?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The present study investigated the cytotoxic activities against MCF-7 and MDA-MB-231 cells, and the potential antioxidant and α-glucosidase inhibitory activities of the extracts of the aerial parts of Trachelospermum jasminoides, a traditional medicinal plant used for the treatment of rheumatic arthralgia in China. In MTT assay and microscopic analysis, the ethyl acetate extract (EAE) of T. jasminoides showed moderate cytotoxic effect against MCF-7 and MDA-MB-231 with IC50 values of 109.54 ± 2.39 μg/mL and 162.68 ± 9.52 μg/mL, respectively, but the water extract (WE) was inactive against both of them. With gallic acid and rutine as equivalents for determination, the total phenolic and total flavonoid contents in the EAE (54.93 ± 0.32 mg GAE/g and 186.75 ± 4.55 mg RE/g, respectively) were higher than those of the WE. The antioxidant activities of the EAE and WE, which were evaluated by reducing power assay, DPPH (2,2-diphenyl-1-picrylhydrazyl) radical scavenging assay and hydroxyl radical scavenging assay, may be directly associated with the high contents of phenols and flavonoids in T. jasminoides. The EAE showed higher inhibitory effect in the α-glucosidase inhibition tests with an IC50 value of 776.81 ± 107.80 μg/mL as compared to the WE with 2607.48 ± 364.31 μg/mL. Furthermore, the antioxidant activity and the inhibitory effects of the fractions against α-glucosidase were concentration-dependent.
Introduction
Cancer is one of the most frequently occurring diseases, increasing to an estimated 18.1 million new cases and causing 9.6 million deaths worldwide in 2018. Globally, breast cancer is the most frequent cancer amongst women, with about 2.1 million newly diagnosed female breast cancer cases in 2018. There have been significant improvements in cancer treatment which enhanced the quality of life and patient survival. However, cancer related deaths are continuously rising [Citation1–3]. In terms of breast cancer cells, the oestrogen receptor (ER) positive breast cancer cells, MCF-7 [Citation4] and oestrogen receptor (ER) negative breast cancer cells, MDA-MB-231 [Citation5], have attracted great interests from researchers.
Free radicals are the well-known inducers of cellular and tissue pathogenesis leading to several human diseases such as cancer, inflammatory disorders, as well as in aging processes [Citation6]. Therefore, the connection between antioxidative and anticancer activities has been widely applied to extant empirical endeavours.
Diabetes is one of the metabolic and non-communicable diseases (NCDs) currently threatening global human health and causing patient death. The International Diabetes Federation (IDF) estimated the global prevalence to be 41.5 million in 2015, or 5.1% in the adult population, and the number is expected to rise to 64.2 million, or 10%, by 2040 [Citation7]. Alpha-glucosidase inhibitors are medicines that inhibit α-glucosidase activity in the intestinal brush border of the small intestine, thus decreasing the hydrolysis of disaccharides and oligosaccharides into glucose and slowing the rise of blood sugar [Citation8]. Acarbose, miglitol and voglibose are commercially available anti-diabetic drugs that control the blood glucose level by inhibiting α-glucosidase activity. However, they all can cause serious side effects such as liver damage, gastrointestinal disorders and adverse reactions. Hence, it is essential to discover more effective and safer new inhibitors from natural products.
T. jasminoides, a member of the family Apocynaceae, is also known as luoshiteng in China and suppresses some inflammatory diseases such as rheumatoid arthritis and abscesses [Citation9–12] and shows anti-cancer and antibacterial activity [Citation13–15]. The anti-breast cancer activity of the extracts of T. jasminoides along with its antioxidant and α-glucosidase inhibitory activities have been poorly reported so far and were explored and reported in this research.
Materials and methods
Collection and identification
The aerial parts of T. jasminoides were harvested from Maoping, Guilin, in July 2017, and identified by Professor Deqing Huang of Guilin Medical University. The voucher specimen (T − tj201707) was deposited in the Experimental Centre of Guilin Medical University. The appearance of T. jasminoides is shown in .
