Abstract
Extracts from the rhizomes of Paris species have been used in Asia to help treat inflammation, infection, analgesia, detoxification, dysentery, etc. Analysis of the chemical components from the rhizomes of Paris vietnamensis showed that they contained polyphenols, flavons, flavonols, flavonoids, tannins and steroidal and triterpenoid saponins. In this study, P. vietnamensis was identified from the sample collected from Sapa, Laocai province, Vietnam and three steroidal glycosides from rhizomes of this species were evaluated for anticancer and antimicrobial activity in vitro. The samples were identified as belonging to P. vietnamensis species through a combination of morphological characteristics and DNA barcodes based on the nucleotide sequence of the ITS and matK. All three steroidal glycosides exhibited strong cytotoxic activity against SK-LU-1, HeLa and MKN7 cell lines with IC50 values ranging from 1.07 to 4.37 µmol L−1, and exhibited high antimicrobial activity against strains of Serratia marcescens, Escherichia coli, Lactobacillus plantarum and Bacillus subtilis. This study provides information on the antibacterial and inhibitory activities of steroidal glycosides derived from the rhizomes of P. vietnamensis collected from Sapa, Laocai province, Vietnam on cancer cell lines. Thus, P. vietnamensis could be used as an alternative to other species of the Paris genus for anticancer and antimicrobial treatment.
Introduction
Traditionally, root and tuber extracts of the Paris species are used for their anti-inflammatory properties, analgesic effects, detoxification and for treatment of some diseases such as cough and epilepsy [Citation1]. Currently, there are eight species of the Paris genus, including P. caobangensis, P. dunniana, P. cronquistii, P. vietnamensis, P. polyphylla, P. delavayi, P. fargesii and P. xichouensis, which are found in many provinces of Vietnam, such as Laocai, Hagiang, Ninhbinh, Thainguyen, Langson, Hoabinh and Thanhhoa. The Paris species are scattered over evergreen tropical forests and in humid and humus soil in mountainous areas at altitudes ranging from 100 m to 1500 m [Citation2]. Rhizomes of Paris plants contain saponin, flavonols, sphingolipids and many other glycosides [Citation1]. Many phytochemicals found in the stem and roots of Paris plants have pharmacological activities, such as alkaloids, flavonoids, saponins, carbohydrates, glycosides, terpenoids, sterols, quinones, phenols and tannins [Citation3]. Saponins in the rhizomes of Paris plants exhibit antibacterial, antiviral and anti-inflammatory action and promote detoxification. Saponins are also a component of some pharmaceuticals used for inhibition of the development of cancer cells [Citation4–6].
Research on saponins has been mainly concentrated on P. polyphylla. The rhizomes of P. polyphylla are rich in phytochemical compounds, and they can be used as a potential source for the isolation of active drug compounds and play important role in medicine. Past studies have provided evidence of the ability of steroidal saponins from P. polyphylla to act as the bioactive compounds that exhibit cytotoxic and antimicrobial activity. For example, there has been studies on the structures of new saponins isolated from the rhizomes of P. polyphylla [Citation7,Citation8] as well as on the phenolic and flavonoid content, antioxidant and antimicrobial activity [Citation9,Citation10] and anticancer activity of steroid saponins in vitro and in vivo [Citation11,Citation12]. However, there are also some reports on the biological and chemical characteristics of saponins from some species of the Paris genus [Citation13–15]. A chemotaxonomic study based on steroidal saponins reported by Wang et al. [Citation14] shows that 70 steroidal saponins have been isolated from various species of the Paris genus. The biological activity of these steroid saponins has been determined to be more active than saponins from P. polyphylla [Citation14]. In this work, we present the results of species identification and the extraction and isolation of three steroidal glycosides of the saponins group from the rhizomes of P. vietnamensis collected from Sapa, Laocai province, Vietnam as well as the evaluation of their cytotoxic and antibacterial activities in vitro.
Materials and methods
P. vietnamensis samples
P. vietnamensis samples were collected from Sapa, Laocai province, Vietnam at an altitude of 1579.6 m at 22°20'29.587″ N; 103°49'10,641″ E. The P. vietnamensis rhizomes were used as the source for extracting saponins and studying their biological activities. P. vietnamensis samples were identified using a comparative morphological method according to Nguyen et al. [Citation2] and the Tropicos website [Citation16]. At the same time, the P. vietnamensis samples were also identified based on DNA barcodes with the nucleotide sequences of the ITS region and matK gene fragment.
