?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this study, we investigated the antioxidant and antimicrobial activities of Isodon amethystoides (Benth.) CY Wu et Hsuan leaf extracts. Leaves were subjected to extraction with acetone, chloroform, ethanol and ethyl acetate. The extraction efficiency of these four solvents ranked as follows: acetone > chloroform > ethanol > ethyl acetate; highest extraction yield of 18.27% was obtained using acetone as the solvent. Total phenolic content and flavonoid content were determined using Folin–Ciocalteau and aluminium chloride (AlCl3) methods, respectively. The acetone extract had the highest total phenolic content (146.77 mg GAE/g dry extract) and flavonoid content (81.57 mg RE/g dry extract) among the four types of extracts. Further, the antioxidant activity was determined by 1,1-diphenyl-2-picrylhydrazyl (DPPH) method and the antimicrobial activity was evaluated using disc-diffusion and broth microdilution assay. The strongest antioxidant activity was observed in the acetone extract with a half minimal inhibitory concentration (IC50) of 0.4024 mg/mL in the DPPH scavenging assay. The acetone and ethyl acetate extracts exhibited strong antimicrobial activity. The strongest antibacterial activity of the acetone extract was observed against Bacillus subtilis and Escherichia coli with a minimum inhibitory concentration (MIC) of 0.10 mg/mL and 0.05 mg/mL, respectively. The acetone extract had high antifungal activity against three of the six agriculturally important pathogenic fungi with MIC ranging from 0.5 mg/mL to 0.8 mg/mL. The antioxidant and antimicrobial activities may be attributed to the high total phenolic and flavonoid contents in the acetone extract. Our findings suggest I. amethystoides leaves as an important source of natural compounds with potential use in agriculture.
Introduction
Genus Isodon (Lamiaceae family) includes several medicinal plants that have been extensively used as a traditional medicine in China. This genus comprises over 100 species [Citation1] distributed mainly in tropical and subtropical Asia [Citation2]. Isodon species have been used for the treatment of various diseases, including pulmonary fibrosis [Citation3] and pneumonia [Citation4]. Of the different species, Isodon amethystoides (Ben-th) Cy Wu et Hsuan has been broadly used as a folk remedy for abscesses, swollen sores and tumors [Citation5]. In the Huaibei region of China, I. amethystoides is called Wang Zao Zi, which is known for its effectiveness in treating festering wounds.
Plant diseases, significantly affecting agricultural production, are a major threat to farmers worldwide [Citation6]. Among these, fungal diseases account for approximately 16% of the yield losses and represent a major threat to global food security [Citation7]. Magnaporthe oryzae is one of the most economically devastating fungi that infect staple food crops like rice, wheat and barley [Citation8]. Some other pathogenic fungi like Aspergillus niger, Macrophoma kuwatsukai Hara, Alternaria kikuchiana Tanaka and Glomerella cingulata (Stonem.) Spauld. et Schrenk cause severe damage to cereals and fruits [Citation9–11]. Use of chemicals is the most common method to control fungal diseases. However, increased use of chemical-based control measures has led to potential ecological and human health risks [Citation12]. The plant kingdom represents a reservoir of bioactive compounds with antimicrobial and antioxidant activities, such as flavonoids, polyphenols, terpenes, coumarins and steroids. Furthermore, studies have indicated plant extracts as safe and effective alternatives [Citation13].
Natural compounds are generally extracted with a proper solvent based on the law of similarity and intersolubility. Therefore, it is of great importance to assess the antioxidant and antimicrobial activities of extracts with different solvents. Studies have reported chemically-active compounds of agricultural importance in several other plants of Lamiaceae family [Citation14, Citation15]. However, the Isodon genus has not been investigated. In the present study, we investigated the antioxidant activity of leaf extracts from I. amethystoides. We also investigated the antimicrobial activity of these extracts against typical gram-positive and gram-negative bacterial strains and six agriculturally important pathogenic fungi.
