?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Lycorine is an isoquinoline alkaloid in Amaryllidaceae with antitumor, antibacterial, antiviral, diuretic and cardiovascular effects. This study explored the antitumor mechanism of lycorine in vitro and in vivo by focusing on its effects on tumor cell membrane fraction and tumor cell membrane structure. The results showed that lycorine had antitumor effect on H22 tumor-bearing mice in vivo and could effectively prolong the survival time of H22 tumor-bearing mice. In vitro, lycorine inhibited the proliferation of HepG-2 cells and induced cell death significantly. Lycorine reduced the total protein content, sialic acid content and cholesterol content on the surface of tumor cell membrane, and reduced the content of some main components of tumor cell membranes. Lycorine reduced the membrane fluidity and cell membrane integrity of tumor cells. In addition, the ion channel (Na+, K+-ATPase, Ca2+, Mg2+-ATPase) activity on the surface of the tumor cell membrane decreased. This contributed to a significant antitumor effect in vitro and in vivo.
Introduction
Liver cancer is one of the most common malignant tumors which threaten human health seriously. Due to the rapid increase in the incidence of liver cancer, effective treatment of liver cancer has always been a major problem worldwide. Lycorine, which is an antitumor compound, has attracted attention for research recently. Lycorine is an isoquinoline alkaloid in Amaryllidaceae, which has antitumor, antibacterial, antiviral, diuretic and cardiovascular effects [Citation1–3]. In recent years, the antitumor effect of lycorine has become a hot topic. Studies have reported that lycorine has significant inhibitory effects on different kinds of cancer cells [Citation4–11]. Therefore, lycorine has a high research value as a potential antitumor drug. However, the mechanism of its antitumor effect is still unclear. Therefore, the purpose of this study was to explore the antitumor mechanism of lycorine on liver cancer in vitro and in vivo by focusing on its effects on tumor cell membrane fraction and tumor cell membrane structure.
Materials and methods
Experimental animals and breeding conditions
This study included 100 Kunming mice (18–22 g), 20 mice per group, equal male-to-female ratio. They were purchased from the Animal Laboratory of Heilongjiang University of Traditional Chinese Medicine (No. 00101003). The mice were kept in a pathogen-free environment at 20 ± 5 °C at a 12/12 h dark/light cycle with free access to food and water.
Ethics statement
All experimental procedures were performed in accordance with PR China Legislation Regarding the Use and Care of Laboratory Animals, and all experiments involving animals were approved by the Animal Care and Use Committee of the Harbin University of Commerce.
Cell lines
Mouse H22 liver cancer cell line was provided by the Anti-Tumor Drug Research Center of the Ministry of Education. Human liver cancer HepG-2 cell line was provided by the Anti-Tumor Drug Research Center of the Ministry of Education.
Experimental instruments and reagents
The following instruments and reagents were used for the experiments: F-7000 fluorescence spectrophotometer (Hitachi Company); 752 UV–visible spectrophotometer (Shanghai Spectral Instrument Co., Ltd.); TGL-22 desktop refrigerated centrifuge (BECKMAN COULTER); EPICS-XL flow cytometer (Beckman-Coulter Company, USA); Olympus IX70 inverted microscope (Olympus Company); lycorine (Shanghai Aladdin Reagent Co., Ltd.); hydroxycamptothecin (Harbin Sanlian Pharmaceutical Co., Ltd.); trypsin (Gibco Company, USA); 3-(4,5)-dimethylthiahiazo (-z-y1)-3,5-di- phenytetrazoliumromide(MTT) (Sigma Company); saponin (Shanghai Aladdin Reagent Co., Ltd.); DPH (Sigma Company);Dimethyl sulfoxide(DMSO)(Shanghai Aladdin Reagent Co., Ltd.);N,N,N-Trimethyl-4-(6-phenyl-1,3,5-hexatrien-1-yl)phenylammonium, p-toluenesulfonate(DPH)(Shanghai Aladdin Reagent Co., Ltd.).
Antitumor effect of lycorine on human hepatoma HepG-2 cells in vitro
Cell culture
HepG-2 cells were inoculated into RPMI 1640 culture medium. Culture bottles were placed in culture boxes, and the solution was changed every three days. After digestion with 0.25% trypsin digestion solution, the cells were transferred for subculture. If used for experiments, the cells could be collected.
