?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study investigated the role of ErbB4, the schizophrenia susceptibility gene, in synaptogenesis and synaptic transmission and its association with the pathogenesis of schizophrenia. We found that there was no significant difference in the size of inhibitory synapsin Gephyrin clusters in the dendrites of gamma-aminobutyric acid (GABA) neurons in the cultured cortex of ErbB4 knockout (ErbB4−/−) mice and ErbB4+/+ mice. The density and area of PSD-95 clusters in GABA-labelled neurons were not significantly different from those in the control group (p > 0.05). Specific pIkBα labelling of the pyramidal neurons and the axon initiation segments showed that the number of neuronal dendrites and the inhibitory postsynaptic proteins Gephyrin in the ErbB4 knockout group were significantly lower than those in the control group, whereas the size of the Gephyrin cluster remained unchanged. The number and area of the VGAT clusters on the somaclonal and axonal surfaces of the pyramidal neurons were also similar. ErbB4 knockout influenced excitatory synapses in intermediate neuronal cells and inhibitory synapses in pyramidal neurons, however, did not affect the inhibitory synapses in intermediate neuronal cells and excitatory synapses in pyramidal neurons. The number and area of PSD-95 clusters on the neuronal surface were significantly lower than those of the 1NMPP1 group, but the number and area of Gephyrin clusters on the neuronal surface were not changed (p > 0.05). These findings demonstrated that ErbB4 gene had no effect on the inhibitory synapses in interneurons and the excitatory synapses in pyramidal neurons.
Introduction
Schizophrenia is a complex disease [Citation1, Citation2] which is caused by disorder of the central nervous system function required to maintain cognitive function and normal social behaviour. The disease can affect perception, thinking, emotion, behaviour and mental activity. If the internal and external environments are not harmonious, and this persists for some time, it is an important feature of the patient’s own lack of cognitive abnormality [Citation3]. Although the most significant clinical aspects of the disease typically only become apparent in late adolescence or early adulthood, much research suggests that schizophrenia is a neurodevelopmental disorder with a strong genetic component [Citation4–6]. Several independent studies have identified that neurotrophin 1 (NRG-1) and its receptor epidermal growth factor 4 (ErbB4) are important susceptibility genes for schizophrenia, however, their exact role in the pathogenesis of schizophrenia remains unknown [Citation7–9]. Studies have shown that NRG-1 and ErbB4 signalling pathways regulate the expansion of inhibitory electrical activity in the mammalian cerebral cortex by autonomously regulating the connectivity of specific gamma-aminobutyric acid (GABA) interneurons [Citation10]. Other studies have suggested different theories for this. For example, ErbB4 expression in the neocortex and hippocampus of mice is mainly confined to certain types of intermediate neurons. ErbB4, in particular, is expressed by many albumin-shaped and basket-like cells. These cells are primarily located at the end of the axon, and posterior synaptic density was regulated by glutamate input [Citation11]. Previous studies have demonstrated that ErbB4 can autonomously promote the formation of synapses in axonal axons of pyramidal cells, and this function may be mediated by NRG1. Furthermore, it has been reported that the expression of ErbB4 in GABA-containing intermediate neurons would regulate the formation of excitatory synapses on the dendrites of these cells [Citation8]. In conclusion, NRG1 and ErbB4 signalling are necessary for postnatal cortical GABA-mediated loops, providing a new perspective for the involvement of these genes in the aetiology of schizophrenia. Further, the NRG-1/ErbB4 signalling pathway can regulate the transmission and differentiation of cerebral cortical neurons and the synaptic integration process to achieve information transmission in the cerebral cortex and complete the brain’s advanced functional activities. However, how to regulate the NRG1/ErbB4 signalling pathway and the underlying mechanism of the development of various neurological loops in the cerebral cortex remain unknown [Citation7, Citation9, Citation12]. It is generally believed that full-length NRG1 is cleaved to form an active NRG-1 ligand that binds to its receptor, ErbB4, thereby activating ErbB4 tyrosine kinase, which in turn affects its ability to act on synaptic transmission and neurons through downstream signalling pathways in neuron phylogenetic regulation [Citation13]. In this study, ErbB4 is used as a target to explore how the schizophrenia susceptibility gene ErbB4 plays a role in synaptic development and prominent transmission, as well as further elucidate its function in schizophrenia and genetic abnormalities.
