Abstract
The present study investigated the inhibitory effect of human umbilical cord mesenchymal stem cells (hucMSCs) on the growth of lung cancer in mice and its relationship with the TLR4/NF-κB signalling pathway. A mouse model of Lewis lung cancer was constructed by inoculating mice with Lewis lung cancer cell suspension under the axilla. Twenty mice were randomly and evenly divided into experimental group and control group. The experimental group received a caudal vein injection of 1 × 106 hucMSCs each week for two consecutive weeks, and the control group, an equal amount of saline at the same time. Two weeks after treatment with hucMSCs, the mice were sacrificed and the tumour volume and tumour weight were recorded. Enzyme-linked immunosorbent assay was used to determine the IL-1, IL-6 and TNF-α contents in lung cancer tissues; real-time polymerase chain reaction, to measure the level of mRNA; and Western blotting, to determine the protein expression. The results showed that injection of hucMSCs inhibited the growth of Lewis lung cancer tissues, reduced Lewis lung cancer tissue weight, enhanced thymus index, and suppressed inflammatory responses. hucMSCs inhibited the expression of inflammatory factors IL-1, IL-6 and TNF-α by activating TLR4/NF-κB signalling pathway, and facilitated the expression of Bax. Moreover, huMSCs induced the expression of apoptogenic factor Bax by activating TLR4/NF-κB signalling pathway, and inhibited the expression of antiapoptotic factor bcl-2. The present study demonstrates that hucMSCs reduce inflammatory responses by mediating TLR4/NF-κB signalling pathway, promote apoptosis of tumour cells, and inhibit tumour proliferation in mice with Lewis lung cancer.
Introduction
Lung cancer is a common malignant tumour in humans. At present, radiotherapy, chemotherapy and surgery are the main treatments for lung cancer, but the survival rate of lung cancer patients is still low [Citation1]. In 2017, the 5-year overall survival rate for lung cancer in the United States was 18% [Citation2]. In China, the 5-year overall survival rate of lung cancer in 2016 was only 15.6% [Citation3]. Therefore, effective treatment of lung cancer and improvement of the survival rate of lung cancer patients are still major challenges.
A promising strategy that has raised hopes as a potential approach to combat different types of tumors is the application of mesenchymal stem cells (MSCs). MSCs are a class of cells originating in the mesoderm, including bone marrow MSCs and adipose MSCs, etc. Human umbilical cord MSCs (hucMSCs) are one of the important sources of MSCs [Citation4,Citation5], and they have strong proliferation and low immunity. Studies have reported that MSCs have homing effect on tumours such as glioma and ovarian cancer, and have anti-tumour effect on blood cancer and breast cancer [Citation6,Citation7]. Therefore, hucMSCs are expected to become an ideal vector for gene therapy and participate in targeted therapy of tumours.
An important aspect of cancer drug research is the elucidation of the underlying molecular mechanisms of action. Toll-like receptors (TLRs) is a family of transmembrane protein receptors, and TLR4 belongs to this family. The occurrence and development of tumours are closely related to chronic inflammation. It is reported that the TLR4/NF-κB signalling pathway plays an important role in inhibiting inflammation and tumorigenesis [Citation8]. In the present study, we investigated the inhibitory effect of hucMSCs on the growth of lung cancer in mice and its relationship with the TLR4/NF-κB signalling pathway.
Materials and methods
Cells
Mouse Lewis lung cancer cells (LLC1; Guangzhou Jennio Biotech Co., Ltd., Guangzhou, China) were cultured in DMEM (Dulbecco's Modified Eagle's Medium) supplemented with 10% foetal bovine serum under 37 °C and 5% CO2. When reaching 95% confluency, the cells were trypsinized and prepared into cell suspension with a density of 1 × 105 cells/mL.
To prepare hucMSCs, matrix tissue mass of umbilical cord in full-term parturients was digested with collagenase IV, and the suspension was collected and centrifuged. The sediments were then digested with 0.05% trypsin and filtered. The filtrate containing cells were used for cell count. After this, the cells were cultured in serum-free and low-glucose DMEM under 37 °C and 5% CO2. The hucMSCs of 3rd–7th passages were used in subsequent experiments.
Animals
Twenty SPF C57BL/6J mice were purchased from Nanjing Biomedical Research Institute of Nanjing University, and used in the present study, including 10 males and 10 females (age, 6 weeks; weight, 18–20 g). The mice were raised in SPF Animal Laboratory under 20–22 °C, 50–70% humidity and 12 h/12 h light and dark cycle. The mice had free access to food and water.