Preparation of extracts
The powdered sample (500 g) was macerated thrice with 95% ethanol at room temperature. The combined ethanol extract was concentrated by using a rotary evaporator to get crude extract (35 g). The ethanol extract was then dispersed in water and sequentially extracted with ethyl acetate (EA) using liquid − liquid partition. After removal of the solvents, two subfractions, namely, EAE (12 g), and WE (23 g), were obtained.
Cell preparation and maintenance
The MCF-7 and MDA-MB-231 cancer cell lines (Shanghai Aulu Biological Technology Co., Ltd, Shanghai, China) were grown in DMEM with supplemented with 10% foetal bovine serum (FBS) (Gibco, 1% antibiotic-antimycotic (10,000 units/mL of penicillin, 10,000 μg/mL of streptomycin and 25 μg/mL amphotericin B) as a complete growth medium (CGM). All consumable materials were purchased from Gibco Thermo Fisher Scientific, USA. The cells were thawed quickly from liquid nitrogen and then 37 °C water bath prior to culture. One millilitre of cells was transferred to a 15-mL centrifuge tube and 1 mL of CGM was added and centrifuged at 201.24 g for 5 min. The supernatant was removed and the pellets were re-suspended with 1 mL of CGM. The 1-mL cell suspension then was transferred to a 25-cm2 cell culture flask. Four millilitres of CGM was added to the flask carefully and the flask was incubated at 37 °C with 5% CO2 in an incubator. Cultured flasks were subcultured into another flask once the cells reached 70 − 80% confluency. The cells were detached with 1 mL of 0.25% trypsin-EDTA after removal of old media and were washed with 2 mL PBS. Cells were checked microscopically daily to ensure that the cells are in healthy condition.
Microculture tetrazolium (MTT) assay
Cytotoxicity assay was prepared with seeding density of 4 × 104 cells/mL of CGM in sterile 96-well flat bottom culture plates. Each well was filled with 200 μL of cell suspension (MCF-7 and MDA-MB-231). This was followed by incubation of plates at 37 °C with 5% CO2 overnight to allow cell attachment. Serially diluted EAE and WE of T. jasminoides (50, 100, 200, 300 and 400 μg/mL) were added into the appropriate wells in four replicates for each concentration. Untreated cells (0 μg/mL) were used as control. In order to observe the inhibitory effect of extracts on MDA-MB-231 and MCF-7 cells, 48 h treatment time was chosen. Therefore, following a 48 h incubation period at 37 °C with 5% CO2 in an incubator, 20 μL of MTT solution was added to the wells and the plates were incubated for an additional 4 h. The medium of each well was carefully aspirated without disturbing the MTT crystals in each well. One hundred and fifty microlitres of dimethylsulphoxide (DMSO) solution was added into each well to dissolve the purple formazan crystals. The optical density (OD) of formazan was proportional to the number of surviving cells that were metabolically active, and was read at 490 nm wavelength using spectrophotometry [Citation16]. Each sample was run in triplicate in one plate and the experiment was repeated three times for validity. After a 4-h incubation period for formazan formation, the OD values, dose-response curves (percentage of cell survival vs. concentration) were generated using linear regression interpolation analysis to obtain IC50 (minimum concentration of hydromethanolic extract that gives 50% survival of MDA-MB-231 and MCF-7 cells). The histogram for cell survivability was constructed by using Excel 2010. The percent of cell survivability was calculated according to the following equation:
The cytotoxic effect against cancer cells was recorded as IC50 and compared with untreated cells. The percentage of cell survivability values against concentration of respective extracts was plotted in order to determine the IC50 values of each extract.
Microscopic analysis by hoechst staining
Morphological changes in the nucleus induced by active EAE treatment were studied by Hoechst 33258 staining. MCF-7 cells (4 × 104/well) were seeded in a 24-well plate and after 24 h of growth cells were treated with different concentrations of EAE for 24 h and cells were fixed with 4% paraformaldehyde for 10 min and then washed with PBS three times and then stained with Hoechst 33258 stain for 5 min and washed with PBS three times. Finally, images were captured by a fluorescent microscope (Nikon ECLIPSE Ti).