Materials and methods
Experimental animals
ErbB4−/− mice were selected as the experimental group, and ErbB4+/+ mice (C57BL/6N) were selected as the control group. Mice lacking the ErbB4 gene result in midembryonic lethality because of defective heart development. To examine later phenotypes, we rescued the heart defects in ErbB4 mutant mice by expressing ErbB4 under a cardiac-specific myosin promoter to ensure the survival of mice (HR ErbB4−/− mice). ErbB4-reporter mouse (C57BL/6N), i.e. ErbB4::Cre ERT2, Rosa::LSL-td Tomato mouse, belonging to ‘Cre-er tool mouse’, was used for labelling of ErbB4+/+ cells.
T796G-ErbB4 mice have an altered base in the mouse gene–the base of the 796th locus of ErbB4 was converted from T (threonine) to G (glycine)–thereby obtaining a specific phosphate point. The mice were obtained from Georgia Regents University (GA, USA).
Ethics statement
All animal experiments in this study were conducted in accordance with the requirements of the Guidelines for the Administration and Use of Laboratory Animals, revised by the American Academy of Experimental Animal Institutes in 2012. During the study, we minimized both the number of animals used and their pain.
Laboratory equipment, pharmaceuticals and reagents
Laboratory equipment
The water bath was purchased from Preresto Leituo (USA), the polymerase chain reaction thermocycler was purchased Bio-Rad (USA), and the nucleic acid electrophoresis instrument was purchased from Thermo Fisher Scientific (USA). Protein electrophoresis instrumentation was purchased from Bio-Rad (USA); the Duc-640 spectrophotometer, from Beckman, Inc. (USA); the microplate reader, from Bio-Rad (USA) and a Teflon homogenizer, from Wheaton Corporation, USA. A desktop operating microscope was purchased from Nikon (USA); the cell incubator was from Thermo Fisher Scientific; the ultra-pure water meter, from Millipore (USA) and the refrigerator, from Thermo Fisher Scientific. A Zeiss LSM51 laser total focused microscope was purchased from Zeiss (Germany).
Pharmaceuticals
AG1487 (article 658552), PD158780 (No. 513035) and 1NMPP1 (item 529581) were purchased from Calbiochem (Germany).
Reagents
The following reagents were prepared: 4% paraformaldehyde solution, 0.2 mol/L phosphate buffered solution, 0.01 mol/L phosphate buffered saline (PBS) solution, as well as the reagents for cell culture, cell transfection, immunoblotting, immunization, chemical solutions and plasmid amplification. The Cyt2-ErbB4-GFP and Cyt2-T796G-GFP plasmids were constructed and validated.
Identification of mouse genotypes
For extraction of mouse genomic deoxyribonucleic acid (DNA), 3 mm of rat tail tissue was used, boiled and centrifuged to collect the supernatant.
The mice genotypes were examined using primers targeting HR ErbB4−/− mice, ErbB4-reporter mice and T796G-ErbB4 mice. The amplified products were detected by electrophoresis of 2% agarose gels (1:10,000).
In vitro culture of mouse cerebral cortical neurons
After the rats were anesthetized, the foetuses were extracted under sterile conditions. The rat foetuses were then cultured, and DNA was extracted and labelled with the mouse genotype. The complete brain was taken from foetal rats, and the cerebral cortex was separated and cut under the optical microscope. After washing with 1× HBSS buffer, 2.5% Trypsin (Corning Cellgro; Cat. No. 25054049) was added to the incubator at 37 °C for digestion. Foetal bovine serum was added, and the supernatant was centrifuged. After washing, the cells were counted. At 4–6 h after inoculation, cells adhered to the dish and were cultured in a serum-free medium for 24 h. Cytarabine was added to inhibit glial cell proliferation.