Detection of lycorine-induced growth inhibition by MTT assay
HepG-2 cells were incubated in 96-well plates at cell concentration of 5 × 104/mL. The cells were further cultured in an incubator at 37 °C and 5% CO2. After 24 h, different concentrations of lycorine (1.55, 3.1, 6.2, 12.4, 24.8 μmol/L) and hydroxycamptothecin (1.4, 2.8, 5.6 μmol/L) were added to the 96-well plates. Six parallel samples were set in each group, and five doses were set in the lycorine group. The control group was added RPMI 1640. After 48 h of continuous culture, the supernatant was removed from the wells, then 100 μL MTT (0.5 mg/mL) solution was added to each hole and cultured in incubator at 37 °C and 5% CO2 for 4 h, then the supernatant was removed and 200 μL DMSO solution was added to the wells. A microoscillator was used to oscillate for 5 min. The optical density (OD) detection wavelength was 570 nm and the half maximal inhibitory concentration (IC50) was calculated.
where ODblank is the average OD value in the blank control group, ODadministration is the average OD value in the administration group.
Morphological observation of tumor cells
HepG-2 cells were incubated in 6-well plates at a concentration of 5 × 104/mL. The cells were further cultured in an incubator at 37 °C and 5% CO2. After 24 h, different concentrations of lycorine (3, 6 and 12 μmol/L) were added into the medium. The positive control group was added 4 μmol/L hydroxycamptothecin, and the negative control group was added RPMI 1640 medium. After 48 h of culture, cell morphology was observed under an inverted microscope.
Observation of cell death morphology by fluorescence microscope
After 48 h of administration, the culture solution was poured out. After fixing at 4 °C for 1 h, the cells were washed with Phosphate buffer saline(PBS) washing solution, stained with Hoechst 33258 staining solution for 30 min in the dark, and photographed under a fluorescence microscope.
Effect of lycorine on the cell death rate of HepG-2 cells
Grouping and administration were done as described earlier. After 48 h of administration, cells were digested and collected with trypsin. The collected cells were immobilized in 70% ethanol solution pre-cooled at –20 °C. Fixation was done at 4 °C for 24 h. Then the upper fixative solution was removed by centrifuge and the samples were washed with PBS solution three times. Finally, 800 μL of propidium iodide (PI) staining solution was added to the collected cells. The cells were incubated at room temperature for 30 min without light. Then the stained suspension was filtered with #300 mesh and detected by flow cytometry.
Effect of lycorine on cell membrane components of HepG-2 cells
After 48 h of administration, cells were collected by trypsinization. The collected cells were hypotonicated with distilled water for 1 h, and 10 mL of distilled water was added to the solution. The supernatant was removed by centrifugation (5000 rpm) twice to obtain a precipitate of tumor cell membranes, which was collected and suspended in physiological saline for use. The protein content was determined using BCA Protein Assay Kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions. The cholesterol content was measured with a cholesterol kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). The sialic acid content was determined by NAD kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
Effect of lycorine on membrane structure and function of HepG-2 cells
Tumor cell membranes collected and suspended in physiological saline as described earlier were used. A fluorescence spectrophotometer was used to determine the membrane fluidity [Citation11].
Effect of lycorine on cell membrane integrity of HepG-2 cells
After 48 h of administration, cells were collected by trypsinization. The collected cells were hypotonicated with distilled water for 1 h. NAD+/NADH Assay Kit with WST-8 (Beyotime, Shanghai, China) was used to determine the cell membrane integrity.
Effect of lycorine on cationic channel activity of HepG-2 cell membrane
After 48 h of administration, cells were collected by trypsinization. The collected cells were hypotonicated with distilled water for 1 h. The supernatant tumor cell membrane was centrifuged and ATPase activity kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) was used for detection.