Total phenolic content (TPC) determination
The TPC in all samples was determined according to the Folin − Ciocalteu method described by Singleton and Rossi with some modifications [Citation17,Citation18]. In brief, 1 mL of sample solutions (10 mg/mL) was mixed with 0.2 mL of Folin − Ciocalteu reagent. After 1 min of incubation at room temperature, 0.3 mL of 20% Na2CO3 aqueous solution was added to the mixtures, followed by the addition of 1.5 mL of distilled water. Then the mixtures were kept in a constant-temperature water bath at 70 °C for 10 min. After cooling to room temperature, the absorbance values were measured at 765 nm. The TPC was expressed as milligrams of gallic acid equivalents per gram (GAE/g) from the calibration curve of a gallic acid standard solution (0–800 μg/mL). All measurements were performed in triplicate.
Total flavonoids content (TFC) determination
The total flavonoids in all samples were determined by a colourimetric method with minor modification [Citation19,Citation20]. Briefly, 0.5 mL of samples (10 mg/mL) was placed in a 10 mL volumetric flask, distilled water was added to make 3 mL, and 0.15 mL of a 5% NaNO2 solution was added. A total of 0.3 mL 10% of AlCl3 solution was added 5 min later, and the mixture was allowed to stand for another 6 min. A total of 1 mL of 1 mol/L NaOH was added, and the total was made up to 5 mL with distilled water. The solution was mixed well again and allowed to stand for 30 min. The absorbance was measured against a blank at 510 nm, and the TFC was determined as rutin equivalent (RE) from the calibration curve of rutin standard solutions (0–800 μg/mL) and was expressed as milligrams of RE/g. All samples were performed in triplicate.
Antioxidant assays
Reducing power assay
The reducing power of samples was measured according to the method of Chen et al. [Citation21] with some modifications. Briefly, 60 μL of sample solution was mixed with 60 μL of phosphate buffer (0.2 mol/L, pH 6.6) and 60 μL of K3Fe(CN)6 (1%, w/v). After through mixing, each solution was incubated at 50 °C for 20 min. After that, the reaction was stopped by adding 60 μL of trichloroacetic acid (10%, w/v). Finally, the mixture was added 30 μL of FeCl3 (0.1%, w/v). After the mixture was kept at room temperature for 10 min, the absorbance was measured at 700 nm. Ascorbic acid was used as a positive control. Higher absorbance value of the reaction mixture indicated increased reducing power. All the tests were performed in triplicates and the results were pooled and expressed as mean values with standard deviation ( SD).
1,1-Diphenyl-2-picrylhydrazyl (DPPH) radical scavenging assay
Antioxidant activity determination of the extracts from T. jasminoides was performed via DPPH radical scavenging as described by Zhao et al. [Citation22] with minor modifications. Sample solution (100 μL) was added to 100 μL of freshly prepared DPPH solution in 96-well plates. The mixture was incubated and the absorbance was measured at 517 nm. Free radical scavenging activity was expressed in terms of the half maximal inhibitory concentration (IC50). DPPH radical scavenging activity was calculated as a percentage using the equation:
where Acontrol is the absorbance of the control and Asample is the absorbance of the sample (extract/ascorbic acid).
Hydroxyl radical scavenging assay
Hydroxyl radical scavenging assay was performed with the method based on Pu et al. [Citation23]. Briefly, 1 mL of FeSO4 (6 mmol/L) and 1 mL of salicylic acid-ethanol (6 mmol/L) were added to 1 mL of T. jasminoides sample in 5 mL eppendorf tubes, and 1 mL of H2O2 (6 mmol/L) was added finally to start the reaction. Then the reaction solutions were incubated at 37 °C for 30 min, and the absorbance was measured at 510 nm. The hydroxyl radical scavenging rate was calculated as follows:
where A0 is the absorbance of the blank sample (water instead of sample) and A1 is the absorbance of the sample, A2 is the background absorbance (water instead of H2O2).