Culture of the HEK293 cell line
The HEK293 cell line [obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA)] was resuspended and incubated at 37 °C. When the cell density reached 80% or above, the HEK293 cell line was subcultured.
Plasmid amplification, extraction and transfection
The competent cells and the plasmid to be transferred were placed on ice for 15 min and then heat shocked for 60 to 90 s. Next, the cells were incubated on ice for 2 min. After Luria–Bertani medium was added, the cells were incubated for 30–45 min and then added to media containing the appropriate antibiotic for selection, cultured at 37 °C overnight and plasmid extraction was performed. HEK293 cells and neuronal cells were transfected.
Immunocytochemistry
The cells were inoculated onto coverslips, and the corresponding drug added. To examine the inhibitory effect of ErbB4 kinase activity on inhibitory synapses of intermediate neurons, the 1NMPP1 was added. Briefly, T796G-ErbB4 mouse embryonic cortical neurons were cultured for 8 days in vitro (DIV8), adding 1NMPP1 to ErbB4 kinase phosphorylation of specific inhibitor. The control group, in which dimethyl sulfoxide was used, was cultured for 17 days (DIV17); after which, neurons were fixed and stained. The samples were then washed rapidly with PBS before treatment with 4% paraformaldehyde for 15 min and incubated with sheep serum for 60 min. Followed by the addition of primary antibody, secondary antibody was added in 0.01 mol/L PBS solution after washing, sealing treatment and incubation at 4 °C under dark preservation. The images were collected by laser confocal microscopy, processed and analysed by ImageJ software (National Institutes of Health, Bethesda, MD).
Western blotting
The cells were pre-inoculated onto Petri dishes and washed with ice-cold PBS solution. Cells were added to cell lysate solution, incubated for 15 min on ice and blended for 60 min. The samples were then centrifuged and temporarily stored at −70 °C.
Mice were sacrificed by cervical dislocation after isoflurane anaesthesia (Vetflurane), and the brain was removed and cut into pieces. Lysis solution was added to the proteins, Teflon homogenizer applied and sufficiently polished after proteolytic cleavage. Then, the samples were centrifuged and protein concentration was measured by BCA Protein Assay Kit (Thermo Scientific™) using a standard curve. The concentration of the sample was taken as the mean protein sample concentration.
Statistical analysis
Data were analysed and processed with SPSS version 20.0 statistical software (IBM, USA). The data are expressed as mean values with standard deviation (.) Statistical differences between the results were determined using the t test; p < 0.05 was defined as statistically significant.
Results and discussion
Identification of gene knockout mice
Except in the heart, the ErbB4 gene was knocked out in HR ErbB4−/− mice. The length of the amplified fragment was about 150 bp for the ErbB4+/+ genotype and about 320 bp for the ErbB4−/− genotype. Heterozygous ErbB4+/+ had an amplified fragment that was 2A strip. Subsequently, knockout mice in the same litter and ErbB4+/+ mice were selected, which were used as the experimental and control groups, respectively.
Effects of ErbB4−/− on GABA neuronal inhibitory synaptic development
There was no significant difference in the number of inhibitory synapsin Gephyrin clusters in the dendrites of GABA neurons in the cultured cortex of ErbB4−/− and ErbB4+/+ mice The results were 0.12 ± 0.02 μm−1 and 0.21 ± 0.01 μm−1 for the experimental and the control groups, respectively; in addition, the area of the Gephyrin cluster was 0.20 ± 0.02 μm2 and 0.19 ± 0.02 μm2, respectively. The results showed that ErbB4−/− did not affect the occurrence or development of GABA neuron inhibitory synapses in neurons, given that the difference was not statistically significant (p > 0.05; , ).
Figure 1. Effect of ErbB4–/– on the area of Gephyrin in inhibitory synapses (A) and on the number of Gephyrin in inhibitory synapses (B).
