Abstract
Biopharmaceutical engineering is a newly established undergraduate degree in China, and a lot of issues regarding students’ training remain to be explored and refined, particularly the curriculum reflecting the industrial needs for students with applied (hands-on) skills, which has been limited. To narrow the gap between industrial demands and students’ training and to provide undergraduates with rational curriculum, in the present investigation, we proposed to reconstruct the curriculum system for applied (hands-on) training of undergraduates of biopharmaceutical engineering based on the life cycle of medicinal products for the first time. A total of 52 subjects on the curriculum were selected and included in different stages of the medicinal products life cycle. After surveying from a recruitment website and biopharmaceutical enterprises, eight applied positions, including drug screening engineer, cell culture engineer, molecular biology technician, fermentation engineer, microorganism engineer, protein purification engineer, protein analysis technician and bio-preparation engineer, were then selected. According to the requirements of each position, 31 subjects were finally chosen from the above-mentioned 52 subjects that are involved in the life cycle of medicinal products. These subjects belong to four clusters and constitute the basis of the curriculum system for applied training of undergraduates of biopharmaceutical engineering. The results obtained from this work are expected to provide evidence for the training of biopharmaceutical students as well as the implementation of collaborative education between university and industry.
Introduction
The fourth industrial revolution will exert profound impacts on the business process, leading to high efficiency and productivity. On the side of products, they will generate more value from data for usage-based design and customization, thereby opening the way to new markets. Hence, the implementation of industry 4.0 will cause the elimination or substantial reduction of lower skilled jobs owing to substitution by automation and digitalization [Citation1], which will demand significant changes in higher engineering education.
Curriculum, the expression of educational ideas in practice, usually exists on three levels: what is programmed for students, what can be delivered to students and what students can experience [Citation2]. The design of a curriculum on an aspect of the process of defining and organizing teaching contents, is a dynamic process due to the fact that its inception usually emerges from previous experiences and needs to be revised in a timely manner according to the social development and progress [Citation3]. Exactly as the concept of sustainability in engineering education [Citation4], higher engineering education should orient the training of students in applied (hands-on) skills and act jointly with industry to design new courses in accordance with the needs of the industry.
Product life cycle (PLC) refers to the entire process of a product, extending from the time it is first available on the market until it is withdrawn [Citation5], and life cycle assessment (LCA), also known as ‘from cradle to grave’ analysis, is a powerful tool for assessment of the environmental effects on products, processes and activities during their life-span [Citation6]. In 2002, Hashimoto expanded the boundaries of PLC to the development of a curriculum in higher education [Citation7], which could be the first time to establish a connection between industry and related subjects on the curriculum. To date, the use of PLC and LCA is likely to be equally applicable to formulate goals in curriculum design for engineering undergraduates [Citation8].
Biopharmaceutical engineering in China is a newly established degree in 2012 after the adjustment of undergraduate degrees in the Ministry of Education of the People’s Republic of China. It belongs to bioengineering, and is an emerging cross-disciplinary degree with strong background of engineering technology based on biotechnology, pharmacy, chemistry, engineering, biology and others [Citation9]. Along with the high-speed development of biopharmaceutical industry in China, biopharmaceutical industry has become one of the most promising areas of economic growth, promoting a surge in demand for highly professional graduates. By the end of 2017, approximately 65 regular universities in China have established the program of biopharmaceutical engineering [Citation10]. However, due to being a newly established degree, little mature school-running experience can be used for reference, especially the design of curriculum for applied (hands-on) training of students according to industrial needs. Thus, the search for an industry-oriented curriculum of biopharmaceutical engineering degree remains an ongoing concern currently.
Suzhou is the most economically advanced prefecture-level city in China, and biopharmaceutical industry is one of its promising mainstay industries. By the end of 2017, approximately 3000 pharmaceutical enterprises were located in Suzhou, and the compound annual growth rate was up to 18%. By 2020, the output value of the biopharmaceutical industry is expected to reach RMB 200-billion yuan [Citation11]. Changshu Institute of Technology (CIT), one of the three undergraduate universities situated in Suzhou is dedicated to the cultivation of students with applied (hands-on) skills. Depending on the advantages of biopharmaceutical industry in Suzhou, the school-running process of biopharmaceutical engineering program at CIT always melts with biopharmaceutical enterprises and the curriculum system was timely refined according to industrial needs. In this paper, taking the recent reconstruction of the curriculum system for biopharmaceutical engineering degree at CIT as an example, the curriculum design for applied (hands-on) training of students of biopharmaceutical engineering based on the life cycle of medicinal products was first reported. It is hoped that this work will provide evidence and reference for other universities and will promote the construction of a sustainable curriculum in the engineering education.