Materials and methods
Plant material
Leaves of one-year old Isodon amethystoides plants were collected on October 13, 2016 from the experimental farm of Huaibei Normal University (E 116.80°, N 33.95°), Anhui Province, China, and identified by Prof. Jianping Xue (Huaibei Normal University). The voucher specimens were deposited to the herbarium of Huaibei Normal University.
Chemicals and microbial strains
Ethanol, acetone, chloroform, ethyl acetate and vitamin C were purchased from Sinopharm Chemical Reagent Co., Ltd. (Beijing, China). All reagents and solvents purchased were of analytical grade.
The microbial strains used for antimicrobial challenge were provided by the Institute of Plant Protection, Anhui Academy of Agricultural Sciences. These included bacterial strains Bacillus subtilis and Escherichia coli and fungal strains Penicillium chrysogenum, Aspergillus niger, Macrophoma kuwatsukai Hara, Alternaria kikuchiana Tanaka, Glomerella cingulata (Stonem.) Spauld. et Schrenk, and Magnaporthe grisea.
Extraction
Leaves collected were air-dried in a shady place at room temperature, ground into powder, and used for extraction with ethanol, acetone, chloroform and ethyl acetate separately. The extraction was performed in an ultrasonic-microwave synergistic extractor for 30 min with set parameters including a material-liquid ratio of 0.3 g/mL, an extraction power of 50 W, and an extraction temperature of 50 °C. The extract was filtered to collect the solution, and the residues were extracted again under the same conditions. The two filtrates were combined and evaporated at low temperature (50 °C) under reduced pressure (10 kPa). The precipitate was weighed and stored at 4 °C for further analysis. Before use, the dried extract was dissolved in the corresponding solvent to a concentration of 1 g/mL.
Determination of total phenolic content
Total phenolic content was measured following a previously published method [Citation16] via the colorimetric Folin–Ciocalteau assay using a UV–Vis spectrophotometer (Unico (Shanghai) Instrument Co., Ltd., Shanghai, China) at a wavelength of 725 nm. The total phenols were expressed as milligrams of gallic acid equivalent (GAE) per gram of dry extract.
Determination of total flavonoid content
Total flavonoid content was measured using the aluminium chloride (AlCl3) colorimetric assay [Citation17]. Here, 200 μL of extract was mixed with 900 μL of ddH2O and 60 μL of 1 mol/L sodium nitrite (NaNO2). The mixture was incubated at room temperature for 5 min; 120 μL of 10% AlCl3 was added to the mixture and incubated at room temperature for 5 min; and 400 μL of 1 mol/L sodium hydroxide (NaOH) was added to the mixture. The absorbance was read at a wavelength of 510 nm, and the flavonoid content was calculated from the standard curve plotted using rutin standard run at a gradient concentration series (0, 0.25, 0.50, 0.75, 1.0, 1.5, and 2.0 mg/mL) against absorbance. The results were presented as milligrams of rutin equivalent to 1 g dry extract (mg RE/g).
Determination of antioxidant activity
The antioxidant activity was determined by the 2,2´-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay. The ability of the samples to scavenge DPPH radicals was determined following the procedure described by Duan et al. [Citation18] with slight modifications. Vitamin C was used as a positive control. For each extract, 2 mL of the diluted sample (a series of concentrations; 80, 60, 40, 20, and 10 mg/mL) was mixed with 2 mL of 121 μmol/L DPPH solution. The reduction (%) of DPPH radicals was determined by reading the absorbance at 517 nm using a UV-VIS spectrophotometer (Unico (Shanghai) Instrument Co. Ltd., Shanghai, China) and using the following formula:
where Asample represents the absorbance in the presence of the test sample and A represents the absorbance of the control containing only methanol and free radicals.