Cancer cell lines and bacterial strains
Cancer cell lines, including the SK-LU-1 cell line (Human lung carcinoma), the HeLa cell line (Human cervical carcinoma) and the MKN7 cell line (Human gastric carcinoma), were used in the cytotoxic assays.
Bacterial strains: Two Gram-negative (Escherichia coli and Serratia marcescens) and four Gram-positive (Staphylococcus aureus, Sarcina lutea, Lactobacillus plantanum and Bacillus subtilis) bacteria were selected for the antibacterial activity assay. They were grown in liquid Luria-Bertani (LB) medium (5 g L−1 yeast extract, 10 g L−1 peptone, 5 g L−1 NaCl, pH 7.0) overnight at 28 °C, and the diluted bacterial suspension (106 mL−1) was ready for detection.
Determining the nucleotide sequence of the ITS region and matK gene
Total DNA was extracted from young leaves [Citation17]. The ITS region and matK fragment were amplified by PCR with primer pairs ITS-F/ITS-R and matK-F/matK-R () according to Kress et al. [Citation18].
Table 1. Nucleotide sequences of the PCR primer pairs.
The thermal cycle of PCR for amplification of ITS was as follows: 4 min at 94 °C, 35 cycles of 30 s at 94 °C, 60 s at 58 °C and 60 s at 72 °C, and a final extension for 10 min at 72 °C. For amplification of the matK fragment: 1 min at 94 °C, 35 cycles of 30 s at 94 °C, 40 s at 53 °C and 40 s at 72 °C, and a final extension for 5 min at 72 °C. PCR products were detected by 1.0% agarose gel electrophoresis and purified using the QIAquick Gel Extraction Kit. The nucleotide sequences of the ITS region and matK were sequenced and analysed using the BLAST in NCBI [Citation19].
Extraction and isolation of steroidal saponins
Crude extracts from 500 g dried rhizomes of P. vietnamensis were cut into pieces with 80% alcohol on a refluxing machine after 24 h and were cleaned with a mixture of ethylacetate and n-butanol to collect pure extracts.
The polyphenols, flavonoids, tannins and saponins were discovered in the solution using colour reactions. Polyphenols were detected by reaction with iron salts (III), flavons and flavonols – with H2SO4, flavonoids – with Mg in HCl solution, tannins – with vanillin/HCl, steroid saponins and triterpenoid saponin were detected by foaming reactions in an alkaline environment (NaOH) or in an acidic environment (HCl). Pure substances were isolated by column chromatography containing silica gel with a particle size of about 63–20 µm. The fraction was extracted in turn with CH2Cl2 and n-butanol (n-BuOH). Segments were analysed by thin layer chromatography.
The substance isolated was identified using nuclear magnetic resonance (NMR) spectroscopy in DMSO-d6 solvents and mass spectra (MS) on the Bruker DRX-500 MHz spectrometers with TMS internal standard. Isolated compounds were dissolved in DMSO-d6 solvent, and experimented on the Bruker DRX-500 MHz spectrometers system with TMS internal standard to afford 1H, 13C, DEPT-135 and 2 D-NMR data. MS spectra were measured on Agilent 1100-LC/MSD Trap mass spectrometers.MS spectral data were afforded ion peaks of compounds for identification of structural compounds.
Cytotoxic assays
Cancer cytotoxicity was determined using the method of Monks et al. [Citation20]. The substances extracted from P. vietnamensis rhizomes were prepared and tested at four concentrations, including 100 µg mL−1, 20 µg mL−1, 4 µg mL−1 and 0.8 µg mL−1. Ellipticine was used as a reference at four concentrations: 10 µg mL−1, 2 µg mL−1, 0.4 µg mL−1 and 0.08 µg mL−1. Further, 10% dimethyl sulfoxide (DMSO) was used as a negative control.
The total protein content of a cell is determined based on the optical density (OD) when the proteins of the cell are stained with sulforhodamine B (SRB). The OD results were read on a wave step of 515–540 nm in an ELISA Plate Reader. OD values are proportional to the amount of SRB, which is attached to protein molecules. The larger the OD value, higher the amount of protein and higher the amount of cells.