Background
Undergraduate degree on biopharmaceutical engineering in CIT
CIT was founded in 1958 and located in Changshu, Suzhou, Jiangsu, China. CIT is a provincial full-time undergraduate university and characterized by training students to master applied skills in industry. There are 54 undergraduate programs of 14 schools that involve seven disciplines such as engineering, science, literature, economics, management, education and arts. Over 20,000 undergraduate students, 100 joint master students and 1000 full-time teachers have been enrolled at CIT [Citation12].
As one of the schools at CIT, the School of Biology and Food Engineering has established five undergraduate programs, including bioengineering, bioscience, biopharmaceutical engineering, food science and engineering and food safety and quality. Among them, bioengineering was founded in 2006, and biopharmaceutical engineering was one of the major orientations. In 2014, biopharmaceutical engineering was applied to the Ministry of Education to become an independent program. In 2015, biopharmaceutical engineering recruited new undergraduate students. After four years of development, the conditions of running schools have gained significant improvements, about 270 undergraduate students, 20 professional teachers and 19 experimental instructors having been enrolled in the biopharmaceutical engineering program of CIT [Citation13].
The life cycle of medicinal products
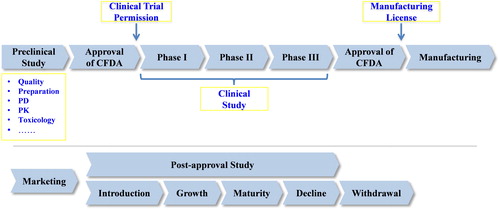
Medicinal products (MPs) mainly comprise three different types of products, including chemical drugs, natural products and biopharmaceutical agents. MPs belong to special commodities by virtue of the fact that their qualities are highly related to the life and health of consumers. Thus, when compared with ordinary good, the research and development, manufacturing, marketing as well as post-approval evaluation of MPs must be subject to strict monitoring. Although, the origin, physicochemical properties, production and quality control of biopharmaceutical agents are quite different from chemical and natural medicines, they share a similar product life cycle. As shown in , the life cycle of MPs primarily includes six stages: (1) Preclinical study: aim of this stage is to obtain the candidate MPs with development potential and to prepare the documentations that are used for the application of clinical trial permission from China Food and Drug Administration (CFDA). Several evaluations, such as bioassay, active ingredient, quality, preparation, pharmacodynamics (PD), pharmacokinetics (PK) and safety should be conducted. Particularly the safety of candidate MPs must be verified by a series of strict assays. In addition, more attention should be equally paid to the issues regarding intellectual properties [Citation14,Citation15]. (2) Clinical study: after receiving clinical trial permission, the phase I clinical trial must be initiated and implemented in hospitals that have been accredited by CFDA. The aim of phase I trials is to explore the maximal tolerated dose and PK properties in human beings, and therefore, healthy volunteers are usually chosen as subjects. The data obtained from phase I trials have to be submitted to the Center for drug evaluation in CFDA for the approval of a phase II trial, which focuses on the PD profiles of candidate MPs in eligible patients. The phase III trial, also known as ‘expanded phase II trial’ orients the confirmation of data obtained from phase II trial, therefore, more subjects, over 300 eligible patients are required [Citation16]. (3) Manufacturing: in China, after phase III clinical trials, if the requirements are met, the manufacturing license can be conferred to applicant, then the licensed MPs can be manufactured in a factory that has been accredited by CFDA according to the requirements of good manufacturing practice (GMP) [Citation17]. Owing to the fact that MPs production requires high professionalism and sophisticated skills, coupled with the diversity of product types, the field of manufacturing should be paid more attention when formulating curriculum for degrees in pharmaceuticals, particularly the training of students with applied (hands-on) skills. (4) Marketing: unlike other products, the marketing of MPs must follow some regulations issued by CFDA, for example, MPs must be sold in hospital and community pharmacies or other medical and health institutions such as centers for disease control and prevention. The propagation of prescription drugs has to be conducted in the medical journals instead of public media [Citation18,Citation19]. (5) Post-approval study: MPs also experience the period of introduction, growth, maturity and decline as other merchandise [Citation5], but every new product, within five years of marketing, must be strictly monitored by CFDA, which is called timeframe for monitoring period of new drugs, or phase IV clinical trial, which demands over 1000 eligible patients [Citation15,Citation16]. (6) Withdrawal: many factors, including patent expiration, changes of disease spectrum, adjustment of industrial policies and so on could lead to the recession of MPs on the market, causing their withdrawal eventually [Citation20].