α-Glucosidase inhibitory assay
The α-glucosidase inhibition potential of T. jasminoides extracts were determined according to the modified methods of previous reports [Citation24,Citation25]. Briefly, 50 μL of diluted sample, 50 μL of phosphate buffer (0.1 mmol/L, pH 6.9) and 100 μL α-glucosidase solution (0.1 U/mL, in 0.1 mol/L phosphate buffer, pH 6.9) were incubated in 96-well plates at 25 °C for 10 min. Then, 50 μL of 5 mmol/L p-nitrophenyl-α-D-glucopyranoside (pNPG) solution (in 0.1 mol/L phosphate buffer, pH 6.9) was added to each well, and the absorbance at 405 nm was recorded with a micro-plate reader after incubated at 25 °C for 5 min. The inhibition percentage was calculated as follows:
where AC is the absorbance of the control (without sample), Ab is the absorbance of the blank sample (without pNPG solution), AS is the absorbance of the sample. Acarbose was used as a positive reference. All tests were performed in triplicate.
Data analysis
All the percentages of cell survivability were expressed as mean values (n = 3) per plate with standard deviation (±SD) and differences among treated and untreated cells were analyzed using analysis of variance (ANOVA) followed by Dunnett’s multiple comparison test. The test was considered statistically significant when p < 0.05 and IBM SPSS Statistics 22 was used to analyze all statistical tests. Data were expressed as mean values of three experiments (n = 3) ± SD and the differences between WE and EAE sample extracts were evaluated using one way ANOVA followed by Tukey’s multiple comparison test. The statistical analysis test was performed using IBM SPSS Statistics 22. Differences were considered statistically significant a level of p < 0.05.
Results and discussion
Anti-proliferation activity
The anti-proliferative potential of the extracts was tested via MTT assay on MCF-7 and MDA-MB-231 cell lines. The effects of WE and EAE of T. jasminoides on the proliferation of MCF-7 and MDA-MB-231 after treatment for 48 h are shown in and . The anti-proliferation effect of EAE was stronger than that of WE, and EAE showed concentration-dependent antiproliferative effects against MCF-7 and MDA-MB-231 tumour cells. The IC50 values for WE and EAE for MCF-7 and MDA-MB-231 cell lines are given in . Han [Citation26] and Li [Citation27] reported that T. jasminoides showed antiestrogenic effect and anti-breast cancer activity. But to our knowledge, this is the first time to investigate the cytotoxic activities against MCF-7 and MDA-MB-231 cells. These results are in accordance with previous findings.
Figure 2. Cell survivability (%) of MCF-7 breast cancer cell line following treatment with WE, EAE extracts and 5-fluorouracil for 48 h. Note: All values were expressed as SD (n = 3). Statistical analysis between treated cells and untreated cells was done using one way ANOVA followed by Dunnett’s multiple comparison test. * p < 0.05, and *** p < 0.001 denoted significant difference as compared to untreated cells (control).
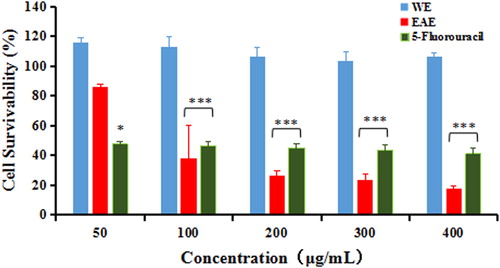
Figure 3. Cell survivability (%) of MDA-MB-231 breast cancer cell line following treatment with WE, EAE extracts and 5-fluorouracil for 48 h. Note: All values were expressed as SD (n = 3). Statistical analysis between treated cells and untreated cells was done using one way ANOVA followed by Dunnett’s multiple comparison test. * p < 0.05, and *** p < 0.001 denoted significant difference as compared to untreated cells (control).
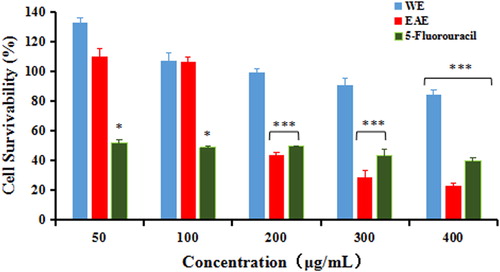
Table 1. IC50 values of WE, EAE and 5-fluorouracil in breast cancer cell lines.
Hoechst 33258 staining to determine the morphological changes in nucleus
In the present study, Hoechst 33258 staining was performed to reveal the apoptosis inducing activity of EAE in MCF-7 cell line. The results of this study confirmed the increase in fluorescence due to chromatin condensation in MCF-7 cells as compared to the vehicle control in a dose-dependent manner (). These results indicated that T. jasminoides could induce apoptosis in breast cancer cell lines.