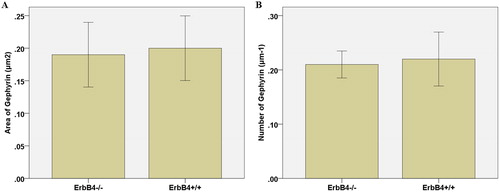
Table 1. Effect of ErbB4−/− on GABA neuronal inhibitory synaptic development.
Effects of ErbB4−/− on the occurrence and development of excitatory synapses in pyramidal neurons
By studying the effect of ErbB4 knockout on the synaptic development of non-GABA neurons, we found that the density and area of PSD-95 clusters in GABA-labelled neurons were not significantly different from those in the control group (p < 0.05; , ). Using pIkBα to specifically label the neurons of the pyramidal neurons and the axon initiation segments, we found that the neuronal dendrites and the inhibitory postsynaptic proteins in the ErbB4 knockout group were reduced by 45% ± 6% and 41% ± 5% in the initial segment of the axons, but the size of the Gephyrin cluster was not significantly changed (p > 0.05; , ). The results showed that ErbB4 knockout influenced dendrites and axons during synaptic development but did not impact the maturation process of synapses. By analysing the number and area of VGAT clusters on the somaclonal and axonal surfaces of the pyramidal neurons, we were able to determine its effect on maturation. The VGAT cluster belongs to the presynaptic protein and can co-label the soma and axon of the pyramidal neurons. VGAT is expressed in GABAergic and glycinergic neurons and is responsible for vesicular storage and subsequent exocytosis of these inhibitory amino acids. While the number of VGAT clusters was significantly reduced, the area of VGAT clusters did not change significantly (p > 0.05; and , ). In summary, it can be concluded that ErbB4 deletion, although leading to a decrease in the number of inhibitory synapses on pyramidal neurons, has no effect on excitatory synapses in pyramidal neurons.
Figure 2. Effect of ErbB4 knockout. Effect of ErbB4 knockout: on the number (A) and size (B) of PSD-95 clusters in GABA-positive neurons; on the number (C) and size (D) of Gephyrin clusters in the dendrites and axons of pyramidal neurons; on the number of VGAT clusters in the soma and axons of pyramidal neurons (E) and on the VGAT cluster area of somaclonal neurons and axons of pyramidal neurons.
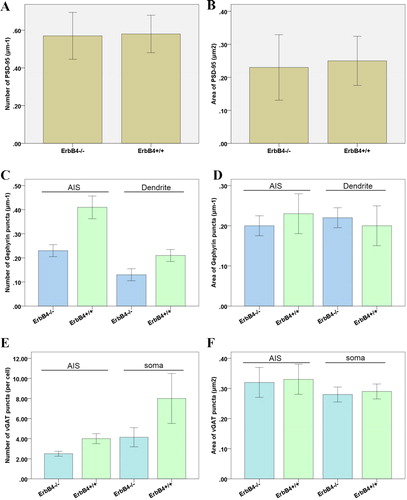
Table 2. The role of ErbB4−/− in the development of excitatory synapses in GABA-labelled neurons.
Table 3. Effect of ErbB4−/− on the number of Gephyrin clusters in dendrites and axons of pyramidal neurons.
Table 4. Effect of ErbB4−/− on Gephyrin cluster size in the dendrites and axons of pyramidal neurons.
Table 5. Effect of ErbB4−/− on the number of VGAT clusters in somaclonal and axons of pyramidal neurons.
Table 6. Effect of ErbB4−/− on VGAT cluster area of pyramidal neurons in soma and axons.