Methods
Selection of subjects on the curriculum based on the life cycle of medicinal products
The selection of subjects on the curriculum for the biopharmaceutical engineering degree was in line with the technical requirements of a medicinal product lifecycle, particularly the stages, such as preclinical study and manufacturing, that need more applied-skills students to participate. Meanwhile, the national standards for bioengineering [Citation21], pharmaceutical engineering [Citation22] and pharmacy teaching [Citation23] were referenced. In addition, the subjects on the curriculum for the biopharmaceutical engineering degree of other universities were also searched from their websites.
Survey on the demands for biopharmaceutical students from a recruitment website
Information on the demands for biopharmaceutical students was obtained by search from www.zhaopin.com. . . . . . . . , a popular recruitment website in China, using the following key words ‘Biopharmaceutical engineering’, ‘Shanghai and Jiangsu province’, ‘Research and development’, ‘Manufacturing’, ‘Quality management’ and ‘Marketing’.
Survey on the demands for biopharmaceutical students from enterprises
In order to further understand the needs of enterprises for biopharmaceutical students, five different types of biopharmaceutical enterprises located in Shanghai, Suzhou and Wuxi were selected as respondents. presents the details of the enterprise survey.
Table 1. Details of enterprise survey.
Relevance of subjects on the curriculum to the applied positions in biopharmaceutical industry
According to the demands of enterprises, the typical applied (hands-on) positions in biopharmaceutical industry were summarized, and then, these positions were matched with the subjects obtained from the life cycle of medicinal products based on the requirements of each position.
Results and discussion
Selection of subjects on the curriculum based on the life cycle of medicinal products
Fifty-two subjects on the curriculum for training biopharmaceutical undergraduates were selected based on the life cycle of medicinal products (). It can be seen that 23 and 13 subjects were included in the stages of preclinical study and manufacturing, respectively, followed by the stage of marketing with seven subjects, clinical study with four subjects, post-approval study with three subjects and withdrawal with two subjects ().
Figure 2. Number of subjects on the curriculum that are involved in different stages of medicinal product life cycle.
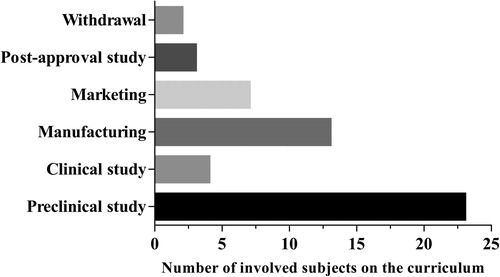
Table 2. Selected curricula for training the biopharmaceutical students based on the life cycle of medicinal products.
Survey on the demands for biopharmaceutical students from recruitment website
After importing the key words ‘Biopharmaceutical engineering’, ‘Shanghai and Jiangsu province’, ‘Research & development’, ‘Manufacturing’, ‘Quality management’ as well as ‘Marketing’, approximately 9006 positions were found, of which 7975 positions were distributed in Shanghai, Suzhou, Nanjing and Wuxi, accounting for 88.6%. As for the educational requirements, PhD degree accounted for 3.1%, master’s degree accounted for 15.0%, whereas undergraduate accounted for 36.9%.
Survey on the demands for biopharmaceutical students from enterprises
The surveyed enterprises represent the emerging types of biopharmaceutical enterprises in China, which are located in Shanghai, Suzhou and Wuxi. As shown in , the undergraduate is one of the most wanted staff members for either production-driven or technology-driven enterprises. The most wanted position is manufacturing technician, followed by product development engineer, suggesting that applied-skills students with strong engineering background may better cater for the needs of the biopharmaceutical industry.