Antimicrobial assay
Referring to the procedure of the National Committee for Clinical Laboratory Standards (NCCLS) [Citation19], the antimicrobial assay was performed via the disc-diffusion method with 100 μL of suspension at a concentration of 2.0 × 108 CFU/mL for bacteria or 2.0 × 104 spores for fungi spread on Mueller-Hinton agar (MHA, Torlak) or malt extract agar (Torlak) in Φ90 mm sterilized Petri dishes. The discs were impregnated with 15 μL of the extract (0.074, 0.156, 0.313, 0.625, 1.250 mg/mL for bacteria and 0.156, 0.313, 0.625, 1.250 mg/mL for fungi) and plated on Petri dishes containing agar. These dishes were incubated at 37 °C for 24 h for bacterial strains and 25 °C for 48 h for fungal strains. Chloramphenicol, streptomycin and nystatin (30 μg for each disc) were used as positive controls for gram-negative, gram-positive and fungal strains, respectively. The solvents were used as negative controls. The inhibition zones were measured to evaluate the antimicrobial activity of each extract.
To precisely measure the antimicrobial activity of the extracts, the minimum inhibitory concentration (MIC) and minimum bactericidal/fungicidal concentration (MBC/MFC) were determined adopting the broth microdilution method following the protocol described by Mihajilov-Krstev et al. [Citation20]. For the bacterial strains, the inoculum was prepared from overnight broth culture and the suspension was adjusted to 0.5 McFarland standard turbidity. A series of doubling dilutions (10–500 mg/mL) were prepared in a 96-well microtiter plate. The microbial concentration in each well was adjusted to 2.0 × 106 CFU/mL for bacteria and 2.0 × 105 spores for fungi. The respective solvent was adopted as the negative control for each extract. The positive controls and incubation conditions were the same as those in the disc-diffusion assay. The minimum inhibitory concentration (MIC) was determined as the lowest concentration completely inhibiting the growth of microbes [Citation19]. The microbial growth was determined by reading the absorbance at 660 nm using a universal microplate reader (BioTek Filters for ELx800). The broth from each well was inoculated by streaking on Mueller Hinton agar (MHA) for bacteria or on malt extract agar (MEA) for fungi to determine the MBC/MFC defined as the lowest concentration of extract at which inoculated microbes were 99.9% killed.
Statistical analysis
Analysis of variance (ANOVA) was used to test the statistical significance (p < 0.05) of the data. Each test had three biological replicates. We considered 95% confidence level for reproducibility of the results.
Results and discussion
Extraction yield, total phenolic content and flavonoid content
A 2.3-fold difference in extraction yield was observed between the four solvents (). The highest extraction yield was observed in acetone (18.27%) followed by ethanol (11.02%), ethyl acetate (8.54%) and chloroform (7.94%). These results indicate a considerable solvent effect on the extraction efficiency of compounds from I. amethystoides. Compared with the other solvent extracts, significantly higher phenolic content (146.77 mg GAE/g dry extract) and flavonoid content (81.57 mg RE/g dry extract) were detected in the acetone extract. More than two-fold difference was observed in these parameters in the acetone extract compared to the chloroform extract. It is probably due to the difference in polarity between the solvents.
Table 1. Effect of solvent on extraction yield, total phenolic content and flavonoid content.
Solvents are selected for extraction based on the polarity of the compounds [Citation21–25]. Generally, solvents with poor polarity are suitable for fatty acids and steroids, whereas those with strong polarity are propitious to phenolic compounds [Citation26]. In the present study, acetone exhibited better extraction efficiency in terms of extract yield, total phenolic content and flavonoid content compared with the other three solvents. This is consistent with the previous reports on high extraction yield and strong biological activity of secondary metabolites in the acetone extracts from Marula leaves [Citation27] and South African Eugenia and Syzygium (Myrtaceae) species [Citation28]. In terms of polarity, acetone is not the strongest among the four solvents tested; it ranks between ethanol and ethyl acetate. This is consistent with the study by Famuyid et al. [Citation28], who reported acetone as a solvent to extract compounds with a wide range of polarities.
Antioxidant activity
In biological systems, excessive free radicals should be removed to prevent oxidative damage. This scavenging effect is related to the combined effect of different secondary metabolites such as phenolic compounds and flavonoids [Citation29, Citation30]. Therefore, the antioxidant activity of plant extracts is usually determined by measuring the free radical scavenging capacity. DPPH is a stable free radical that is effective in antioxidant activity evaluation based on a hydrogen atom transfer reaction [Citation31–33]. This assay is sensitive and depends on substrate polarity as well as on hydrogen donation and/or radical scavenging activity. It indicates the capacity of plant extracts to scavenge free radicals and to donate hydrogen atoms or electrons independent of any enzymatic activity [Citation34].