Inhibitory effect of ErbB4 kinase activity on inhibitory synapses of intermediate neurons
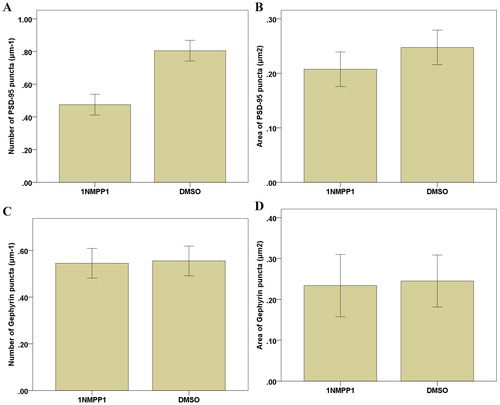
The cerebral cortical neurons of ErbB4−/− mice and ErbB4 reporter mice were cultured in vitro. The results showed that ErbB4 gene knockout influenced excitatory synaptic interneurons, pyramidal neuron inhibitory synaptic occurrence and development. However, ErbB4 knockout did not affect inhibitory synaptic interneurons or pyramidal neuron excitability synapse. The effect of ErbB4 inhibitor AG1487 and PD158780 kinase activity was examined after treatment; both inhibitory and excitatory synaptic intermediate cells and pyramidal neurons were affected. Compared to the control group, we found that addition of 1NMPP1 to the experimental group resulted in a significantly reduced PSD-95 cluster number and area by 25% ± 3% and 21% ± 3%, respectively (), suggesting that ErbB4 kinase activity was specifically blocked and the inhibition of intermediate neuron excitatory synapse formation and development. However, the number and area of Gephyrin clusters on the surface of interneurons were not changed (p > 0.05; ). This indicated that the specific inhibition of kinase activity in ErbB4 did not inhibit the occurrence or development of inhibitory synapses in interneurons. These results suggested that ErbB4 kinase activity was involved in the occurrence and development of excitatory synapses in interneurons, but not in the regulation or development of inhibitory synapses in interneurons.
Synaptic PSD and gephyrin protein level in cultured pyramidal neurons in vitro
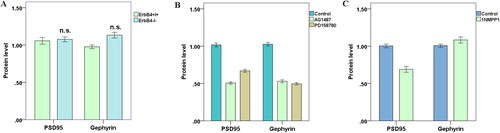
Western blotting was used to detect the protein levels of synaptic PSD and Gephyrin in the control and experimental groups of mouse pyramidal neurons in vitro. showed that there was no significant change in the levels of excitatory postsynaptic protein PSD95 or inhibitory postsynaptic protein Gephyrin in the ErbB4−/− group compared to the control group. This indicated that although ErbB4 regulated the development of neuronal synapses, the expression level of synapse proteins in neurons did not change significantly. Following AG1478 and PD158780 treatment, we found that the levels of PSD and Gephyrin proteins in neurons declined sharply (), which coincided with the previous observation that these two inhibitors could widely inhibit the development of different types of synapses between neurons. After addition of 1NMPP1 to T796G-ErbB4 murine cortical neurons to specifically inhibit ErbB4 kinase activity, we found that PSD95 protein levels decreased while the amount of Gephyrin protein did not change significantly (), further demonstrating that ErbB4 kinase activity only regulated excitatory synapses in interneurons and did not affect the development of inhibitory synapses between neurons.
Figure 4. Synaptic PSD and Gephyrin protein levels in pyramidal neurons cultured in vitro. (A) In the ErbB4–/– group, there was no significant change in PSD95 and Gephyrin protein levels (p > 0.05); (B) AG1487 and PD158780 downregulated neuronal PSD95 and Gephyrin protein levels (p < 0.05); (C) After 1NMPP1 treatment, PSD95 protein levels decreased (p < 0.05), while Gephyrin protein levels did not change (p > 0.05).
Neuronal cells rely on excitatory synapses and inhibitory synapses to contact and transmit signals and ultimately form the brain’s neural loop [Citation14]. This study explored the effect of ErbB4, a susceptible gene of schizophrenia, on the excitability and inhibitory synaptogenesis and development of neurons in the cerebral cortex. Additionally, this study revealed how ErbB4 tyrosine kinase in ErbB4 was involved in the synaptogenesis and development of neuronal cells through the study of ErbB4 kinase mutant model mice (T796G-ErbB4) for the first time at the synaptic level.