Cytotoxicity was expressed as the concentration of drug that inhibited cell growth by 50%. Inhibitory concentration 50% (IC50) is the concentration of the sample at which it can inhibit 50% of cells, free radicals, or enzymes. The substance is considered to have good activity when IC50 ≤5 µM [Citation21].
Antibacterial assays
In order to determine the antibacterial activity of saponin, 50 µL of the diluted bacterial suspension (106 mL−1) was brushed on 0.5-cm-thick LB plates. The LB plates were perforated with 0.5-cm-diameter holes, and each hole was supplemented with 100 µL of three steroidal glycoside solutions extracted and isolated from the P. vietnamensis rhizomes with different concentrations (0.1, 0.4, 0.8, 1.0 and 1.5 mg mL−1) or with DMSO for the control. The inhibition activity of saponin against bacterial growth was observed after incubation at 28 °C for two days. The antibacterial levels were determined by the diameter of the inhibition zones (in millimetres) around the wells.
Results and discussion
P. vietnamensis species identification from samples collected from Sapa, Laocai, Vietnam
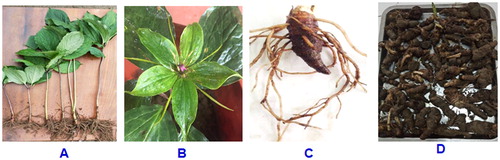
Using a comparative morphological method according to Nguyen et al. [Citation2] and based on the description of the Tropicos website [Citation16], the samples collected from Sapa, Laocai, Vietnam were initially identified as a P. vietnamensis species ().
Figure 1. Some morphological characteristics of (A) P. vietnamensis: the P. vietnamensis trees in young stage, (B) flower and tepals of the plants at the mature stage, (C) the fresh rhizome, and (D) dried rhizomes.
Results of amplification of the ITS region and matK fragment by PCR are showed in . The PCR products of Paris samples using the ITS primer pair consisted of a DNA band about 0.7 kb in size (), while the matK primer pair resulted in a DNA band about 0.8 kb in size (. PCR products were used to determine the nucleotide sequence of the ITS region and matK. The obtained results showed that the ITS region was 675 bp in length and matK was 787 bp in length. The ITS region isolated from P. vietnamensis plants includes a portion of 18S rDNA gene fragment, the non-coding segment ITS1,5.8S rDNA gene, non-coding segment ITS2 and a portion of the 28S rDNA gene fragment.
Figure 2. Result of electrophoresis PCR products to test (A) ITS region and (B) matK gene. M: 1.0 kb DNA marker; 1, 2, 3, 4: Paris samples collected in Sa Pa, Lao Cai province, Vietnam.

The results of BLAST analysis based on the nucleotide sequence of the ITS region and matK showed that our collected samples belonged to P. vietnamensis. The ITS sequences isolated from Paris samples in Sapa were 95% homologous to the two ITS sequences of P. vietnamensis (GenBank accession numbers DQ486018 and JF977362) [Citation22,Citation23]. The ITS sequences of our collected samples have been published in the GenBank with accession number LT853586 [Citation24]. Thus, it can be concluded that the ITS region isolated from samples of the Paris genus in Sapa (Vietnam) is the ITS region of P. vietnamensis species.
The similarity rate of the matK sequences isolated from the Paris samples in Sa Pa was 96% in comparison to the sequence of the matK of P. vietnamensis (GenBank accession number KX784050) [Citation25]. Thus, these results demonstrated that the gene isolated from samples of the Paris genus in Sapa (Vietnam) is the matK of P. vietnamensis species. The matK sequence has been published in the GenBank with accession number LT853583 [Citation26].