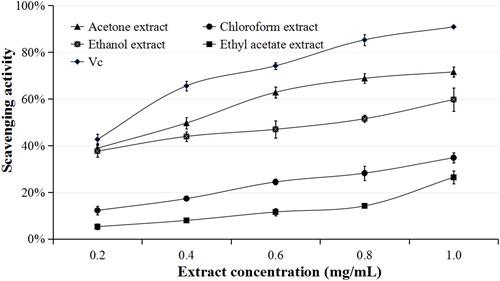
The antioxidant activity of the extracts from I. amethystoides leaves is presented in and . The DPPH scavenging percentage increased with increase in extract concentration. The concentration-dependent activity indicates the efficiency of the DPPH scavenging assay. In our study, acetone > ethanol > chloroform > ethyl acetate was the ranking in DPPH scavenging activity observed for each concentration tested (). We determined IC50 values of 0.4024, 1.5507, 0.6788, and 2.1225 mg/mL for acetone, chloroform, ethanol and ethyl acetate extracts, respectively.
Figure 2. IC50 values for DPPH scavenging ability of leaf extracts of Isodon amethystoides. Vc, vitamin C.
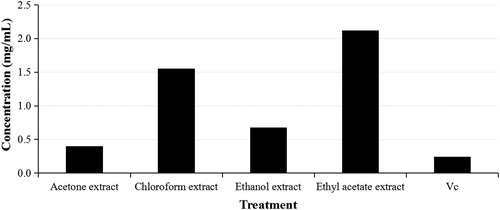
IC50 is an important indicator to intuitively display the antioxidant activity of an extract through the DPPH scavenging assay. The lower the IC50 value, the stronger the antioxidant activity of the plant extract [Citation35]. There are reports on antioxidant activity results from secondary metabolites like phenolic and non-phenolic compounds [Citation36]. High DPPH scavenging effect was observed in the acetone extract (with IC50 closer to the positive control, vitamin C). This is consistent with the phenolic and flavonoid contents detected from I. amethystoides leaf extracts with acetone as the solvent. However, the ranking of the antioxidant activity was not fully consistent with that of the phenolic and flavonoid contents. These results suggest the presence of other secondary metabolites contributing to the antioxidant activity in I. amethystoides leaves, agreeing with previously published phytochemical compositions including diterpenoids, flavonoids, phenolic acids, triterpenoids, volatile oils, ursolic acid and β-sitosterol [Citation37, Citation38].
Antimicrobial activity
Antibiotics and germicides effectively control infective pathogens and have been widely applied in agricultural production [Citation39]. However, drug resistance accompanies the use of antibiotics and germicides. Medicinal plant resources are promising alternatives to solve this problem. During explant disinfection in tissue culture experiments, I. amethystoides leaves sampled from fields required only a simple disinfectant treatment compared with other plant species (unpublished data), which implies the potential of I. amethystoides in plant disease control.
In this study, the antimicrobial activity of I. amethystoides extracts was tested on six agriculturally important pathogenic fungi of rice, pear and orange as well as on typical gram-positive and gram-negative bacterial strains (). The extracts with four solvents exhibited antibacterial activity against two bacterial strains with different inhibition zone diameters. The acetone extract gave the best inhibitory effect against B. subtilis and E. coli with 10 mm and 12 mm inhibition zones, respectively. MICs of the extracts with different solvents ranged from 0.1 mg/mL to 0.3 mg/mL for both bacterial strains (). The antibacterial activity was even equivalent to that of the standard antibiotics chloramphenicol and streptomycin used as positive controls against gram-positive and gram-negative bacteria respectively. The strongest antibacterial activity against B. subtilis and E. coli was observed in the acetone extract, with a MIC of 0.10 mg/mL and 0.05 mg/mL, respectively. These results demonstrate the antibacterial effectiveness of I. amethystoid
Figure 3. MIC and MBC/MFC of leaf extracts of Isodon amethystoides against challenged strains. Chloramphenicol, streptomycin and nystatin (30 μg for each disc) were used as positive controls for gram-negative, gram-positive and fungal strains, respectively.