Table 3. Requirements and demands of surveyed biopharmaceutical enterprises for the students.
Relevance of subjects on the curriculum to the applied positions in biopharmaceutical industry
According to the needs of biopharmaceutical enterprises, there are eight applied (hands-on) positions that are mainly included in the processes of manufacturing and product development, such as drug screening engineer, cell culture engineer, molecular biology technician, fermentation engineer, etc. (). The job requirements of each position were also listed in , and the program-related subjects derived from the life cycle of medicinal products were selected and matched with the job requirements of each position, respectively.
Table 4. Typical applied positions and requirements in biopharmaceutical industry as well as the major-related curricula derived from the life cycle of medicinal products.
Thirty-one subjects on the curriculum were finally chosen for the applied (hands-on) training of students of biopharmaceutical engineering (). These subjects can be classified into four clusters including chemistry, biology, bioengineering and pharmaceutical engineering according to their subject attributes.
Table 5. Curriculum clusters for training the applied students of biopharmaceutical engineering.
In recent years, the market share of biopharmaeutical agents has been greatly growing. Taking monoclonal antibodies as an example, until 2014, 47 monoclonal antibodies had already been approved on the market by the Food and Drug Administration (FDA) and the European Medicines Agency (EMA). The global sales of monoclonal antibodies quickly increased from ∼ $39 billion to ∼ $75 billion from the year 2008 to 2013. Under this rate of increase, the global sales of monoclonal antibodies are expected to reach $125 billion by 2020 and $139 billion by 2024 [24].
The rapid development of biopharmaceutical industry has profoundly influenced the pharmacy education. In the European Union, the biotechnology-related courses have been set up in the pharmacy curriculum at most universities, 60% of which have chosen to incorporate different biotechnological topics and subjects throughout the pharmacy curriculum [Citation25]. In China, an emerging undergraduate degree, biopharmaceutical engineering was approved to be established by the Ministry of Education in 2012. Until 2017, 65 regular universities in China had established the degree of biopharmaceutical engineering [Citation10]. However, as a newly established degree, many issues about students’ training need to be explored and refined. Therefore, in the present investigation, taking the recent improvement of the curriculum system for biopharmaceutical engineering program at CIT as an example, the curriculum design for applied (hands-on) training of students of biopharmaceutical engineering based on the product life cycle was reported for the first time.
As shown in , the first step of this work was to choose the potential subjects on the curriculum that are used for training the students of biopharmaceutical engineering. In order to fully reflect the comprehensiveness of curriculum, the life cycle of medicinal products was taken as a basis for subject selection, owing to the fact that the PLC spans the entire process from a product becoming first available on the market until it is withdrawn, and PLC analysis has been applied to formulate goals in the curriculum design for engineering undergraduates [Citation8]. It can be seen from and that 52 subjects on the curriculum were selected based on the life cycle of biopharmaceutical products, 23 of which were involved in the stage of preclinical study, because the quality of a medicinal product is highly related to the life and health of consumers, and before marketing, all the products must be subject to strict evaluation and assessment [Citation26]. Meanwhile, some basic subjects on the curriculum, such as analytical chemistry, organic chemistry, cell biology, biochemistry, molecular biology etc., were also included in this stage. Thirteen subjects with strong engineering characteristics were involved in the stage of manufacturing, and the course of ethics in engineering was also included considering the importance of this subject in cultivating the professional ethics of students [Citation27]. Surveys on the demands for biopharmaceutical students were then conducted from a popular recruitment website and enterprises, respectively. With regard to the survey from the recruitment website, ‘Shanghai’ and ‘Jiangsu Province’ were chosen as the location key words due to the fact that in China, these areas belong to economically developed regions that attract employment of many students every year. About 88.6% of the biopharmaceutical positions were centered in the core cities of Yangtze River Delta region including Shanghai, Suzhou, Nanjing and Wuxi, suggesting that these cities have converged many biopharmaceutical enterprises with different types, and there is a strong demand for students in these cities.
Figure 3. Diagram of curriculum design for training applied-skills students of biopharmaceutical engineering based on the product life cycle.