The Paris genus has 22 species, and in Vietnam eight species have been recorded in the North and the Central highlands, one among them is P. vietnamensis (Takht) Li [Citation2]. P. vietnamensis is a flowering plant described by Li in 1984 that belongs to the Paris genus in the Melanthiaceae family [Citation27]. Based on morphological characteristics of stems, roots and leaves, a plant can be initially identified as P. vietnamensis. However, it is difficult to distinguish between certain species of the Paris genus correctly based only on morphology. Thus, we used DNA barcodes with the ITS region and the matK gene to identify the P. vietnamensis collected from Sapa (Laocai), Vietnam. The ITS region of the nuclear genome and matK of the chloroplast genome are highly conserved; thus, they can be used to distinguish species with similar morphological characteristics. The ITS region includes internal transcribed spacer regions (ITS1 and ITS2), 5.8S ribosomal RNA gene, a portion of the 18S ribosomal RNA gene, and a portion of the 28S ribosomal RNA gene [Citation28,Citation29]. The matK gene encodes the maturase enzyme involved in the elimination of introns during RNA transcription [Citation30,Citation31]. At present, the ITS region and matK are considered the most useful markers for identifying and evaluating plant phylogenesis [Citation28,Citation29]. In our study, we identified P. vietnamensis through a combination of morphological characteristics and DNA barcodes based on the nucleotide sequences of the ITS region and matK. The species identification results are the basis for evaluating and exploiting bioactive substances from P. vietnamensis.
Determination of some secondary compounds from P. vietnamensis
A total of 71.83 g of crude extracts were obtained from 500 g dried rhizomes, and after treatment of crude extracts, 40.695 g of pure extract were obtained, reaching a rate of 8.14%. Substances with different bioactivities were identified in the extract by colour reactions. The polyphenols, flavons and flavonols, flavonoids, tannins, steroid saponins and triterpenoid saponins were detected in extracts from the rhizomes of P. vietnamensis (Supporting material Figure S1). The results show that the extract from the rhizomes of P. vietnamensis plants contained two saponin groups: steroid saponins and triterpenoid saponins.
Isolation and identification of saponin compounds of pure extracts from the P. vietnamensis rhizomes
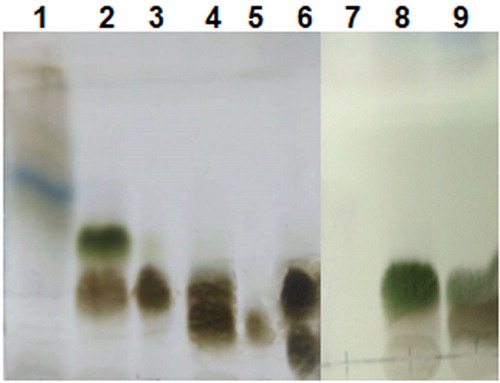
From 20 g of the extract, six segments were obtained by column chromatography using silica gel as an absorbent with the dichloromethane/methanol solvent system at a ratio of 9:1. The pieces were tested by a thin layer chromatography to look for clear clues of substances separated, not coincided (). From this result (), three pure substances were collected, including the first white substance collected from the third piece, the second white solid substance collected from the fifth piece and the second segment. Due to the new position and different colour of the second segment, we continued to run the barcode column containing the second segment with a washing solvent system of dichloromethane/methanol at the ratio of 4:1 (v/v), the collected product was the third substance (. The colour reaction used in this work was based on published data and use of reference standards in comparison with the colour reaction of similar compounds isolated from some other plants.
Figure 3. Results of thin layer chromatographic analysis: from the 1st to 6th segments that were separated by the dichloromethane/methanol solvent system at a 9:1 ratio and from the 7th to 9th segments that were separated by the dichloromethane/methanol solvent system at a 4:1 ratio.
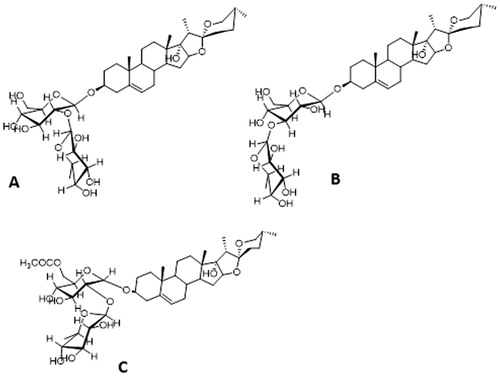
Three isolated pure substances were analysed using NMR nuclear resonance spectra in DMSO-d6 solvent and mass spectrometry (MS). The MS spectrum of the first substance with an ion peak of m/z 757 [M + H2O−1] has been analysed and compared with the results of Xie et al. [Citation32].This substance was identified as 25(R)-spirost-5-en-3β,17a-diol-3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside, which was isolated for the first time from Polygonatum prattii species (. The ion peak of the second substance is m/z 757 [M + H2O−1]. Comparison of the nuclear magnetic resonance spectrum of the second substance with the compound isolated from Dracaena thalioides [Citation5] revealed complete homology, and this substance was identified as 25(R)-spirost-5-en-3β,17a-diol-3-OaL-rhamnopyranosyl-(1→3)-β-D-glucopyranoside (. The ion peak of the third substance was m/z 779 [M-H]–. By comparing the results of nuclear magnetic resonance spectrum, the third substance was identified as 25(R)-spirost-5-en-3β,17a-diol-3-OaL-rhamnopyranosyl-(1→2)-6-acetyl-β-D-glucopyranoside (. This result is consistent with a study conducted by Liu et al. [Citation15]. Thus, the three saponin types extracted from Paris vietnamensis collected from Sapa, Laocai province, Vietnam are steroidal glycosides.
