmembrane domains of the up-regulated (MG565980) and down-regulated (MG565978) genes were predicted using TMpred sofrware, it was observed that the transmembrane domain profile is identical. This confirms that the two genes have the same function but one of them was shut down and the other was not affected by the viral stress (). This assumption agrees with the report by Fu et al. [Citation50] that some abnormalities were observed in the leaves of Chinese cabbage infected with Turnip mosaic virus (TuMV), among them membrane vesiculation and breakdown.
The up-regulation of kinesin-like protein
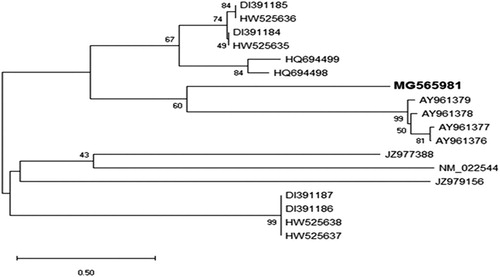
Although kinesin-like protein gene (Acc# MG565981) with a molecular weight about 750 bp was detected in the mock-inoculated plants in low intensity, this band was noticed with high intensity in the infected plants at 15 dpi post inoculation (). NCBI-BLAST and phylogenetic analysis revealed that it is closely related to the kinesin-like protein gene (Acc# XM_004233996) isolated from tomato Heinz 1706 cultivar in the UK with 98% similarity (). The bioinformatics analysis detected three motifs in the kinesin-like protein sequence (). These motifs are the Protein kinase C phosphorylation site (three aa), the Casein kinase II phosphorylation site (four aa) and the N-myristoylation site (6 aa) ().
Figure 4. Phylogenetic analysis showing the genetic relationship between the nucleotide sequence of the tomato (S. lycopersicum) kinesin-like protein gene (MG565981) and other kinesin-like protein genes available in GenBank. The phylogeny was tested with 2000 bootstrap replicates based on the UPGMA statistical method.
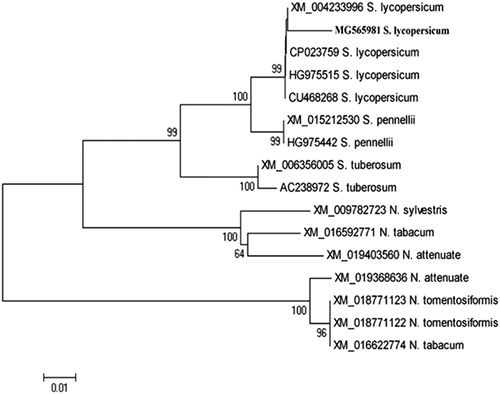
Figure 5. DNA nucleotide sequence of tomato kinesin-like protein gene. The deduced amino acid sequence of the protein is shown below the DNA sequence. Potential Protein kinase C phosphorylation site (three aa), Casein kinase II Phosphorylation site (four aa) and N-myristoylation site (six aa) are shadowed and underlined. Black colour indicates interaction between two sites.
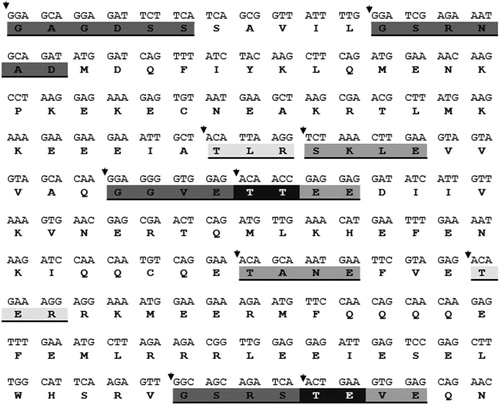
Bioinformatics tools have been widely used to analyse and identify motifs from DNA and protein sequences [Citation51]. Keating and Striker [Citation52] reported that the cellular protein phosphorylation process has major effects on both viral infection and replication. Moreover, the addition of phosphate group by kinases can regulate the stability, activity and interactions of a viral protein with other cellular and viral proteins [Citation53]. Interestingly, the kinesin gene showed about 60% similarity with one of the up-regulated chloroplast genes (MG565979) but their transmembrane profiles are different (), which confirmed that the two genes were structurally similar but functionally different as previously reported [Citation54]. Evidently, viruses transport their macromolecules like RNA and proteins from cell to cell through the cell membrane [Citation55,Citation56].