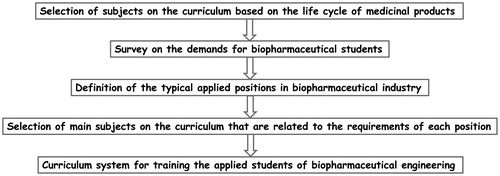
As for the educational requirements, undergraduate accounted for 36.9%, indicating that applied-skills students are still one of the most wanted types of personnel in biopharmaceutical industry. In the next survey from enterprises, five different types of biopharmaceutical enterprises located in Shanghai, Suzhou and Wuxi were selected, after interviewing with different participants of each enterprise or questionnairing, the most wanted personnel were found to be manufacturing technicians and product development engineers (). Based on the results of surveys, the research and production processes as well as the characteristics of biopharmaceutical products, eight typical applied (hands-on) positions in biopharmaceutical industry, including drug screening engineer, cell culture engineer, molecular biology technician, fermentation engineer, microorganism engineer, protein purification engineer, protein analysis technician and bio-preparation engineer, were identified, and 31 subjects were chosen from the 52 subjects on the curriculum that are involved in different stages of the life cycle of biopharmaceutical products according to the requirements of each position (). Finally, these subjects on the curriculum were divided into four clusters (chemistry, biology, bioengineering and pharmaceutical engineering) based on their subject attributes to constitute the curriculum system for applied (hands-on) training of the students of biopharmaceutical engineering ().
Herein, the competencies of biopharmaceutical engineering graduates were summarized into eight applied (hands-on) positions at biopharmaceutical enterprises according to the industrial needs. The related subjects on the curriculum that can be used to develop these competencies were selected based on the life cycle of medicinal products, which could contain similar stages for biopharmaceutical, chemical and natural medicines, but the specific subjects involved are different from each other, particularly the stages of preclinical study and manufacturing. For example, pharmacy students should focus on the knowledges of basic pharmacology, medicine design and analysis, medicinal chemistry and biopharmaceutics, microbiological and quality assurance aspects of medicine production [Citation28]. On the other hand, based on the similar product life cycle, to acquire the industry-needed competencies, biopharmaceutical engineering students should pay more attention to bioengineering-related subjects on the curriculum, such as cell engineering, antibody engineering, bioreactor engineering and fermentation engineering as well as some subjects of pharmaceutical engineering, including industrial pharmaceutics, principle of chemical engineering, biopharmaceutical process and pharmaceutical equipment and engineering design.
Conclusions
The major contribution of the present study was to explore the curriculum design for the applied (hands-on) training of students of biopharmaceutical engineering based on PLC and to match the selected subjects on the curriculum with the requirements of each applied (hands-on) position, narrowing the gap between industrial needs and students’ training. The results obtained from the present work are likely to provide evidence and reference for the training of biopharmaceutical students as well as the implementation of collaborative education between school and enterprise. However, this work only provides guidelines for the subjects to be included in the curriculum system for the training of biopharmaceutical students. Future studies on the topics of each subject, teaching devices, teaching methods and integrating mechanism of industry and education etc. could be also considered.
Disclosure statement
The authors declare that there is no conflict of interest.
Additional information
Funding
References
- Cevik Onar S, Ustundag A, Kadaifci Ç, et al. The changing role of engineering education in Industry 4.0 era. In: Ustundag A,Cevikcan E, editor. Industry 4.0: managing the digital transformation. Springer series in advanced manufacturing. New York (NY): Springer; 2018. p. 137–151.
- Prideaux D. Curriculum design. BMJ. 2003;326(7383):268–270.
- Toral SL, Martínez-Torres MR, Barrero F, et al. An electronic engineering curriculum design based on concept-mapping techniques. Int J Technol Des Educ. 2007;17(3):341–356.
- Kumar V, Haapala KR, Rivera JL, et al. Infusing sustainability principles into manufacturing/mechanical engineering curricula. J Manuf Syst. 2005;24(3):215–225.
- Rink DR, Swan JE. Product life cycle research: a literature review. J Bus Res. 1979;7(3):219–242.
- Roy P, Nei D, Orikasa T, et al. A review of life cycle assessment (LCA) on some food products. J Food Eng. 2009;90(1):1–10.
- Hashimoto K. Product life cycle theory: a quantitative application for casino courses in higher education. Int J Hosp Manag. 2003;22(2):177–195.