Establishment of tumor model of H22 tumor-bearing mice
The mice were disinfected in the area of the abdominal cavity, inoculated with 200 μL H22 liver cancer cells intraperitoneally, and kept for 5–8 days under the same conditions. Enlargement of mouse abdominal cavity indicated the accumulation of peritoneal fluid and verified the success of the tumor model. The abdomen of mice was disinfected under aseptic conditions, and the ascites of mice inoculated successfully were extracted with a medical syringe. Usually, the peritoneal tumor fluid of mice was pale yellow. The ascetic fluid tumor cell status was observed by microscope and the cell count was scored using a cell counting plate. The tumor cell viability was tested by the trypan blue exclusion method to ensure the survival rate was above 95%. Finally, the cell concentration was adjusted to 5 × 106/mL using 4 °C sterile saline. Each mouse was subcutaneously inoculated with 0.2 mL of the tumor fluid in the axillary region of the right forelimb [Citation12, Citation13].
Grouping and administration of H22 tumor-bearing mice
One hundred Kunming mice with successful tumor inoculation were randomly divided into five groups, 20 mice in each group: negative control group (tumor model without treatment), positive control group (tumor model with hydroxycamptothecin) and three lycorine groups with high (40 mg/kg), medium (20 mg/kg) and low dose (10 mg/kg) of lycorine. Another 20 Kunming mice without tumor inoculation were placed as normal controls. Lycorine was injected intraperitoneally after 24 h. Hydroxycamptothecin was administered in the positive control group at a dose of 6 mg/kg. Saline injection was administered in the negative control group. The animals were kept under the same conditions, and continuous administration was done once a day for 7 days.
Antitumor effect of lycorine on H22 tumor-bearing mice
The procedures were done on an ice bath. After 24 h of withdrawal, the mice were weighed and sacrificed by cervical dislocation. The mice were immobilized in supine position, and the chest was disinfected with iodine and alcohol. Then the tumors were dissected and weighed. Finally, the tumor inhibition rate in the groups injected with lycorine was calculated by the following formula:
Effect of lycorine on life extension of H22 tumor-bearing mice
After the last drug administration, the average survival time of the lycorine group and negative control group was recorded. The observation time of the lycorine group was set at 30 days (If it exceeded this time, it was calculated as 30 days). The survival time of H22 tumor-bearing mice in the lycorine group and the negative control group was recorded, and the life extension rate of H22 tumor-bearing mice in the lycorine group was calculated as follows:
Collection of tumor cells from H22-bearing mice
H22 tumor-bearing mice were treated for seven consecutive days, and 1 mL of ascites was extracted from the abdominal cavity of the mice on day 8. Samples were centrifuged (2000 r/min for 10 min) and the supernatant was removed. If there were more impurities, such as red blood cells in ascites of mice, the samples were washed with 0.85% NH4Cl solution. Following centrifugation (2000 r/min for 10 min), the samples were washed three times with normal saline solution and the supernatants were removed. Finally, the precipitated tumor cells were collected for use.
Determination of cell death rate of tumor cells of H22 tumor-bearing mice
The collected H22 tumor-bearing mouse tumor cells were uniformly mixed with PBS, washed once and the supernatant was removed by centrifugation. The cell pellet was resuspended with a –20 °C 70% ethanol solution and placed in a 4 °C refrigerator for fixation for 24 h. Following centrifugation (2000 r/min for 10 min), the supernatant was removed and 800 μL PI dye solution were added. After incubation at room temperature for 30 min in the dark, the samples were filtered using a nylon mesh and the dead cells were detected by flow cytometry.
Data analysis
Data analysis was performed using SPSS19.0, and the results are expressed as mean values with standard deviation (). Comparisons between groups were done by one-way analysis of variance (ANOVA).
Results and discussion
Lycorine inhibited the proliferation of human hepatocellular carcinoma HepG-2 cells in MTT assay in vitro
Incubation with different concentrations of lycorine for 48 h significantly inhibited HepG-2 cells proliferation in the MTT assay (). Within a certain range, the inhibition of the proliferation induced by lycorine was positively correlated with the dose and the IC50 was 5.73 μmol/L. Three concentrations of lycorine (3 μmol/L, 6 μmol/L and 12 μmol/L) were set as low, medium and high dose groups according to IC50. Briefly, 4 μmol/L hydroxycamptothecin was set as the positive control group.