After 7 days of feeding, each mouse was inoculated with Lewis lung cancer cell suspension under the axilla (0.2 mL). Two weeks later, mice with visible transplanted tumours were included as successful Lewis lung cancer transplantation model mice. The mice were randomly and evenly divided into control group and experimental group. The experimental group received a caudal vein injection of 1 × 106 hucMSCs each week in two consecutive weeks, day 0 being the day of the first injection. The control group received a caudal vein injection of an equal amount of saline at the same time: one in the first week and another one in the second week. After two weeks, the mice were sacrificed by cervical dislocation. After weighing each mouse, tumour and thymus tissues were excised and weighed. Thymus index was calculated by the equation: thymus index = thymus weight (g)/body weight (10 g). Diameter (d) of tumours was measured by vernier callipers to calculate the radius (r), and tumour volume (V) was calculated by equation: V (cm3) = (4/3)πr3. Tumour weight was directly measured by an electronic scale.
Ethics of experimentation
All animal experiments were conducted according to the ethical guidelines of Lishui People’s Hospital. All efforts were made to minimise animal suffering.
Enzyme-linked immunosorbent assay (ELISA)
The concentrations of interleukin (IL)-1, IL-6 and tumour necrosis factor alpha (TNF-α) in tumour tissues were determined by respective ELISA kits (Elabscience Biotechnology Co., Ltd, Wuhan, China).
Real-time polymerase chain reaction (RT-PCR)
RNA extraction kit was used for the extraction of RNA in tissues and cells. After reverse transcription for the synthesis of cDNA, RT-PCR was performed for quantification. The primers used for TLR4, NF-κB, Bax, Bcl-2, IL-1, IL-6, TNF-α and β-actin are shown in . The following amplification programme (Takara, Dalian, China; 7300 Fast Real-Time PCR System, Thermo Fisher Scientific, Waltham, MA, USA) was used: initial denaturisation at 95˚C for 30 s; 40 cycles of denaturation at 95˚C for 5 s, annealing at 60˚C for 31 s, and elongation at 72˚C for 45 s.
Table 1. Primer sequences for RT-PCR.
Western blotting
Tumour tissues were washed with phosphate-buffered saline, and lysed with tissue lysis buffer for extraction of total proteins. Polyacrylamide agarose gel electrophoresis was performed and the proteins were transferred onto PVDF (polyvinylidene difluoride) membrane. The membrane was incubated with TLR4 antibody (1:500; Abcam, Cambridge, UK), NF-κB antibody (1:1000; Abcam, Cambridge, UK), Bax antibody (1:1000; Abcam, Cambridge, UK), bcl-2 antibody (1:1000; Abcam, Cambridge, UK), and β-actin antibody (1:10,000; Abcam, Cambridge, UK) overnight. After washing with TBST (tris-buffered saline and Tween 20 buffer), the membrane was incubated with secondary antibody (Abcam, Cambridge, UK) at room temperature for 1 h. After washing with TBST again, the membrane was developed by ECL (enhanced chemiluminescence) in dark. The grayscales of protein bands were analysed using Image-J software. Relative expression levels of TLR4, NF-κB, Bax and bcl-2 proteins were calculated against β-actin.
Statistical analysis
The results were analysed using SPSS 22.0 statistical software (IBM, Armonk, NY, USA). The data were expressed as mean values with standard deviations (± SD). Comparison between two groups was carried out using Student’s t-test. Comparison of more than two groups was performed by one-way analysis of variance followed by Student–Newman–Keuls posthoc test. p < 0.05 indicated statistically significant differences.
Results and discussion
With the increase of environmental pollution and smoking, lung cancer has become the main cause of death from malignant tumours [Citation9]. Clinical treatment for lung cancer is limited and the overall 5-year survival rate of lung cancer patients is less than 15% [Citation10]. As a non-immunogenic cancer, lung cancer is resistant to the immune system [Citation11]. The immune system protects the body from cancer by identifying cancer cells. Tumour microenvironment is a chronic inflammatory process, which can promote the occurrence and development of tumours. It is believed that continuous progression of inflammatory responses and activation of the body’s immune system are closely related to tumours [Citation12].