Moreover, kinesin genes are maternal DNA and are one of the superfamily of microtubule motor proteins found in all eukaryotic organisms; they are important for chloroplast movement and anchoring to the plasma membrane [Citation57]. Accordingly, the high expression level of the kinesin gene in infected plants could be considered a marker for plant defence against viral infection, and this assumption was in agreement with the previous results [Citation58] that the kinesin-like proteins play a fundamental role in the oriented deposition of cellulose microfibrils and the strength of the cell wall. In addition, both of the mitochondrial and chloroplast genes are associated in guiding the cell during pathogen attacks. This suggestion agrees with Kong and Hanley-Bowdoin [Citation57], who reported that kinesin-like protein interacts with the geminivirus AL1 protein in a yeast 2-hybrid assay. Moreover, Kellmann et al. [Citation59] showed that interconnection between, movement protein (MP) of Tomato spotted wilt tospovirus (TSWV) (NSm) and the kinesin-like protein permits intracellular movement of TSWV nucleocapsids through utilising the cytoskeleton or vesicle sorting systems. Consequently, plants, as well as viruses transport their macromolecules like RNA and proteins from cell to cell through plasmodesmata [Citation55,Citation56], and such transport activities could also require kinesins [Citation14].
To study whether the kinesin gene was one of the defence genes in the plant or if it belonged to this large family, the obtained DNA sequence of kinesin was compared with 18 defence genes in the GenBank database (). It was observed that 19 genes were divided into two lineages. The first lineage was split into two main clusters including 11 genes, with kinesin among them. The second cluster was grouped into two groups and kinesin was isolated in one group, with high similarity to the other four genes (2nd group). The four defence genes are isolated from Arabidopsis. The results showed that this gene might have a special defensive function against viral infections. We assumed that the similarities between the kinesin gene and the other defence genes indicate that the kinesin gene may play an important role in the plant defence against viral infections.
Figure 6. Phylogenetic analysis showing the genetic relationship between nucleotide sequence of the kinesin-like protein gene (MG565981) and other 18 defensin genes available in GenBank. The kinesin had high similarity to the four defencing genes isolated from Arabidopsis plant. The phylogeny tested with bootstrap method with 2,000 replications and generated based on UPGMA statistical method.
Conclusions
Based on the present studies and in line with previous reports, we concluded that chloroplast and kinesin-like protein genes are targets during TMV infection, and the virus is able to modulate some of them either in its movement or during propagation. There are different copies of the chloroplast genes that were affected by the viral infection; some of these genes may shut down, whereas others are up-regulated. The gene selectivity depends on the epitopes existing either on the expressed proteins on the chloroplast membrane or on the viral coat protein. Furthermore, studies of the pathways in which chloroplast and kinesin-like protein genes are involved may elucidate the mechanisms of tomato tolerance to the viral infection and can lead us to a more comprehensive understanding of tomato–TMV interactions.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
The author(s) received no specific funding for this work.
References
- Abdelkhalek A, ElMorsi A, AlShehaby O, et al. Identification of genes differentially expressed in Iris yellow spot virus infected onion. Phytopathol Mediterr. 2018;57(2):334–340.
- Pal KK, Gardener M. Biological control of plant pathogens. Plant Health Instruct. 2006; doi:10.1094/PHI-A-2006-1117–02.
- Hanson P, Chen JT, Cou CG, et al. Tomato production. Asian Vegetable Res Dev Center 2001;62:8–9.
- Lugasi A, Biro L, Hovarie J, et al. Lycopene content of foods and lycopene intake in two groups of the Hungarian population. Nutr Res. 2003;23(8):1035–1044.
- Giovannoni JJ. Fruit ripening mutants yield insights into ripening control. Curr Opin Plant Biol. 2007;10(3):283–289.
- Prabahar A, Swaminathan S, Loganathan A, et al. Identification of novel inhibitors for tobacco mosaic virus infection in Solanaceae plants. Adv Bioinformatics. 2015;2015:1.
- Scholthof K, Adkins S, Czosnek H, et al. Top 10 plant viruses in molecular plant pathology. Mol Plant Pathol. 2011;12(9):938–954.
- Whitham SA, Yang C, Goodin MM. Global impact: elucidating plant responses to viral infection. Mol Plant Microbe Interact. 2006;19(11):1207–1215.
- Bhattacharyya D, Chakraborty S. Chloroplast: the Trojan horse in plant-virus interaction. Mol Plant Pathol. 2018;19(2):504–518.
- Caplan JL, Kumar AS, Park E, Padmanabhan MS, et al. Chloroplast stromules function during innate immunity. Dev Cell. 2015;34(1):45–57.
- Ganusova EE, Rice JH, Carlew TS, et al. Altered expression of a chloroplast protein affects the outcome of virus and nematode infection. Mol Plant Microbe Interact. 2017;30(6):478–488.
- Reddy AS, Day IS. Kinesins in the Arabidopsis genome: a comparative analysis among eukaryotes. BMC Genomics 2001;2(1):2.
- Lee YR, Liu B. Cytoskeletal motors in Arabidopsis. Sixty-one kinesins and seventeen myosins . Plant Physiol. 2004;136(4):3877–3883.