Figure 4. The structure of three steroidal glycosides isolated from rhizomes of P. vietnamensis: (A) 25(R)-spirost-5-en-3β,17a-diol-3-O-α-L-rhamnopyranosyl-(1→2)-β-D-glucopyranoside; (B) 25(R)-spirost-5-en-3β,17a-diol-3-O-α-L-rhamnopyranosyl-(1→3)-β-D-glucopyranoside; (C)25(R)-spirost-5-en-3β,17a-diol-3-OaL-rhamnopyranosyl-(1→2)-6-acetyl-β-D-glucopyranoside.
Cytotoxic activity of three steroidal glycosides against cancer cell lines
Three steroidal glycosides, extracted and isolated from the rhizomes of Paris vietnamensis, were assayed on three cancer cell lines, including SK-LU-1 cell line (Human lung carcinoma), HeLa cell line (Human cervical carcinoma) and MKN7 cell line (Human gastric carcinoma) (). The results in show that the three steroidal glycosides are all inhibitors of the cancer cell lines SK-LU-1, HeLa and MKN7 to different degrees, with IC50 values ranging from 1.07 ± 0.13 to 4.37 ± 0.59 (µM).
Table 2. Cytotoxicities of three steroidal glycosides from P. vietnamensis against the human cancer cell lines in vitro (IC50, µmol L–1).a
According to Wang et al. [Citation3] and Rajsekhar et al. [Citation33], the rhizomes of the P. polyphylla Paris genus are rich in important medicinal phytochemical compounds, such as alkaloids, flavonoids, saponins, carbohydrates, glycosides, cardiac glycosides, terpenoids, sterols, quinones, phenols and tannins. From the rhizomes of P. vietnamensis, we have also identified bioactive compounds such as polyphenols, flavons and flavonols, flavonoids, tannins, steroid saponins and triterpenoid saponins. These results reinforced the conclusion that rhizomes from species in the Paris genus contain many valuable compounds for medicine.
Saponins are natural plant glycosides with strong foam-forming properties. The glycosides have medicinal activity, such as antitumour, antiviral, antifungal, antiallergy, immune-modulating and hypoglycemic activities [Citation34]. Since 1980, Sheo et al. [Citation8] have reported that four new saponins, polyphyllins C, D, E and F, from the tubers of P. polyphylla have been isolated, and their structures have been identified. When studying the steroidal saponins from P. polyphylla var. Yunnanensis, Wu et al. [Citation7] have isolated eleven saponins, along with seven known steroidal saponins from the rhizomes of Paris polyphylla var. yunnanensis, and their chemical structures were elucidated. These compounds were tested for cytotoxic effects on human nasopharyngeal carcinoma epithelial cells, and seven compounds displayed more potent inhibitory effects than cisplatin (the positive control) [Citation7]. Moreover, this research provided evidence of the role of steroidal saponins as main bioactive compounds that exhibit cytotoxicity. Steroidal saponins trigger apoptosis via intrinsic and extrinsic pathways [Citation35].