Injection of hucMSCs inhibits the growth of Lewis lung cancer tissues in vivo
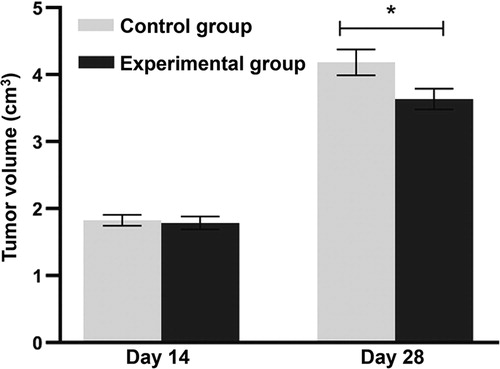
MSCs have strong self-renewal ability and the potential to differentiate into osteocytes, adipocytes, etc. In addition, MSCs have low immunogenicity and can inhibit inflammation, tissue damage and tumour development. Therefore, MSCs have broad prospects in clinical application. It is reported that hucMSCs reduce tumour volume [Citation13]. Fat-derived MSCs can regulate the AKT signalling pathway, inhibit proliferation of hepatocellular carcinoma cells and promote apoptosis of hepatocellular carcinoma cells [Citation14]. Moreover, hucMSCs can inhibit the proliferation of hepatocellular carcinoma HCCC-9810 cells through the IGF-1R/PI3K/AKT pathway [Citation15]. These studies demonstrate that MSCs can inhibit the occurrence and development of tumours and have potential application value in the treatment of tumours. To examine the potential effect of hucMSCs on lung cancer tissue volume, the experimental group received a caudal vein injection of 1 × 106 hucMSCs each week and the control group received an equal amount of saline at the end of week two after injection. The data showed that on days 0–14, the tumour volume in the experimental group was not different from that in the control group (p > 0.05 at all time points). From day 25 on, the tumour volume in the experimental group was significantly lower than that in the control group on each subsequent day (p < 0.05 at all time points) (). The result suggests that injection of hucMSCs inhibits (slows down) the growth of Lewis lung cancer tissues in vivo.
Figure 1. Lewis lung tumour volumes in mouse model. Experimental group (n = 10) received caudal vein injection of 1 × 106 hucMSCs each week for consecutive two weeks. Control group (n = 10) received caudal vein injection of equal amount of saline at the same time. *p < 0.05 compared with control group at the same time point.
Treatment with hucMSCs reduces Lewis lung cancer tissue weight and enhances thymus index of mice
To evaluate the tumour weight and thymus index in the two groups, the mice were sacrificed by cervical dislocation on day 28. The data showed that the tumour weight in the experimental group was significantly lower than that in the control group (p < 0.05), whereas the thymus index in the experimental group was significantly higher than that in the control group (p < 0.05) (). The result indicates that treatment with hucMSCs reduces Lewis lung cancer tissue weight and enhances thymus index of mice.
Table 2. Comparison of tumour weight and thymus index.
Treatment with hucMSCs inhibits inflammatory responses in mice with Lewis lung cancer
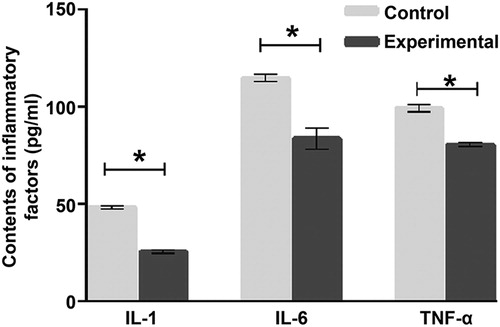
To measure the contents of IL-1, IL-6 and TNF-α, ELISA was performed. The data showed that the contents of IL-1, IL-6 and TNF-α in tumour tissues from the experimental group were significantly lower than those from the control group, respectively (p < 0.05 for all) (). The result indicates that treatment with hucMSCs inhibits inflammatory responses in mice with Lewis lung cancer.
Figure 2. Contents of inflammatory factors IL-1, IL-6 and TNF-α in tumour tissues from mice. Experimental group (n = 10) received caudal vein injection of 1 × 106 hucMSCs each week for consecutive two weeks. Control group (n = 10) received caudal vein injection of equal amount of saline at the same time. ELISA was used to determine IL-1, IL-6 and TNF-α contents in lung cancer tissues. *p < 0.05.