Table 1. Lycorine inhibited HepG 2 cell proliferation in vitro.
Lycorine induced morphological changes in HepG-2 cells in vitro observed by inverted microscopy and fluorescence microscopy
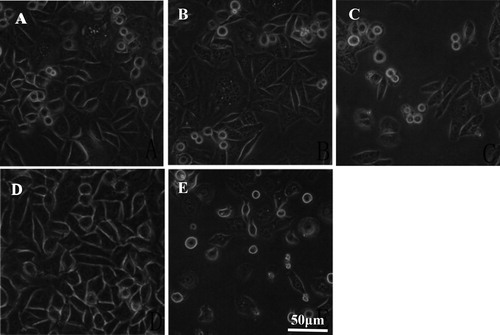
The cells in the negative control group were in good condition, with adherent growth and clear outline under inverted microscope (). After 48 h of administration, the cells in the positive control (hydroxycamptothecin) group grew sparsely, and most of the cells were suspended in the medium. In the lycorine administration group, with the increase in the dose, the number of cells with adherent growth gradually decreased, the cell growth was sparse, and the shape became more and more irregular. These effects were most obvious in the high-dose group, and some cells were ruptured.
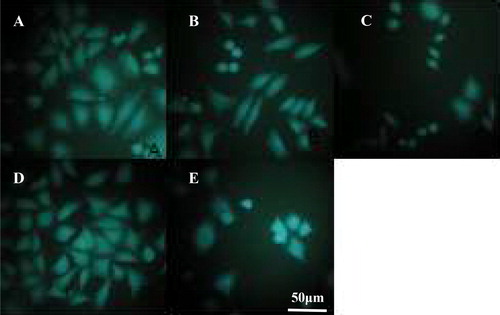
Figure 1. Morphology of HepG-2 cells with or without lycorine treatment in vitro. Inverted microscopy (magnification 10 × 40). Cells treated with lycorine 3 μmol/L (A), 6 μmol/L (B) or 12 μmol/L (C); Control (D); Cells treated with 4 μmol/L HCPT (E).
The results from the fluorescence microscopy observation () showed that the tumor cells in the negative control group were closely arranged, the overall contour was clear and intact, and the nucleus showed uniform fluorescence. The cells in the positive control (hydroxycamptothecin) group showed obvious rupture of the outer membrane, the nucleus narrowed, and the overall contour of the cells was blurred.
Lycorine-induced cell death rate in HepG-2 cells was detected by flow cytometry
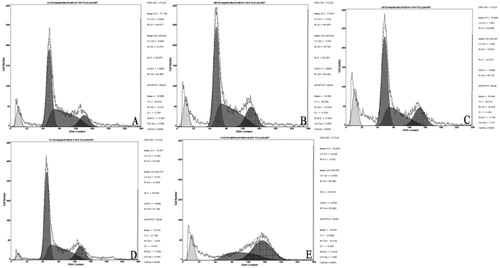
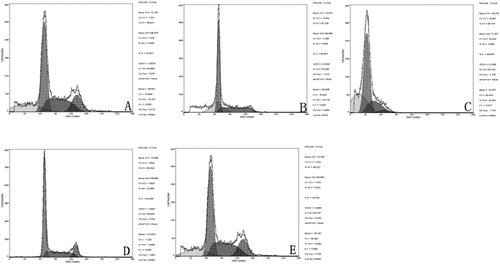
Flow cytometry analysis () showed that, after 48 h of hydroxycamptothecin administration, the cell death rate in the positive control group was (18.53 ± 0.07)%. The cell death rate in the groups with low, medium and high doses of lycorine was (7.77 ± 0.13)%, (10.59 ± 0.26)% and (12.95 ± 0.14)%, respectively. The cell death rate increased with the concentration of lycorine, and it was dose-dependent. There was a significant difference of mortality between the lycorine treated groups and the negative control group (p < 0.01).
Lycorine decreased the total protein, sialic acid and cholesterol content of HepG-2 cell membranes
After 48 h of administration, lycorine reduced the total protein content, sialic acid content, and cholesterol content in the tumor cell membranes (). The decrease was inversely proportional to the applied dose. The total protein content of the tumor cell membranes in each treatment group was significantly different from that of the negative control group (p < 0.01).