Six pennogenyl saponins were isolated from the rhizomes P. quadrifolia by Gajdus et al. [Citation36], among which pennogenyl saponins 5 and six exhibited cytotoxic activity against HL-60, HeLa and MCF-7 tumour cells with IC50 values of 1.0 to 3.2. Wen et al. [Citation13] investigated saponins from P. fargesii var. brevipetala and found that its rhizomes contained pennogenyl saponins as the main components, with a small amount of diosgenin saponins. The cytotoxic activities of Saponin H were on HepG2, A549, RPE and L929 cells, revealing remarkable cytotoxic activity on A549 cells with an IC50 value of 1.53 ± 0.08 μg mL−1 [Citation13]. Zhu et al. [Citation12] explored the antitumour effects of PSI, II, VI and VII, and the results revealed that PSI, II, VI and VII inhibited the proliferation of PC-9-ZD cells and induced significant cell apoptosis. Studies on the anticancer effects of steroidal saponins on experimental mice by Yan et al. [Citation11] showed that the apoptosis rate in tumour cells was increased in comparison to cells in control mice, and that typical apoptosis was induced in a dose-dependent manner. In a report by Liu et al. [Citation15], four new spirostanol saponins were isolated from the rhizomes of P. vietnamensis and their chemical structures were determined. All of these saponins have cytotoxicity against human glioblastoma U87MG and U251 cell lines [Citation15].
According to Puwein et al. [Citation35], cancer is one of the leading causes of mortality in the world. There are many synthetic drugs available for cancer treatment with a number of undesirable side effects. P. polyphylla plants are an alternative therapeutic herbal drug that is comparatively less toxic [Citation35]. However, assessment of the medical and pharmacological properties of steroidal saponins suggests that P. thibetica, P. vietnamensis, P. delavayi and P. pseudothibetica contain saponins more active than P. polyphylla. Therefore, these four Paris species could be used as an alternative to P. polyphylla [Citation14].
In our study, an in vitro approach was used to confirm the cytotoxic activity and antibacterial activity of three steroidal glycosides isolated from the rhizomes of P. vietnamensis. These steroidal glycosides were tested for toxic activity against the cancer cell lines SK-LU1, HeLa and MKN7. The results of the cytotoxic assay showed that all three steroid glycosides had strong inhibitory activity on cancer cell lines, and the IC50 value ranged from 1.07 to 4.37 µM. The steroid glycosides of P. vietnamensis could therefore be used to create new drugs.
Antibacterial activity of three steroidal glycosides
The antibacterial effect of these steroidal glycosides was tested at different concentrations using both Gram-negative (E. coli, L. plantanum and S. marcescens) and Gram-positive (B. subtilis) bacteria via the agar diffusion method. However, these steroidal glycosides had no bactericidal effects on Gram-positive bacteria, such as S. aureus and S. lutea (). The three steroidal glycosides showed the highest inhibition zones against B. subtilis at all concentrations (Supporting material Figure S2).
Table 3. Antimicrobial activity of three steroidal glycosides on different bacterial strains.
Previous research has suggested that P. polyphylla is a potent antimicrobial agent. The main compounds responsible for its antimicrobial activity are steroidal saponins, which are concentrated mostly in its rhizomes. Six new steroidal saponins from P. polyphylla var. yunnanensis showed the best activity with Propionibacterium acnes at an MIC value of 3.9 μg mL−1 [Citation9]. These compounds showed antimicrobial activity against a wide range of bacteria, such as Agrobacterium tumefaciens, B. subtilis, E. coli, S. aureus, Helicobacter pylori, Xanthomonas vesicatoria, Staphylococcus haemolyticus and Pseudomonas [Citation37]. Mayirnao and Bhat [Citation10] have reported that the rhizome extract of P. polyphylla species exhibited significant antimicrobial activity, and it was observed that at concentration of 5 mg mL−1 the zone of inhibition was 30.66–31.33 in E. coli and S. aureus. Our research on the antimicrobial effects of three steroidal glycosides from the rhizomes of P. vietnamensis confirmed the results from previous studies on the extracts of P. polyphylla. The three steroidal glycosides identified from P. vietnamensis have the potential to be used as substitutes for chemical antibiotic drugs.
Conclusions
Based on a combination of the morphological characteristics and DNA barcodes, samples P. vietnamensis were identified prior to chemical composition analysis and assessment of bioactive compounds. The experimental results revealed the anticancer and antimicrobial activity of three steroidal glycosides that were isolated from the rhizomes of P. vietnamensis collected from Sapa, Laocai province, Vietnam. These three steroidal glycosides have strong cytotoxic activity against SK-LU-1, HeLa and MKN7 cell lines with IC50 value ranging from 1.07 to 4.37 µM. They also exhibit high antibacterial activity against S. marcescens, E. coli, L. plantanum and B. subtilis. Therefore, it is necessary to continue such studies in order to identify new bioactive compounds from the rhizomes of P. vietnamensis for anticancer and antimicrobial treatment.