Figure 4. Detection of DNA damage by EAE induced apoptosis in tumour cells by Hoechst 33258 staining. Note: MCF- 7 cells were seeded in 24-well culture plate and allowed to grow for 24 h. After treatment for 24 h with different concentrations of T. jasminoides. Cells were stained with Hoechst 33258 following a standard protocol and observed using fluorescence microscopy. Scale bar = 100 μm.

Total phenolic and flavonoid content
The total phenolic content of WE and EAE were 50.27 ± 1.42 mg GAE/g and 54.93 ± 0.32 mg GAE/g of dry powder, respectively (). Obviously, the WE and EAE had a similar phenolic content. The flavonoid content of the extracts is shown in . The EAE (186.75 ± 4.55 mg RE/g of dry powder) possessed higher total flavonoid contents than WE (76.33 ± 1.19 mg RE/g of dry powder). The extracting ratio of total flavonoids from T. jasminoides by an ultrasonic wave cooperated method was 3.22% [Citation28], which was a bit higher than that achieved by our ethanol soaking extraction. It is commonly considered that medicinal plants with high amounts of phenols and flavonoids have potential antioxidant actions [Citation29,Citation30], which prompted us to evaluate the antioxidant activity.
Table 2. Total phenolic content, flavonoid content and antioxidant effects of T. jasminoides extracts.
Antioxidant activities in vitro
Reducing power
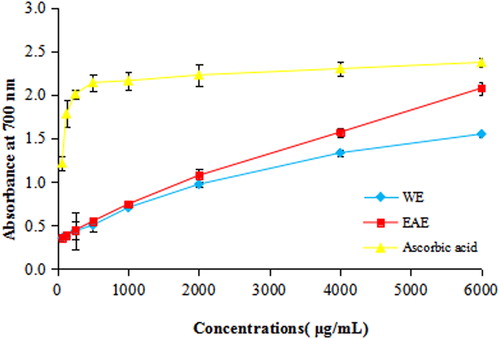
The reducing capacity of the extracts against Fe3+/ferricyanide complex to the ferrous form may serve as a significant indicator of antioxidant capacity [Citation31,Citation32]. The existence of reductants is the key of the reducing power; they exhibit their antioxidant activities through the action of breaking the free radical chain by donating a hydrogen atom. The reduction of the Fe3+/ferricyanide complex to the ferrous form occurs due to the presence of reductants in the solution [Citation33]. The reducing power was determined at 700 nm and the absorbance values are shown in and . The reducing power of all the samples increased with increasing concentrations. Between the two fractions, EAE exhibited higher reducing power than WE. At 6000 μg/mL, the absorbance of WE, EAE and ascorbic acid were 1.548 ± 0.276, 2.077 ± 0.071 and 2.373 ± 0.055, respectively, which indicated that the reducing power of EAE had already reached 87.5% of that of ascorbic acid. This result indicated that the EAE of T. jasminoides possessed higher reducing power in comparison with the extracts from other plants that have been reported in recent years such as Momordica charantia, Gynura procumbens and so on [Citation34,Citation35].
Table 3. OD values of extracts/drug at 700 nm.
DPPH radical scavenging activity
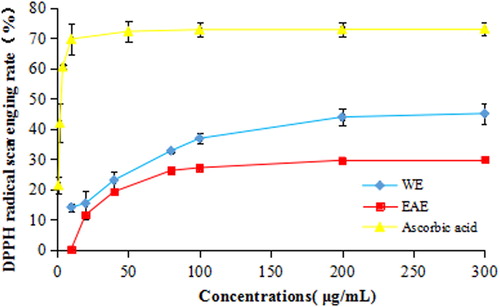
DPPH is one of the few stable and commercially available organic nitrogen radicals [Citation36]. The DPPH assay is a very common spectrophotometric method to determine the antioxidant activity of natural products [Citation37]. The DPPH scavenging activity of the extract from T. jasminoides is shown in . And the half maximal inhibitory concentration (IC50) decreased in the order of EAE (749.89 ± 51.92 μg/mL) > WE (282.43 ± 30.66 μg/mL) > ascorbic acid (5.29 ± 1.42 μg/mL) (). At 100 μg/mL, the scavenging rates of WE, EAE and ascorbic acid were 36.81 ± 1.75%, 27.10 ± 0.54% and 72.76 ± 2.38%, respectively. In comparison with other plants of the family Apocynaceae, the DPPH radical scavenging activity of T. jasminoides was higher than that reported for Hunteria umbellata [Citation38], and a little weaker than that of Dregea volubilis [Citation39].