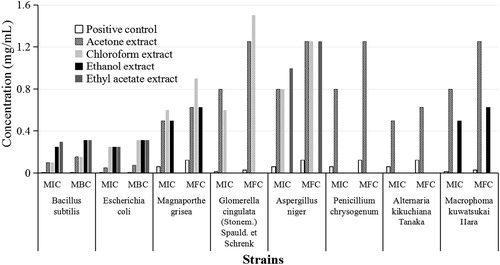
Table 2. Antimicrobial activity of leaf extracts of Isodon amethystoides.
es leaf extracts. The observed antibacterial effect is in agreement with other reports that some Isodon species have antibacterial activities, e.g. I. excisa for oral bacteria, and I. serra for Staphylococcus epidermidis [Citation40, Citation41].
Among the six challenged agriculturally important pathogenic fungi, G. cingulata, A. niger and P. chrysogenum were not sensitive to I. amethystoides extracts in the four solvents tested, whereas the other three fungi presented sensitivity. The extracts in acetone, chloroform and ethyl acetate exhibited inhibitory activity against M. grisea. The acetone extract had an inhibition zone closer to that of the positive control nystatin. The ethyl acetate and acetone extracts exhibited antifungal activities against A. kikuchiana and M. kuwatsukai; the ethyl acetate extract had an inhibition zone (4 mm) equivalent to that of the positive control (). MIC values of I. amethystoides leaf extracts to M. grisea, A. kikuchiana and M. kuwatsukai were between 0.5 mg/mL and 0.8 mg/mL (), which implies that I. amethystoides leaf extracts may be effective in controlling rice and pear diseases.
Among the various extracts, the acetone extract showed the largest inhibition zone and lowest IC50 value in both antibacterial and antifungal challenges. These findings were consistent with the distribution of total phenolic compounds and flavonoids in I. amethystoides leaf extracts with different solvents in our experiments. To our knowledge, this is the first report on the inhibitory effect of Isodon genus against agriculturally important pathogenic fungi, which indicates I. amethystoides leaves as a potential source of plant-derived products.
Conclusions
Solvent significantly influenced extraction yield, total phenolic content, and flavonoid content of I. amethystoides leaf extracts. We can conclude that acetone is most suitable for extracting secondary metabolites from this plant. I. amethystoides leaf extracts demonstrated antioxidant activity and antimicrobial activity particularly against agriculturally important pathogenic fungi such as M. grisea, A. kikuchiana, and M. kuwatsukai. Our findings have proven I. amethystoides as a potent source of biologically active compounds that can be used to control plant diseases. Further studies should be carried out on identifying compounds from the leaf extracts, then determining which one accounting for the antimicrobial activity, and accordingly unraveling the metabolic pathway through transcriptome analysis for synthetic biology research.
Disclosure statement
The authors declare that there are not conflicts of interests.
Additional information
Funding
References
- Harley RM, Atkins S, Budantsev AL, et al. Labiatae In: Kadereit, J.W., editor. The families and genera of vascular plants, VI (Lamiales). Berlin: Springer; 2004. p. 167–275.
- Yu XQ, Maki M, Drew BT, et al. Phylogeny and historical biogeography of Isodon (Lamiaceae): rapid radiation in south-west China and Miocene overland dispersal into Africa. Mol Phylogenet Evol. 2014;77:183–194.
- Yang F, Cao YR, Zhang J, et al. Glaucocalyxin A improves survival in bleomycin- induced pulmonary fibrosis in mice. Biochem Bioph Res Co. 2017;482(1):147–153.
- Zhao F, Sun M, Zhang W, et al. Comparative transcriptome analysis of roots, stems and leaves of Isodon amethystoides reveals candidate genes involved in Wangzaozins biosynthesis. BMC Plant Biol. 2018;18(1):272. [cited 2019 Sep 29]. Available from:
- Chen ZJ, Li YS, Zhou JY, et al. Immunopharmacological effect of Rabdosia amethystoides (benth.) Hara in Mice. Chin Pharm J 2006;41:908–910.