Table 2. Effect of Lycorine on cell membrane components of hepatocellular carcinoma HepG-2 cells in vitro ( n = 6).
Lycorine reduced the fluidity and integrity of HepG-2 cell membranes
As shown in after 48 h of administration of lycorine, the fluidity and integrity of the tumor cell membranes decreased. The degree of impairment was negatively correlated with doses (p < 0.01).
Table 3. Effect of lycorine on tumor cell membrane fluidity and integrity ( n = 6).
Lycorine reduced the activity of Na+, K+-ATPase and Ca2+, Mg2+-ATPase in HepG-2 cell membranes
The results are shown in . Lycorine reduced the activity of Na+, K+-ATPase and Ca2+, Mg2+-ATPase in HepG-2 cell membranes. This decrease became more pronounced as the concentration of lycorine increased.
Table 4. Effect of lycorine on tumor cell membrane ATP activity in vitro ( n = 6).
Lycorine exerted antitumor effect in vivo
The results () showed that the growth of H22 mouse tumors was inhibited both in the lycorine group and the positive control group, which could prolong the survival time of H22 mice. This suggests that lycorine exerted significant antitumor effect. The inhibition rates in the low, medium and high dose lycorine groups were 23.36%, 36.50% and 56.93%, respectively. The life prolongation rates in the low, medium and high dose lycorine groups were 29.09%, 50.19% and 69.08%, respectively.
Table 5. Effect of lycorine on tumor growth and length of life of H22 tumor-bearing mice ( n = 20).
Lycorine enhanced the cell death rate of tumor cells in H22 tumor-bearing mice in vivo
After the action of lycorine on H22 tumor-bearing mice, the cell death of tumor cells was detected by flow cytometry (). The results showed that the cell death rate in the positive control group was (14.88 ± 0.09)%. The cell death rates in the groups that received low, medium and high doses of lycorine were (19.24 ± 0.11)%, (24.77 ± 0.06)% and (32.88 ± 0.11)%, respectively. The cell death rate of tumor cells increased with the concentration of lycorine in a dose-dependent manner. The difference between the lycorine groups and the negative control group was statistically significant (p < 0.01).
Lycorine reduced the total protein content, sialic acid content and cholesterol content of tumor cell membranes in vivo
The lycorine administration groups had lower total protein, sialic acid and cholesterol content in the tumor cell membranes of H22 tumor-bearing mice, which were inversely proportional to the dose (). There was a significant difference between the lycorine-administered groups and the negative control group, as well as the lycorine-administered group and the positive control group (p < 0.01).
Table 6. Effect of lycorine on H22 tumor-bearing mice tumor cell membrane composition ( n = 20).
Lycorine decreased the cell membrane fluidity and integrity in H22 tumor-bearing mice
Compared with the normal group, the cell membrane fluidity decreased in each dose group (). Compared with the negative group, the cell membrane fluid–mosaic structure of the lycorine-administered groups and the positive control group was observed. The cell membrane integrity was decreased in each dose group compared with the normal group. Compared with the negative group, the integrity of cell membrane of the lycorine-administered group and the positive control group decreased (p < 0.01).
Table 7. Effect of lycorine on tumor cell membrane fluidity and membrane integrity in H22 tumor-bearing mice ( n = 20).
Lycorine decreased the Na+, K+-ATPase and Ca2+, Mg2+-ATPase activity of tumor cell membranes in vivo
The results are shown in . The lycorine administration groups showed decreased Na+, K+-ATPase and Ca2+, Mg2+-ATPase activities in the tumor cell membranes in a dose-dependent manner. The difference between the drug-administered groups and the negative group was statistically significant (p < 0.01).
Table 8. Lycorine impact on tumor cell membrane ATP activity.