Author contributions
Conceived and designed the experiments: LTKV, MHC, TTTV, KVP, QHN. Performed the experiments: TTTV, QHN, KVP, LTNN, DTN. Performed analyses and wrote the article: LTNN, QHN, LTKV, HMC. Did the proof-reading: LTKV, HMC.
Supplemental Material
Download PDF (559.6 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Do TL. Medicinal plants and medicines in Viet Nam. Vietnam: Medical Publishing House; 2004.
- Nguyen QN, Pham TH, Phan VT, Hoang VT. Taxonomy of the genus Paris L. (Melanthiaceae) in Vietnam. J Biol (Vietnam). 2016;38:333–339. doi.
- Wang YC, Yi TY, Lin KH. In vitro activity of Paris polyphylla Smith against enterovirus 71 and coxsackievirus B3 and its immune modulation. Am J Chin Med. 2011;39(06):1219–1234.
- Zhang J, Shen T, Wang Y, et al. Chemical assessment of wild Paris rhizome from Southwest China. Afr J Pharm Pharmacol. 2012;6(40):2802–2807.
- Tang LY, Wang ZJ, Wu HW, et al. Steroidal glycosides from the underground parts of Dracaena thalioides and their cytotoxic activity. Phytochemistry. 2014;107:102–110.
- Wei JC, Gao WY, Yan XD, et al. Chemical constituents of plants from the genus Paris. Chem Biodivers. 2014;11:1277–1297.
- Wu X, Wang L, Wang H, et al. Steroidal saponins from Paris polyphylla var. yunnanensis. Phytochemistry. 2012;81:133–143.
- Sheo BS, Thakur RS, Hans RS. Spirostanol saponins from Paris polyphylla, structures of polyphyllin C, D, E and F. Phytochemistry. 1980;21:2925–2929.
- Qin XJ, Sun DJ, Ni W, et al. Steroidal saponins with antimicrobial activity from stems and leaves of Paris polyphylla var. yunnanensis. Steroids. 2012;77(12):1242–1248.
- Mayirnao H, Bhat A. Evaluation of antioxidant and antimicrobial activity of Paris polyphylla SM. Asian J Pharm Clin Res. 2017;10(11):315–319.
- Yan LL, Zhang YJ, Gao WY, et al. In vitro and in vivo anticancer activity of steroid saponins of Paris polyphylla var. yunnanensis. Exp Oncol. 2009;31(1):27–32.
- Zhu XH, Jiang H, Li J, et al. Anticancer effects of Paris Saponins by apoptosis and PI3K/AKT pathway in Gefitinib-Resistant Non-Small Cell Lung Cancer. Med Sci Monit. 2016;22:1435–1441.
- Wen F, Yin H, Chen C, et al. Chemical characteristics of saponins from Paris fargesii var. brevipetala and cytotoxic activity of its main ingredient, paris saponin H. Fitoterapia. 2012;83(4):627–635.
- Wang Y, Gao W, Li X, et al. Chemotaxonomic study of the genus Paris based on steroidal saponins. Biochem Syst Ecol. 2013;48:163–173. https://doi.org/10.1016/j.bse.2012.12.011
- Liu Y, Wang M, Liu K, et al. New Steroidal saponins from the rhizomes of Paris vietnamensis and their cytotoxicity. Molecules. 2018;23(3):588. [cited 2019 Aug 06].
- Li H. Paris vietnamensis (Takht.) Tropicos.org. [Internet]. Saint Louise (MI): Missouri Botanical Garden. Available from: http://www.tropicos.org/Name/18404296
- Saghai-Maroof MA, Soliman KM, Jorgensen RA, et al. Ribosomal DNA spacer-length polymorphisms in barley: Mendelian inheritance, chromosomal location, and population dynamics. Proc Natl Acad Sci U S A. 1984;81(24):8014–8018.
- Kress JW, Wurdack KJ, Zimmer EA, et al. Use of DNA barcodes to identify flowering plants. Proc Natl Acad Sci U S A. 2005;102(23):8369–8374.