hucMSCs inhibit the expression of inflammatory factors IL-1, IL-6 and TNF-α by activating TLR4/NF-κB signalling pathway, and facilitate the expression of Bax mRNA
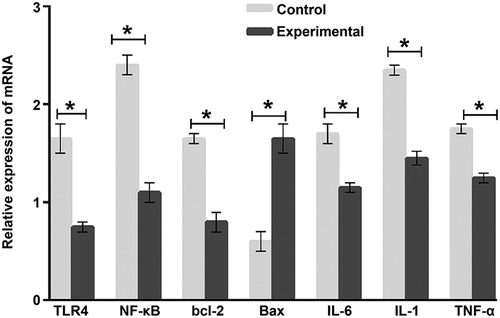
TLRs play important roles in innate immune system, and activate the immune system by identifying specific molecules. TLRs can recognise endogenous and exogenous ligands. TLR4 mainly depends on MyD88 pathway to activate downstream NF-κB phosphorylation, leading to increased release of inflammatory factors such as IL-1, IL-6 and TNF-α. TLR4 is widely expressed in lung cells, bone marrow and infiltrating cells. TLR1-6 and TLR9 are highly expressed in airway epithelial cells [Citation16–18]. The expression of TLR2, TLR4, TLR7/8 and TLR9 in lung cancer was significantly higher than that in normal lung [Citation19,Citation20]. Therefore, activation of the TLR4 pathway in the immune system may be a new strategy for cancer treatment. To determine the expression of mRNA, RT-PCR was carried out. The data showed that the Bax mRNA level in the experimental group was significantly higher than that in the control group (p < 0.05), whereas the levels of TLR4, NF-κB, bcl-2, IL-1, IL-6 and TNF-α mRNA in the tumor tissues from the experimental group were significantly lower than those in the control group (p < 0.05) (). The results suggest that hucMSCs inhibit the expression of inflammatory factors IL-1, IL-6 and TNF-α by activating TLR4/NF-κB signalling pathway, and facilitate the expression of Bax mRNA.
Figure 3. Relative expression of TLR4, NF-κB, Bcl-2, Bax, IL-6, IL-1 and TNF-α mRNA in tumour tissues from mice. Experimental group (n = 10) received caudal vein injection of 1 × 106 hucMSCs each week for consecutive two weeks. Control group (n = 10) received caudal vein injection of equal amount of saline at the same time. RT-PCR was used to measure the level of mRNA. *p < 0.05.
huMSCs induce the expression of apoptogenic factor Bax by activating TLR4/NF-κB signalling pathway, and inhibit the expression of antiapoptotic factor bcl-2
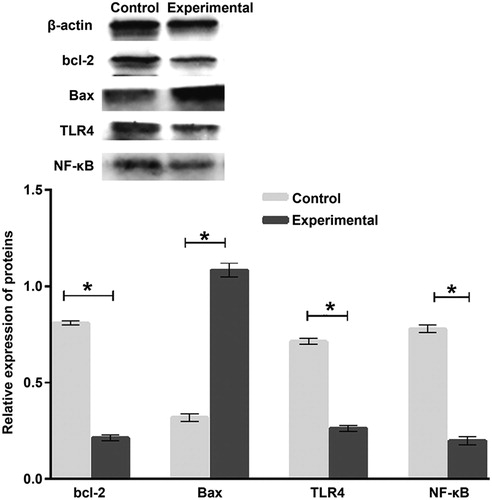
To determine the expression of proteins, Western blotting was employed. The data showed that the expression of Bcl-2, TLR4 and NF-κB proteins in the experimental group was significantly lower than that in the control group (p < 0.05), whereas Bax protein expression in the experimental group was significantly higher than that in control group (p < 0.05) (). This result indicates that huMSCs induce the expression of apoptogenic factor Bax by activating TLR4/NF-κB signalling pathway, and inhibit the expression of antiapoptotic factor bcl-2.
Figure 4. Relative expression of Bcl-2, Bax, TLR4 and NF-κB proteins in tumour tissues from mice. Experimental group (n = 10) received caudal vein injection of 1 × 106 hucMSCs each week for consecutive two weeks. Control group (n = 10) received caudal vein injection of equal amount of saline at the same time. Western blotting was used to determine protein expression. *p < 0.05.
Conclusions
The present study demonstrates that hucMSCs significantly inhibit the growth of lung cancer tumours, reduce tumour volume and mass, and decrease the expression of inflammatory factors in mice with Lewis lung cancer. Of note, hucMSCs reduce inflammatory responses by mediating the TLR4/NF-κB signalling pathway, promote expression of the apoptogenic factor Bax, and inhibit tumour proliferation in mice with Lewis lung cancer.
Author contributions
LL, JP, ZC and ZY contributed to the design of the study. LL, JP, XC, EG, CX and HZ performed the experiments. LL and JP analysed the data. LL, JP, ZC and ZY interpreted results and prepared the manuscript. The final version of the manuscript has been read and approved by all authors.