- Richardson DN, Simmons MP, Reddy AS. Comprehensive comparative analysis of kinesins in photosynthetic eukaryotes. BMC Genomics. 2006;7(1):18.
- Li J, Xu Y, Chong K. The novel functions of kinesin motor proteins in plants. Protoplasma 2012;249(S2):95–100.
- Hirokawa N, Noda Y, Tanaka Y, et al. Kinesin superfamily motor proteins and intracellular transport. Nat Rev Mol Cell Biol. 2009;10(10):682–696.
- Verhey KJ, Meyer D, Deehan R, et al. Cargo of kinesin identified as JIP scaffolding proteins and associated signaling molecules. J Cell Biol. 2001;152(5):959–970.
- Lee YR, Li Y, Liu B. Two Arabidopsis phragmoplast-associated kinesins play a critical role in cytokinesis during male gametogenesis. Plant Cell. 2007;19(8):2595–2605.
- Poirier GM, Pyati J, Wan JS, et al. Screening differentially expressed cDNA clones obtained by differential display using amplified RNA. Nucleic Acids Res. 1997;25(4):913–914.
- Kuno N, Muramatsu T, Hamazato F, et al. Identification by large-scale screening of phytochrome-regulated genes in etiolated seedlings of Arabidopsis using a fluorescent differential display technique. Plant Physiol. 2000;122(1):15–24.
- Wang ZK, Sun QX. Primary study on the relationship between differential gene expression patterns in roots at jointing stage and heterosis in agronomic traits in a wheat dialed cross. Sci Agric Sin. 2003;36:473–479.
- Behiry S, Ashmawy N, Abdelkhalek A, et al. Compatible- and incompatible-type interactions related to defense genes in potato elucidation by Pectobacterium carotovorum. J Plant Dis Prot. 2018;125(2):197–204.
- Andleeb S, Amin I, Bashir A, et al. Transient expression of betaC1 protein differentially regulates host genes related to stress response, chloroplast and mitochondrial functions. Virol 2010;7(1):373–385.
- Hafez EE, Abdelkhalek AA, Abeer SA, et al. Altered gene expression: induction/suppression in leek elicited by iris yellow spot virus infection (IYSV) Egyptian isolate. Biotechnol Biotechnol Equip. 2013;27(5):4061–4068.
- Abdelkhalek A, Sanan-Mishra N. A comparative analysis of the suppressor activity of tobacco mosaic virus proteins in the tomato plant. Jordan J Biol Sci Short Commun. 2018;11(4):469–473.
- Hofmann K, Stoffel W. TMbase-a database of membrane spanning proteins segments. Biol Chem Hoppe Seyler. 1993;374:166.
- Das PP, Lin Q, Wong SM. Comparative proteomics of tobacco mosaic virus-infected Nicotiana tabacum plants identified major host proteins involved in photosystems and plant defence. J Proteomics. 2019;194:191–199.
- Alonso JM, Gorzny ML, Bittner AM. The physics of tobacco mosaic virus and virus-based devices in biotechnology. Trends Biotechnol. 2013;31(9):530–538.
- Abdelkhalek A, Sanan-Mishra N. Differential expression profiles of tomato miRNAs induced by tobacco mosaic virus. J Agr Sci Technol. 2019;21(2):475–485.
- Nassar EA, El-Dougdoug KA, Osman ME, et al. Characterization and elimination of a TMV isolate infecting Chrysanthemum plants in Egypt. Int J Virol. 2012;8:14–26.
- Cohn J, Sessa G, Martin GB. Innate immunity in plants. Curr Opin Immunol. 2001;13(1):55–62.
- Baebler S, Witek K, Petek M, et al. Salicylic acid is an indispensable component of the Ny-1 resistance-gene-mediated response against Potato virus Y infection in potato. J Exp Bot. 2014;65(4):1095–1109.
- ElMorsi A, Abdelkhalek A, AlShehaby O, et al. Pathogenesis-related genes as tools for discovering the response of onion defence system against Iris yellow spot virus infection. Botany 2015;93(11):735–744.
- Caplan JL, Mamillapalli P, Burch-Smith TM, et al. Chloroplastic protein NRIP1 mediates innate immune receptor recognition of a viral effector. Cell 2008;132(3):449–462.
- Zhao J, Liu Q, Zhang H, et al. The RubisCO small subunit is involved in Tobamovirus movement and Tm-22-mediated extreme resistance. Plant Physiol. 2013;161(1):374–383.
- Zhao J, Zhang X, Hong Y, et al. Chloroplast in Plant-Virus Interaction. Front Microbiol. 2016;7:1565
- Manfre A, Glenn M, Nunez A, et al. Light quantity and photosystem function mediate host susceptibility to turnip mosaic virus via a salicylic acid-independent mechanism. Mol Plant Microbe Interact. 2011;24(3):315–327.