Hydroxyl radical scavenging activity
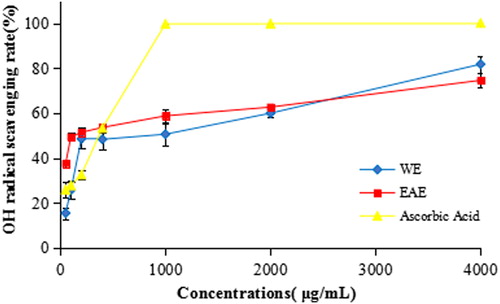
Hydroxyl radicals can damage nucleic acids, lipids carbohydrates and amino acids. They are also considered the most harmful free radicals among the reactive oxygen species (ROS) [Citation40]. Therefore, removing hydroxyl radicals is quite important for the antioxidant defence in living cell systems. As illustrated in and , the WE and EAE exhibited moderate scavenging activities against hydroxyl radicals. At 1000 μg/mL, the hydroxyl radical scavenging activities of WE, EAE and ascorbic acid were 50.57 ± 4.99%, 58.76 ± 2.82% and 99.76 ± 0.16%, with IC50 values of 585.40 ± 123.23 μg/mL, 202.02 ± 22.42 μg/mL and 208.05 ± 3.69 μg/mL, respectively. For comparison, the IC50 value of the extracts of the flowers of Dregea volubilis was 170.67 ± 0.98 мg/mL [Citation39], which was a bit lower than that of the EAE of T. jasminoides.
α-Glucosidase inhibitory activity
In the method used here in vitro, α-glucosidase hydrolizes p-nitrophenyl-α-D-glucopiranoside to glucose and the yellow p-nitrophenol which can be determined with spectrophotometry at 405 nm [Citation41]. The in vitro α-glucosidase inhibitory studies demonstrated that the extracts of T. jasminoides had inhibitory activity. As is shown in , the α-glucosidase inhibitory activities of WE, EAE and acarbose were 47.17 ± 0.92%, 54.34 ± 0.75% and 74.40 ± 0.75%, respectively, at the concentration of 800 μg/mL. The enzyme inhibition effect of the EAE was higher than that of WE with IC50 value of 776.81 ± 107.80 μg/mL and 2607.48 ± 364.31 μg/mL, respectively, as compared to the standard acarbose (100.85 ± 10.84 μg/mL). In comparison with the extracts from other plants of the family Apocynaceae that have been reported in recent years, the IC50 values of б-glucosidase inhibition of T. jasminoides were lower than that of Hunteria umbellata (184.35μµg/mL) [Citation38], and higher than that of Dregea volubilis (3780.09 ± 21.19 мg/mL) [Citation39]. These differences may be ascribed to the nature of the phenolics and flavonoids of the extracts, which were proved to be effective inhibitors of б-glucosidases [Citation42].
Table 4. α-Glucosidase inhibitory activity of extracts/drug.
Conclusions
In summary, two fractions (WE and EAE) were gained from ethanol soaking extraction of T. jasminoides. Their cytotoxic, antioxidant and α-glucosidase inhibitory activities were investigated. The anti-breast cancer study demonstrated that EAE induced apoptosis mediated cell death in MCF-7 or MDA-MB-231 cells in vitro. In contrast, EAE of T. jasminoides may be a potential candidate for oestrogen receptor (ER) positive breast cancer agent because it is more cytotoxic to MCF-7 cancer cell lines as compared to MDA-MB-231. Thus T. jasminoides may have a potential to be used in complementary and alternative medicine for the treatment of breast cancer and other types of cancer. High TPC and TFC were found in the extracts of T. jasminoides. The high amounts of phenolics and flavonoids were probably in correlation with good antioxidant activity in T. jasminoides. The two fractions both exhibited good reducing power and scavenging capacities on DPPH and hydroxyl radicals in a concentration-dependent manner. EAE exhibited higher reducing power and similar OH radical scavenging activity compared with that of WE. However, WE exhibited better DPPH radical scavenging activity than EAE. Moreover, it can be concluded that WE and EAE of T. jasminoides inhibited the activity of α-glucosidase, which may be attributed to the presence of phytochemicals such as flavonoids and tannins in these plant extracts. Further study is required to isolate the specific compounds from T. jasminoides that may be responsible for this effect.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ferlay J, Colombet M, Soerjomataram I, et al. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int J Cancer. 2019;144(8):1941–1953.
- Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424.
- Global Burden of Disease Cancer Collaboration. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4:1553–1568.
- Holliday DL, Speirs V. Choosing the right cell line for breast cancer research. Breast Cancer Res. 2011;13(4):215–221.
- Tate CR, Rhodes LV, Segar HC, et al. Targeting triple-negative breast cancer cells with the histone deacetylase inhibitor panobinostat. Breast Cancer Res. 2012;14(3):R79–R93.
- Halliwell B. Free radicals, antioxidants and human diseases; curiosity, cause, or consequence? Lancet 1994;334:721–724.
- International Diabetes Federation, Diabetes Atlas (IDFDA). IDF diabetes atlas. 7th ed. Brussels: IDFDA; 2015.
- Hao WX, Wang M, Lv M. The inhibitory effects of Yixing black tea extracts on α-glucosidase. J Food Biochem. 2017;41(1):e12269.
- Li RW, Lin GD, Myers SP, et al. Anti-inflammatory activity of Chinese medicinal vine plants. J Ethnopharmacol. 2003;85(1):61–67.
- Lee MH, Lee JM, Jun SH, et al. In-vitro and in-vivo anti-inflammatory action of the ethanol extract of Trachelospermi caulis. J Pharm Pharmacol. 2007;59(1):123–130.
- Sheu MJ, Chou PY, Cheng HC, et al. Analgesic and anti-inflammatory activities of a water extract of Trachelospermum jasminoides (Apocynaceae). J Ethnopharmacol. 2009;126(2):332–338.
- Kim HS, Kim AR, Lee JM, et al. A mixture of Trachelospermi caulis and Moutan cortex radicis extracts suppresses collagen-induced arthritis in mice by inhibiting NF-κB and AP-1. J Pharm Pharmacol. 2012;64(3):420–429.
- Nishibe S, Han YM. Chemical constituents from Trachelosperomum jasminoides and its anticancer activity. World Phytomed. 2002;17:57–58.
- Qian XJ, Jin YS, Chen HS, et al. Trachelogenin, a novel inhibitor of hepatitis C virus entry through CD81. J Gen Virol. 2016;97(5):1134–1144.
- Shin HS, Bae MJ, Jung SY, et al. Enhancing effect of trachelogenin from Trachelospermi caulis extract on intestinal barrier function. Biol Pharm Bull. 2015;38(11):1707–1713.
- Baharum Z, Akim AM, Taufiq-Yap YH, et al. In vitro antioxidant and antiproliferative activities of methanolic plant part extracts of Theobroma cacao. Molecules 2014;19(11):18317–18331.
- Singleton VL, Rossi JA. Colorimetry of total phenolics with phosphomolybdic phosphotungstic acid reagent. Am J Enol Vitic 1956;16:144–158.
- Iqbal S, Younas U, Chan KW, et al. Proximatecomposition and antioxidant potential of leaves from three varieties of mulberry (Morus sp.): a comparative study. Int J Mol Sci. 2012;13:6651–6664.
- Dewanto V, Wu XZ, Adom KK, et al. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J Agric Food Chem. 2002;50(10):3010–3014.
- Chan KW, Iqbal S, Khong NMH, et al. Preparation of deodorized antioxidant rich extracts from 15 selected spices through optimized aqueous extraction. J Med Plants Res. 2011;5:6067–6075.
- Chen RZ, Liu ZQ, Zhao JM, et al. Antioxidant and immunobiological activity of water-soluble polysaccharide fractions purified from Acanthopanax senticosu. Food Chem. 2011;127(2):434–440.