- Yasuhara K, Morozov A, Kilgore D, et al. Considering life cycle during design: a longitudinal study of engineering undergraduates. Proceedings of the 2009 American Society for Engineering Education Conference and Exhibition; 2009 June 14–17; Austin, Texas. Seattle, WA: University of Washington, Center for the Advancement of Engineering Education; 2009. Available from: https://peer.asee.org/5508
- Ministry of Education of the People’s Republic of China. General higher education undergraduate professional setting regulations (Promulgated in 2012) [cited 2012 Sept 18]. Available from: http://old.moe.gov.cn/publicfiles/business/htmlfiles/moe/s3882/201210/xxgk_143152.html
- Li HB, Fu M, Wu D, et al. Study on the training mode of biopharmaceutical major in Huaihua university. J Huaihua Univ. 2018;37(11):95–97.
- Sina Medicine. Research on the development of biopharmaceutical industry in Suzhou [cited 2019 Jan 10]. Available from: https://med.sina.com/article_detail_103_2_58886.html
- Changshu Institute of Technology. Introduction of Changshu Institute of Technology [cited 2019 Jul 01]. Available from: http://www.cslg.edu.cn/html/article_list_4.html
- Changshu Institute of Technology. Introduction of School of Biology and Food Engineering [cited 2019 Jul 01]. Available from: https://swxy.cslg.edu.cn/xxgk/xyjj.html
- China Food and Drug Administration. Good Laboratory Practice (SFDA Order No. 2) [cited 2003 Aug 06]. Available from: http://samr.cfda.gov.cn/WS01/CL1031/24472.html
- China Food and Drug Administration. Provisions for Drug Registration (SFDA Order No. 28) [cited 2007 Jul 07]. Available from: http://samr.cfda.gov.cn/WS01/CL1031/24529.html
- China Food and Drug Administration. Good Clinical Practice (SFDA Order No. 3) [cited 2003 Aug 6]. Available from: http://samr.cfda.gov.cn/WS01/CL1031/24473.html
- China Food and Drug Administration. Good Manufacturing Practice (Revised edition 2010, MOHC Order No. 79) [cited 2011 Jan 17]. Available from: http://samr.cfda.gov.cn/WS01/CL0053/58500.html
- China Food and Drug Administration. Good Supplying Practice (CFDA Order No. 13) [cited 2015 Jul 01]. Available from: http://samr.cfda.gov.cn/WS01/CL1031/123040_7.html
- China Food and Drug Administration. Law of the People’s Republic of China on Pharmaceutical Administration [cited 2015 Apr 24]. Available from: http://samr.cfda.gov.cn/WS01/CL0784/124980.html
- Vondeling GT, Cao Q, Postma MJ, et al. The impact of patent expiry on drug prices: a systematic literature review. Appl Health Econ Health Policy. 2018;17(2):255–256.
- Teaching Steering Committee of Institutions of Higher Learning. The national standards for bioengineering teaching. Beijing (BJ): Ministry of Education of the People’s Republic of China; 2018.
- Teaching Steering Committee of Institutions of Higher Learning. The national standards for pharmaceutical engineering teaching. Beijing (BJ): Ministry of Education of the People’s Republic of China; 2018.
- Teaching Steering Committee of Institutions of Higher Learning. The national standards for pharmacy teaching. Beijing (BJ): Ministry of Education of the People's Republic of China; 2018.
- Yang O, Qadan M, Ierapetritou M. Economic analysis of batch and continuous biopharmaceutical antibody production: a review. J Pharm Innov. 2019;14:1–19.
- Savova A, Mitov K, Stoimenova A, et al. Pharmaceutical biotechnology education in the pharmacy curriculum at European universities. Biotechnol Biotecnol Equip. 2012;26(4):3187–3191.
- Yao X, Ding J, Liu Y, et al. The new drug conditional approval process in china: challenges and opportunities. Clin Ther. 2017;39(5):1040–1051.
- David RH. Ethics instruction in engineering education: a (mini) meta-analysis. J Eng Educ. 2001;90(2):223–229.
- Sosabowski MH, Gard PR. Pharmacy education in the United Kingdom. Am J Pharm Educ. 2008;72(6):130. DOI:10.5688/aj7206130