- Dardick C. Comparative expression profiling of Nicotiana benthamiana leaves systemically infected with three fruit tree viruses. Mol Plant Microbe Interact. 2007;20(8):1004–1017.
- Lu J, Du ZX, Kong J, et al. Transcriptome analysis of Nicotiana tabacum infected by cucumber mosaic virus during systemic symptom development. PLoS One. 2012;7(8):e43447.
- Kundu S, Chakraborty D, Kundu A, et al. Proteomics approach combined with biochemical attributes to elucidate compatible and incompatible plant-virus interactions between Vignamungo and mungbean yellow mosaic India virus. Proteome Sci. 2013;11(1):15.
- Mochizuki T, Ogata Y, Hirata Y, et al. Quantitative transcriptional changes associated with chlorosis severity in mosaic leaves of tobacco plants infected with cucumber mosaic virus. Mol Plant Pathol. 2014;15(3):242–254.
- Shimizu T, Satoh K, Kikuchi S, et al. The repression of cell wall-and plastid-related genes and the induction of defense-related genes in rice plants infected with rice dwarf virus. Mol Plant Microbe Interact. 2007;20(3):247–254.
- Rodriguez M, Munoz N, Lenardon S, et al. The chlorotic symptom induced by sunflower chlorotic mottle virus is associated with changes in redox-related gene expression and metabolites. Plant Sci. 2012;196:107–116.
- Wu L, Wang S, Chen X, et al. Proteomic and phytohormone analysis of the response of maize (Zea mays L.) seedlings to sugarcane mosaic virus. PLoS One. 2013;8(7):e70295.
- Bhattacharyya D, Prabu G, Reddy KK, Kushwaha NK, et al. A geminivirus beta satellite damages the structural and functional integrity of chloroplasts leading to symptom formation and inhibition of photosynthesis. J Exp Bot. 2015;66(19):5881–5895.
- Reuveni M, Debbi A, Kutsher Y, et al. Tomato yellow leaf curl virus effects on chloroplast biogenesis and cellular structure. Physiol Mol Plant Pathol. 2015;92:51–58.
- Fraser R. Effects of two TMV strains on the synthesis and stability of chloroplast ribosomal RNA in tobacco leaves. Mol Gen Genet. 1969;106(1):73–79.
- Lehto K, Tikkanen M, Hiriart JB, et al. Depletion of the photosystem II core complex in mature tobacco leaves infected by the flavum strain of tobacco mosaic virus. Mol Plant Microbe Interact. 2003;16(12):1135–1144.
- Krenz B, Jeske H, Kleinow T. The induction of stromule formation by a plant DNA-virus in epidermal leaf tissues suggests a novel intra- and intercellular macromolecular trafficking route. Front Plant Sci. 2012;3:291.
- Fu DY, Hong J, Chen JS, et al. Accumulation of coat protein of Turnip mosaic virus in host chloroplasts and its effect on PS II activity. Zhi Wu Sheng Li Yu Fen Zi Sheng Wu Xue Xue Bao. 2004;30(1):34–40.
- Hafez EE, El-Morsi AA, El-Shahaby OA, Abdelkhalek AA. Occurrence of iris yellow spot virus from onion crops in Egypt. VirusDisease 2014;25(4):455–459.
- Keating JA, Striker R. Phosphorylation events during viral infections provide potential therapeutic targets. Rev Med Virol. 2012;22(3):166–181.
- Jakubiec A, Jupin I. Regulation of positive-strand RNA virus replication: the emerging role of phosphorylation. Virus Res. 2007;129(2):73–79.
- Sanchez F, Wang X, Jenner CE, et al. Strains of Turnip mosaic potyvirus as defined by the molecular analysis of the coat protein gene of the virus. Virus Res. 2003;94(1):33–43.
- Reddy AS. Molecular motors and their functions in plants. Int Rev Cytol. 2001;204:97–178.
- Kim JY. Regulation of short-distance transport of RNA and protein. Curr Opin Plant Biol. 2005;8(1):45–52.
- Kong LJ, Hanley-Bowdoi L. A geminivirus replication protein interacts with a protein kinase and a motor protein that display different expression patterns during plant development and infection. Plant Cell. 2002;14(8):1817–1832.
- Zhong R, Burk D, Morrison WH, et al. A kinesin-like protein is essential for oriented deposition of cellulose microfibrils and cell wall strength. Plant Cell. 2002;14(12):3101–3117.
- Kellmann JW, von Bargen S, Heinze C, et al. Isolation of a DnaJ homologous protein from Arabidopsis thaliana interacting with NSm, the tomato spotted wilt tospovirus movement protein. Beiträge zur Züchtungsforschung, Quedlinburg: Bundesanstalt für Züchtungsforschung an Kulturpflanzen 2000;6:96–100.