- Savary S, Ficke A, Aubertot JN, et al. Crop losses due to diseases and their implications for global food production losses and food security. Food Sec. 2012;4(4):519–537.
- Mahmuti M, West JS, Watts J, et al. Controlling crop disease contributes to both food security and climate change mitigation. Int J Agr Sustain. 2009;3:189–202.
- Almeida F, Rodrigues ML, Coelho C. The still underestimated problem of fungal diseases worldwide. Front Microbiol. 2019;10:214. [cited 2019 Sep 29].
- Barna B, Leiter É, HegeduS N, et al. Effect of the Penicillium chrysogenum antifungal protein (PAF) on barley powdery mildew and wheat leaf rust pathogens. J Basic Microbiol. 2008;48(6):516–520.
- Chao X, Wang C, Ju L, et al. Multiple locus genealogies and phenotypic characters reappraise the causal agents of apple ring rot in China. Fungal Divers. 2015;71:215–231.
- Zhang X, Xi H, Lin K, et al. Aspergillus leaf spot of field bindweed (Convolvulus arvensis L.) caused by Aspergillus niger in China. SpringerPlus 2016;5(1):605. [cited 2019 Sep 29].
- Hughes DJ, West JS, Atkins SD, et al. Effects of disease control by fungicides on greenhouse gas emissions by UK arable crop production. Pest Manag Sci. 2011;67(9):1082–1092.
- Sayago JE, Ordoñez RM, Kovacevich LN, et al. Antifungal activity of extracts of extremophile plants from the Argentine Puna to control citrus postharvest pathogens and green mold. Postharvest Biol Tec. 2012;67:19–24.
- Karamiosboo R, Khodaverdi M, Aliakbari F. Antibacterial effect of effective compounds of Satureja hortensis and Thymus vulgaris essential oils against Erwinia amylovora. J Agr Sci Tech 2010;12:35–45.
- Isaac GS, Abu-Tahon MA. In vitro antifungal activity of medicinal plant extracts against Fusarium oxysporum F. Sp Lycopersici race 3 the causal agent of tomato wilt. Acta Biol Hung. 2014;65(1):107–118.
- Duan Y, Zhao F, Li H, et al. Evaluation of aqueous chlorine dioxide for disinfecting plant explants. In Vitro Cell Dev Biol-Plant. 2016;52(1):38–44.
- Zhang R, Zeng Q, Deng Y, et al. Phenolic profiles and antioxidant activity of litchi pulp of different cultivars cultivated in Southern China. Food Chem. 2013;136(3–4):1169–1176.
- Duan Y, Su Y, Chao E, et al. Callus-mediated plant regeneration in Isodon amethystoides using young seedling leaves as starting materials. Plant Cell Tiss Organ Cult. 2019;136(2):247–253.
- NCCLS-National Committee for Clinical Laboratory Standards. 2003. Performance Standards for Antimicrobial Susceptibility Testing: eleventh Informational Supplement. Document M100-S11 National Committee for Clinical Laboratory Standard, Wayne, PA, USA.
- Mihajilov-Krstev T, Radnovic D, Kitic D, et al. Antimicrobial activity of Satureja Hortensis L. essential oil against pathogenic microbial strains. Biotechnol Biotec Eq. 2009;23(4):1492–1496.
- Rebey IB, Bourgou S, Debez IBS, et al. Effects of extraction solvents and provenances on phenolic contents and antioxidant activities of cumin (Cuminum cyminum L.) seeds. Food Bioprocess Technol. 2012;5:2827–2836.
- Kchaou W, Abbès F, Blecker C, et al. Effects of extraction solvents on phenolic contents and antioxidant activities of Tunisian date varieties (Phoenix dactylifera L.). Ind Crop Prod. 2013;45:262–269.
- Ngo TV, Scarlett CJ, Bowyer MC, et al. Impact of different extraction solvents on bioactive compounds and antioxidant capacity from the root of Salacia chinensis L. J Food Quality. 2017;1:1–8.