Ethical approval and consent to participate
All procedures performed in this study were approved by the Ethics Committee of Lishui People’s Hospital. Written informed consent was obtained from all patients or their families.
Consent for publication
Written informed consents for publication of any associated data and accompanying images were obtained from all patients or their parents, guardians or next of kin.
Acknowledgements
The authors wish to thank their department and research team for their help and dedication.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Michels S, Wolf J. Stratified treatment in lung cancer. Oncol Res Treat. 2016;39(12):760–766.
- Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA: a cancer journal for clinicians. 2017;67(1):7–30.
- Woodard GA, Jones KD, Jablons DM. Lung cancer staging and prognosis. Cancer Treat Res. 2016;170:47–75.
- Abbaspanah B, Momeni M, Ebrahimi M, et al. Advances in perinatal stem cells research: a precious cell source for clinical applications. Regen Med. 2018 ;13(5):595–610.
- Bieback K, Brinkmann I. Mesenchymal stromal cells from human perinatal tissues: From biology to cell therapy. World J Stem Cells. 2010; 2(4):81–92.
- Choi SA, Lee JY, Wang KC, et al. Human adipose tissue-derived mesenchymal stem cells: characteristics and therapeutic potential as cellular vehicles for prodrug gene therapy against brainstem gliomas. Eur J Cancer (Oxford, England: 1990). 2012;48(1):129–137.
- Mader EK, Butler G, Dowdy SC, et al. Optimizing patient derived mesenchymal stem cells as virus carriers for a Phase I clinical trial in ovarian cancer. J Transl Med. 2013;11(1):20–20.
- Lamrani M, Sassi N, Paul C, et al. TLR4/IFNgamma pathways induce tumor regression via NOS II-dependent NO and ROS production in murine breast cancer models. Oncoimmunology 2016; May5(5):e1123369.
- Yang Z, Zhu L, Li F, et al. Bone marrow stromal cells as a therapeutic treatment for ischemic stroke. Neurosci Bull. 2014;30(3):524–534.
- Zhao H, Yao Y, Yang H, et al. Hormone therapy as a management strategy for lung metastasis after 5 years of endometrial cancer: A case report and literature review. Medicine 2017;96(51):e9223.
- Ferrari SM, Fallahi P, Galdiero MR, et al. Immune and Inflammatory Cells in Thyroid Cancer Microenvironment. Int J Mol Sci 2019;20(18): E4413.
- Schöniger S, Böttcher D, Theuß T, et al. Expression of Toll-like receptors 2, 4 and 6 in equine endometrial epithelial cells: A comparative in situ and in vitro study. Res Vet Sci. 2017;112:34–41.
- Leng L, Wang Y, He N, et al. Molecular imaging for assessment of mesenchymal stem cells mediated breast cancer therapy. Biomaterials 2014;35(19):5162–5170.
- Zhao W, Ren G, Zhang L, et al. Efficacy of mesenchymal stem cells derived from human adipose tissue in inhibition of hepatocellular carcinoma cells in vitro. Cancer Biother Radio. 2012;27(9):606–613.
- Yulyana Y, Ho IA, Sia KC, et al. Paracrine factors of human fetal MSCs inhibit liver cancer growth through reduced activation of IGF-1R/PI3K/Akt signaling. Molec Ther. 2015;23(4):746–756.
- Mills JT, Schwenzer A, Marsh EK, et al. Airway epithelial cells generate pro-inflammatory tenascin-C and small extracellular vesicles in response to TLR3 stimuli and rhinovirus infection. Front Immunol 2019;10:1987.
- Lewandowska-Polak A, Brauncajs M, Jarzębska M, et al. Toll-like receptor agonists modulate wound regeneration in airway epithelial cells. Int J Mol Sci 2018; 19(8): E2456.
- Gowing SD, Chow SC, Cools-Lartigue JJ, et al. Gram-negative pneumonia augments non-small cell lung cancer metastasis through host toll-like receptor 4 activation. J Thorac Oncol 2019;S1556-0864(19):30653–30657.
- Yu L, Chen S. Toll-like receptors expressed in tumor cells: targets for therapy. Cancer Immunol Immunother. 2008;57(9):1271–1278.
- Cherfils-Vicini J, Platonova S, Gillard M, et al. Triggering of TLR7 and TLR8 expressed by human lung cancer cells induces cell survival and chemoresistance. J Clin Invest. 2010;120(4):1285–1297.