Antitumor mechanism of lycorine on liver cancer cells
Lycorine is a monomeric active ingredient which is widely distributed in Amaryllidaceae and has antitumor, antiviral and sedative pharmacological effects. The antitumor effect of lycorine has become a research hotspot in recent years. It has been reported that lycorine can effectively inhibit the growth of various cells and induce apoptosis [Citation14–20]. The cell biofilm system plays an important regulatory role in the process of cell apoptosis. During the process of cell apoptosis, many apoptosis-related events are completed by the cell biofilm system [Citation21, Citation22]. The main cell structure in this process includes the cell membrane, which plays a very important role in the process of accepting, integrating and amplifying the apoptosis signal.
In this study, we first investigated the antitumor effect of lycorine on H22 tumor-bearing mice by inhibiting tumor growth. It was found that lycorine could effectively inhibit the growth and proliferation of tumor cells in H22 tumor-bearing mice. Through the above experiments, it can be proved that lycorine has an obvious antitumor effect on tumor cells of H22 tumor-bearing mice.
The MTT assay was used to detect the inhibitory effect of lycorine on the proliferation of HepG-2 cell line. The IC50 was calculated to be 5.73 μmol/L. Under an inverted microscope, the cells in the lycorine treated group were rounded and floated, and the cells in the high-dose group were broken. These phenomena indicate that lycorine can induce cell death of human hepatoma HepG-2 cells [Citation23]. Under a fluorescence microscope, the tumor cells in the negative control group were closely arranged, the overall contour was clear and intact, and the nucleus showed uniform fluorescence. The cells in the positive control hydroxycamptothecin group were obviously ruptured and the nucleus was shrunk. The administration group found that the phenomenon of sparse cell growth was more pronounced as the concentration of the drug was increased. Flow cytometry was used to detect the cell death rate of HepG-2 cells treated with lycorine. The cell death rate of each administration group was significantly different from that of the negative control group (p < 0.01). Therefore, it can be explained that lycorine has a cell death-inducing effect on HepG-2 cells.
Compared with the negative control group, the cell membrane protein content of the lycorine-administered group and the positive control group decreased, and there was a significant difference compared with the negative control group. The membrane protein content of the lycorine group was negatively correlated with the dose (p < 0.01). It can be speculated that lycorine reduced the total protein content in the tumor cell membrane of H22 mice, which is the main component of the cell membrane, reducing the physiology of the cell membrane structure, physical and chemical substance binding sites and transportation of intracellular and extracellular environment substances [Citation24]. Compared with the negative control group, the sialic acid content of the cell membrane of the lycorine-administered group and the positive control group decreased (p < 0.01). The sialic acid content of the cell membrane of the lycorine group was negatively correlated with the dose (p < 0.01). It can be speculated that the decrease of sialic acid content in tumor cell membrane may be due to the decrease of total protein content on the membrane of tumor cells, resulting in a decrease in the attachment point of sialic acid glycosylation, thereby affecting the adhesion of sialic acid and reducing the sialic acid content of tumor cell membrane [Citation25].
Compared with the negative control group, the plasma membrane cholesterol levels of the lycorine-administered group and the positive control group decreased. Compared with the negative control group, there was a significant difference. The plasma membrane cholesterol content of the lycorine group was negatively correlated with the dose (p < 0.01). It can be inferred that lycorine can reduce the basic skeleton of the tumor cell membrane by reducing the content of cholesterol on the tumor cell membrane of H22 tumor-bearing mice, thereby affecting the normal physiological function of the tumor cell membrane, exerting an antitumor effect [Citation26].
Membrane lipid fluidity of cell membranes is an important indicator for determining whether or not the cell membrane changes. The membrane lipid fluidity of tumor cells was detected after the detection of lycorine in H22 tumor-bearing mice. The experimental results showed that compared with the normal group, the cell membrane fluidity decreased in each dose group. Compared with the negative control group, the cell membrane fluidity of the lycorine-administered group and the positive control group decreased, compared with the negative control group. There was a significant difference of cell membrane fluidity between the lycorine group and control group, which was negatively correlated with the dose (p < 0.01). It can be speculated that lycorine can reduce the fluidity of the tumor cell membrane, affecting the tumor cells to exert normal physiological functions, and thus achieve the purpose of antitumor effect [Citation10, Citation27].