- Singariya P, Kumar P, Mourya K. Comparative bioactivity of dhaman grass root extracts in different polar solvents against plant and human pathogens. Int J Green Pharm. 2012;6(3):248. [cited 2019 Sep 29].
- Mohammadi M, Alaei M, Bajalan I. Phytochemical screening, total phenolic and flavonoid contents and antioxidant activity of Anabasis setifera and Salsola tomentosa extracted with different extraction methods and solvents. Orient Pharm Exp Med. 2016;16(1):31–35.
- Dirar AI, Alsaadi DHM, Wada M, et al. Effects of extraction solvents on total phenolic and flavonoid contents and biological activities of extracts from Sudanese medicinal plants. S Afr J Bot. 2019;120:261–267.
- Loff JN. Antibacterial activity of Marula (Sclerocarya birrea (A. rich.) Hochst. subsp. caffra (Sond.) Kokwaro) (Anacardiaceae) bark and leaves. J Ethnopharmacol 2001;76(3):305–308.
- Famuyide IM, Aro AO, Fasina FO, et al. Antibacterial and antibiofilm activity of acetone leaf extracts of nine underinvestigated South African Eugenia and Syzygium (Myrtaceae) species and their selectivity indices. BMC Complem Altern M 2019;19:141. [cited 2019 Sep 29].
- Jovtchev G, Stankov A, Georgieva A, et al. Cytotoxic and genotoxic potential of Bulgarian Rosa alba L. essential oil - in vitro model study. Biotechnol Biotec Eq. 2018;32(2):513–519.
- Fayemi PO, Ozturk I, Kaan D, et al. Bioactivities of phytochemicals in Callistemon citrinus against multi-resistant foodborne pathogens, alpha glucosidase inhibition and MCF-7 cancer cell line. Biotechnol Biotec Eq. 2019;33(1):764–778.
- Bandonienė D, Murkovic M, Pfannhauser W, et al. Detection and activity evaluation of radical scavenging compounds by using DPPH free radical and on-line HPLC-DPPH methods. Eur Food Res Technol. 2002;214(2):143–147.
- Kedare SB, Singh RP. Genesis and development of DPPH method of antioxidant assay. J Food Sci Technol. 2011;48(4):412–422.
- Li W, Hosseinian FS, Tsopmo A, et al. Evaluation of antioxidant capacity and aroma quality of breast milk. Nutrition 2009;25(1):105–114.
- Mileva M, Kusovski V, Krastev D, et al. Chemical composition, in vitro antiradical and antimicrobial activities of Bulgarian Rosa alba L. essential oil against some oral pathogens. Int J Curr Microbiol App Sci 2014;3:11–20.
- Michiels JA, Kevers C, Pincemail J, et al. Extraction conditions can greatly influence antioxidant capacity assays in plant food matrices. Food Chem. 2012;130(4):986–993.
- Mabrouki H, Duarte CMM, Akretche DE. Estimation of total phenolic contents and in vitro antioxidant and antimicrobial activities of various solvent extracts of Melissa officinalis L. Arab J Sci Eng. 2018;43(7):3349–3357.
- Li GY, Wang YL, Xu ZP, et al. Studies on the chemical constituents of Isodon amethystoides (Ben-th) Cy Wu et Hsuan. Acta Pharm Sin 1981;9:667–671.
- Sun HD, Xu Yl, Jiang B. Diterpenoids from Isodon Species. Beijing: Science Press; 2001.
- Kaye KS, Gales AC, Dubourg G. Old antibiotics for multidrug-resistant pathogens: from in vitro activity to clinical outcomes. Int J Antimicrob Agents. 2017;49(5):542–548.
- Liu F, Zhu XH, Wang Q. The inhibition of Rabdosia excisa on oral bacteria. Pharm J Chin PLA 2010;26:421–423.
- Mo XL, Qiu WF, Huang SS, et al. Antibacterial and antifungal activities of different plant resources of herba Isodon serra. Mod Chin Med 2016;18:980–984.