- NCBI. Basic Local Alignment Search Tool (Blast) [Internet]. Bethesda (MD): National Center for Biotechnology Information. Available from: https://blast.ncbi.nlm.nih.gov/Blast.cgi
- Monks A, Scudiero D, Skehan P, et al. Feasibility of a high-flux anticancer drug screen using a diverse panel of cultured human tumor cell lines. J Natl Cancer Inst. 1991;83(11):757–766.
- Hughes JP, Rees S, Kalindjian SB, et al. Principles of early drug discovery. Br J Pharmacol. 2011;162(6):1239–1249.
- Tang MX, Wang L, Weng Z. Paris vietnamensis internal transcribed spacer 1, 5.8S ribosomal RNA gene, and internal transcribed spacer 2, complete sequence. GenBank: DQ486018.1. 2007 May 1. Available from: https://www.ncbi.nlm.nih.gov/nuccore/DQ486018
- Li DZ, Gao LM, Li HT, et al. Paris vietnamensis voucher JYH77C 18S ribosomal RNA gene, partial sequence; internal transcribed spacer 1, 5.8S ribosomal RNA gene, and internal transcribed spacer 2, complete sequence; and 28S ribosomal RNA gene, partial sequence. GenBank: JF977362.1. 2012 Jul 12. Available from: https://www.ncbi.nlm.nih.gov/nuccore/JF977362
- Vu TTT, Dinh HT, Ho TM, et al. Paris vietnamens is genomic DNA sequence contains 18S rRNA gene, ITS1, 5.8S rRNA gene, ITS2, 28S rRNA gene, isolate Sapa. GenBank: LT853586.1. 2017 May 12. Available from: https://www.ncbi.nlm.nih.gov/nuccore/LT853586
- Huang Y, Li X, Yang Z, et al. Paris vietnamensis chloroplast, complete genome. GenBank: KX784050.1. 2017 May 12. Available from: https://www.ncbi.nlm.nih.gov/nuccore/KX784050
- Vu TTT, Dinh HT, Ho TM, et al. Paris vietnamensis chloroplast matK gene for maturase K, isolate Sapa. GenBank: LT853583.1. 2017 May 12. Available from: https://www.ncbi.nlm.nih.gov/nuccore/LT853583.1
- Li H. Paris vietnamensis (Takhtajan). Acta Bot Yunnan. 1984;6:357.
- van de Wiel CCM, van der Schoot J, van Valkenburg JLCH, et al. DNA barcoding discriminates the noxious invasive plant species, floating pennywort (Hydrocotyle ranunculoides L.f.), from non-invasive relatives. Mol Ecol Resour. 2009;9(4):1086–1091.
- Vijayan K, Tsou CH. DNA barcoding in plants: taxonomy in a new perspective. Curr Sci. 2010;99:1530–1540.
- Huang Y, Li X, Yang Z, et al. Analysis of complete chloroplast genome sequences improves phylogenetic resolution in Paris (Melanthiaceae). Front Plant Sci. 2016;7:1797.
- Song Y, Wang S, Ding Y, et al. Chloroplast genomic resource of Paris for species discrimination. Sci Rep. 2017;7(1):3427. [cited 2019 Aug 06].
- Xie BB, Liu HY, Ni W, et al. Ypsilandrosides C-G, five new spirostanol saponins from Ypsilandra thibetica. Steroids. 2009;12:950–955.
- Rajsekhar PB, Arvind Bharani RS, Jini Angel K, et al. Extraction of Paris polyphylla Rhizome using different solvents and its phytochemical studies. Int J Adv Res Biol Sci. 2016;8:18–21.
- Lacaille-Dubois MA, Wagner H. A review of the biological and pharmacological activities of saponins. Phytomedicine. 1996;2(4):363–386.
- Puwein A, Thomas SC, Singha LI. A review on Paris Polyphylla smith: as an effective and alternative treatment of Cancer. Int J Pharm Sci Invent. 2018;7:6–12.
- Gajdus J, Kaczyński Z, Kawiak A, et al. Isolation and identification of cytotoxic compounds from the rhizomes of Paris quadrifolia L. Phcog Mag. 2014;10:S324–S333.
- Zhao J, Mou Y, Shan T, et al. Antimicrobial metabolites from the endophytic fungus Pichia guilliermondii isolated from Paris polyphylla var. yunnanensis. Molecules. 2010;15(11):7961–7970.