- Zhao QS, Xie BX, Yan J, et al. In vitro antioxidant and antitumor activities of polysaccharides extracted from Asparagus officinalis. Carbohyd Polym. 2012;87(1):392–396.
- Pu XY, Ma XL, Liu L, et al. Structural characterization and antioxidant activity in vitro of polysaccharides from Angelica and Astragalus. Carbohyd Polym. 2016;137:154–164.
- Wan CP, Yuan T, Cirello AL, et al. Antioxidant and α-glucosidase inhibitory phenolics isolated from highbush blueberry flowers. Food Chem. 2012;135(3):1929–1937.
- Dang PH, Nguyen NT, Nguyen HX, et al. α-Glucosidase inhibitors from the leaves of Embelia ribes. Fitoterapia 2015;100:201–207.
- Han YM. Chemical constituents and anticancer activities of Trachelospermum jasminoides. Drugs Clin. 2002;17:57–58.
- Li M. Research progress in the pharmacological activities of Trachelospermum jasminoides. Technol Econ Guide 2017;25:158–159.
- Zhao CC, Wang LD, Cheng W, et al. Research on the extraction process of total flavonoids from Chinese starjasmine stem by ultrasonic wave cooperated method. J Anhui Agr Sci. 2011;39:2080–2081.
- Safari M, Ahmady-Asbchin S. Evaluation of antioxidant and antibacterial activities of methanolic extract of medlar (Mespilus germanica L.) leaves. Biotechnol Biotechnol Equip. 2019;33:372–378.
- Nordin ML, Abdul Kadir A, Zakaria ZA, et al. In vitro investigation of cytotoxic and antioxidative activities of Ardisia crispa against breast cancer cell lines, MCF-7 and MDA-MB-231. BMC Complement Altern Med. 2018;18(1):87–96.
- Yildirim A, Mavi A, Oktay M, et al. Comparison of antioxidant and antimicrobial activities of tilia (Tilia Argentea Desf Ex DC), sage (Salvia Triloba L.), and black tea (Camellia Sinensis) extracts. J Agric Food Chem. 2000;48:5030–5034.
- Zou YP, Lu YH, Wei D. Antioxidant activity of a flavonoid-rich extract of Hypericum perforatum L. in vitro. J Agric Food Chem. 2004;52(16):5032–5039.
- Stähelin HB, Gey KF, Eichholzer M, et al. Plasma antioxidant vitamins and subsequent cancer mortality in the 12-year follow-up of the prospective basel study. Am J Epidemiol. 1991;133(8):766–775.
- Raghu KL, Ramesh CK, Srinivasa TR, et al. DPPH scavenging and reducing power properties in common vegetables. Res J Pharm, Biol Chem Sci. 2010;1:399–406.
- Li JE, Wang WJ, Zheng GD, et al. Physicochemical properties and antioxidant activities of polysaccharides from Gynura procumbens leaves by fractional precipitation. Int J Biol Macromol. 2017;95:719–724.
- Burits M, Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother Res. 2000;14(5):323–328.
- Mensor LL, Menezes FS, Reis AS, et al. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother Res. 2001;15(2):127–130.
- Oboh G, Adebayo AA, Ademosum AO. Erection-stimulating, anti-diabetic and antioxidant properties of Hunteria umbellata and Cylicodiscus gabunensis water extractable phytochemicals. J Complement Integr Med. 2017;15:20160164.
- Das B, De A, Das M, et al. A new exploration of Dregea volubilis flowers: Focusing on antioxidant and antidiabetic properties. S Afr J Bot. 2017;109:16–24.
- Reiter RJ, Melchiorri D, Sewerynek E, et al. A review of the evidence supporting melatonin's role as an antioxidant. J Pineal Res. 1995;18(1):1–11.
- Sulistiyani SM, Sari YP. Inhibition of α-glucosidase activity by ethanolic extract of Melia azedarach L. leaves. IOP Conf Ser Earth Environ Sci. 2016;31:012025.
- Bothon F, Debiton E, Avlessi F, et al. In vitro biological effects of two antidiabetic medicinal plants used in Benin as folk medicine. BMC Complement Altern Med. 2013;13(1):51–59.