The normal cell membrane has a self-repairing ability in the isotonic solution to form a closed shadow bubble. This ability can restore the barrier effect of the cell membrane on the cation absorption and macromolecular operation in the extracellular environment of the cell membrane. After detecting the inhibitory effect of lycorine on H22 tumor-bearing mice, it was found that the cell membrane integrity of each dose group was lower than that of the normal group. The cell membrane integrity of the drug-administered group and the positive control group decreased, and there was a significant difference compared with the negative control group. The cell membrane integrity of the lycorine group was negatively correlated with the dose (p < 0.01). It has been verified by the previous experimental results that the sialic acid content on the tumor cell membrane was reduced, and the integrity of the tumor cell membrane was indeed regulated by the sialic acid content on the tumor cell membrane [Citation28].
The occurrence and development of tumors are related to the activity of sodium ion channels. The increase of sodium pump activity is one of the mechanisms of cell carcinogenesis. When the balance of sodium ion concentration is destroyed in the body tissues, the concentration of sodium ions inside and outside the cells will change. The imbalance between the internal and external environment of the cell directly leads to the metabolism of the cell, and the proliferation of the cell is out of control. The study found that the main reason for the increase of sodium pump activity is: increased intracellular sodium concentration in cancerous cells, which stimulates the surface of the cell membrane sodium pump to enhance its activity. It was found that all doses of lycorine could reduce Ca2+ on tumor cell membrane. The activities of Mg2+-ATPase and Na+, K+-ATPase decreased the cation channel activity of tumor cells, and destroyed the cation balance in tumor cells, and the ATPase activity was negatively correlated with the dose (p < 0.01) [Citation29].
Conclusions
The obtained results indicated that lycorine has an antitumor effect on H22 tumor-bearing mice and HepG2 cells. Lycorine could reduce the content of the main components of tumor cell membranes, including reduce the total membrane protein content, tumor cell membrane cholesterol content and tumor cell membrane sialic acid content. We suggest that the Chinese medicine lycorine can change the structure of the tumor cells, disrupt the structural integrity of the tumor cell membranes and interfere with the tumor cell growth. Normal physiological function inhibits tumor cell growth and division. This suggested mechanism of action of lycorine as an antitumor drug from the perspective of membrane pharmacology needs to be verified in subsequent experiments.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Tasker SZ, Cowfer AE, Hergenrother PJ. Preparation of structurally diverse compounds from the natural product lycorine. Org Lett. 2018;20(18):5894–5898.
- Roy M, Liang L, Xiao X, et al. Lycorine: a prospective natural lead for anticancer drug discovery. Biomed Pharmacother. 2018;107:615–624.
- Hu Z, Wang Z, Liu Y, et al. Leveraging botanical resources for crop protection: the isolation, bioactivity and structure–activity relationships of lycoris alkaloids. Pest Manage Sci. 2018;74(12):2783–2792.
- Jiang QQ, Liu WB. Lycorine inhibits melanoma A375 cell growth and metastasis through the inactivation of the PI3K/AKT signaling pathway. Med Sci (Paris). 2018;34(F1):33–38.
- Ji Y, Yu M, Qi Z, et al. Study on apoptosis effect of human breast cancer cell MCF-7 induced by lycorine hydrochloride via death receptor pathway. Saudi Pharm J. 2017;25(4):633–637.
- Zeng H, Fu R, Yan L, et al. Lycorine induces apoptosis of A549 cells via AMPK-mammalian target of rapamycin (mTOR)-S6K signaling pathway. Med Sci Monit. 2017;23:2035–2041.
- Zhang W, Cui EH. Study on effect of lycorine in inducing apoptosis of pulmonary carcinoma cell A549. Zhongguo Zhong Yao Za Zhi. 2015;40(16):3278–3282.
- Li X, Xu P, Wang C, et al. Synergistic effects of the immune checkpoint inhibitor CTLA-4 combined with the growth inhibitor lycorine in a mouse model of renal cell carcinoma. Oncotarget. 2017;8(13):21177–21186.
- Cao Z, Yu D, Fu S, et al. Lycorine hydrochloride selectively inhibits human ovarian cancer cell proliferation and tumor neovascularization with very low toxicity. Toxicol Lett. 2013;218(2):174–185.
- Li L, Dai HJ, Ye M, et al. Lycorine induces cell-cycle arrest in the G0/G1 phase in K562 cells via HDAC inhibition. Cancer Cell Int. 2012;12(1):49.
- Liu J, Hu JL, Shi BW, et al. Up-regulation of p21 and TNF-alpha is mediated in lycorine-induced death of HL-60 cells. Cancer Cell Int. 2010;10:25.
- Ji CF, Ji YB. Effect of asparagus polysaccharide on erythrocyte membrane components and fluidity in S180 mice. Tianjin Tradit Chin Med. 2009;26(06):479–482.
- Ji YB, Ji CF, Ma HT. Effects of ethanol extracts from qinglongyi on the biochemical function of tumor cell membrane in H22 mice. Chin J Tradit Chin Med. 2005;30(07):531–534.
- Ji YB, Wang SH, Gao SY, et al. Effects of anemone on membrane fluidity and membrane protein level in H22 tumor-bearing mice. J Chin Herb Med. 2005;0(02):239–241.
- Ji YB, Wang SH, Gao SY, et al. Effect of anemone on sialic acid and occlusion of cell membrane of H_(22) tumor-bearing mice. J Chin Herb Med. 2005;(01):79–81.
- Ji YB, Ji CF. Effect of astragalus polysaccharide on erythrocyte immune function in tumor model mice. J Mod Food Sci Technol. 2013;29(09):2042–2046, 2052.
- Song GL, Wang JB, Du QB, et al. Study on changes of S180 sarcoma cell membrane characteristics by auricularia auriculata polysaccharide. Chin J Pharm. 2012;47(04):255–261.
- Hu QL, Feng ZY. Effect of polysaccharides from cactus on lipid fluidity of tumor cell membrane. Mod Chin. Tradit Med. 2006;(11):17–19.
- Ji YB, Ma HT. Effect of qinglongyi extract on cell membrane fluidity in tumor-bearing mice. J Chin Herb Med. 2004;(12):70–73.
- Ji YB, Gao SY, Kong Q. Effect of seaweed polysaccharide on the fluidity of tumor cell membrane. J Chin Herb Med. 2002;(05):54–56.
- Ji YB, Chen HJ, Ji CF. Effect of qinglongyi polysaccharide on ATPase activity of H22 tumor cells. J Drug Eval. 2006;(06):412–415.
- Ji YB, Gao SY, Wang HL, et al. Effect of total alkaloid of nightingale on the activity of sodium pump and calcium pump of tumor cell membrane was studied. World J Sci. Technol. 2006;(04):40–43.
- Wang ZJ, Qiao P, Fan HK, et al. Effect of pyrimidine on sodium pump activity of S180, EAC and H22tumor cell membrane in mice. Chin J Pract Neurol Dis. 2006;0(02):17–18.
- Evdokimov NM, Lamoral-Theys D, Mathieu V, et al. In search of a cytostatic agent derived from the alkaloid lycorine: synthesis and growth inhibitory properties of lycorine derivatives. Bioorg Med Chem. 2011;19(23):7252–7261.
- Chen S, Jin G, Huang K-M, et al. Lycorine suppresses RANKL-induced osteoclastogenesis in vitro and prevents ovariectomy-induced osteoporosis and titanium particle-induced osteolysis in vivo. Sci Rep. 2015;5:12853.
- Roy M, Liang L, Xiao X, et al. Lycorine downregulates HMGB1 to inhibit autophagy and enhances bortezomib activity in multiple myeloma. Theranostics. 2016;6(12):2209–2224.
- Ilavenil S, Kaleeswaran B, Sumitha P, et al. Protection of human erythrocyte using Crinumasiaticum extract and lycorine from oxidative damage induced by 2-amidinopropane. Saudi J Biol Sci. 2011;18(2):181–187.
- Wang Y-H, Wan Q-L, Gu C-D, et al. Synthesis and biological evaluation of lycorine derivatives as dual inhibitors of human acetylcholinesterase and butyrylcholinesterase. Chem Cent J. 2012; 6:96.
- Misaghian N, Ligresti G, Steelman LS, et al. Targetting the leukemic stem cell: the Holy Grail of leukemia therapy. Leukemia. 2009;23(l):25–42.