Recent studies [Citation15, Citation16] have shown that more than 99% of ErbB4+/+ cells in mouse cerebral cortical neurons are GABA neurons, and ErbB4 is generally not expressed in excitatory neurons. In this study, ErbB4 was found to be involved in the regulation of the development of excitatory synapses in middle neurons. It was further demonstrated that ErbB4 did not participate in the regulation of excitatory synapses of pyramidal neurons. There was no significant change in the number or area of excitatory synapses in non-GABA intermediate neurons of ErbB4−/− in vitro. This was consistent with the results of in vivo experiments after knocking out the ErbB4 gene in Cre-ErbB4f/f mice [Citation17]. After deletion of the ErbB4 gene in the cerebral cortex, the excitatory presynaptic protein GluA1 and the number of clusters were reduced. Some researchers added NRG1 to promote the occurrence and development of excitatory synapses in the middle neurons of GABA neurons cultured in vitro, revealing that this effect was due to the combination of ErbB4-positive intracellular domain proteins with PSD-95 clusters in the middle neurons. Moreover, it suggested that the binding may be related to the tyrosine kinase activity of ErbB4 [Citation18, Citation19].
There are few studies about the relationship between ErbB4 and GABA interneurons in synaptogenesis and development, and the results are controversial [Citation20, Citation21]. NRG1 was found to have no effect on the density or area of inhibitory postsynaptic protein (Gephyrin) in GABA neurons in hippocampal neurons in vitro [Citation22], whereas knockout of the ErbB4 gene reduced GAD65-positive neurons. The inhibitory effect of the presynaptic protein VGAT cluster was detected on the fluorescence intensity, but it did not change the number of VGAT clusters [Citation23]. One study showed that the ErbB4 gene did not participate in the development or progression of inhibitory synapses in the cerebral cortex of basilar neurons [Citation24]. In this study, we found that the density and size of Gephyrin clusters in GABA intermediate neurons of HR and ErbB4−/− mice in vitro were significantly changed. Although ErbB4+/+ cells in the neurons of the cerebral cortex are intermediate neurons, only 2/3 of the GABA interneurons belong to ErbB4-positive cells. The use of the specific ErbB4 inhibitor 1NMPP1 in T796G-ErbB4 mice did not result in development of inhibitory synapses in GABA-positive neurons. In summary, ErbB4 tyrosine kinase activity does not affect the occurrence or development of synapses in GABA intermediate neuronal cells.
Vascular cortical PV-positive cells typically include two types of light-shaped cells and basket-like cells. Light-shaped cells are usually distributed in the anatomical neurons of the excitatory neurons, forming axis-axis synapses, while the basket-like cells are usually distributed in the excitatory neurons and dendrites, forming axis-tree synapses.
In this study, we found that the number of Gephyrin clusters in the dendritic neurons of the pyramidal neurons and the number of Gephyrin clusters in the intermediate neurons were reduced in the cultured ErbB4−/− neurons, and the same was true in the covens of VGAT. This indicated that ErbB4 regulated the occurrence and development of inhibitory synapses in pyramidal neuronal cells. A recent study reported that the synaptogenesis and development of ErbB4−/− hippocampus basket cells did not affect synaptogenesis or development in mice [Citation17].
At the same time, to determine whether ErbB4 tyrosine kinase activity affects the occurrence or development of synaptogenesis of pyramidal cells, this study examined the specific inhibition of ErbB4 kinase activity by 1NMPP1 in cultured T796G-ErbB4 neurons in vitro. It was found that there was no effect on the density or area of Gephyrin clusters in neuronal axons and dendrites, and the expression of Gephyrin proteins in cultured neurons in vitro was not changed.
Conclusions
In summary, this study explored how the schizophrenia susceptibility gene ErbB4 played a role in synaptic development and prominent delivery, as well as its association with schizophrenia and genetic abnormalities. The results showed that the ErbB4 gene had no effect on the occurrence or development of inhibitory synapses in middle neurons and similarly had no effect on the occurrence or development of excitatory synapses in pyramidal neurons.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Fromer M, Roussos P, Sieberts SK, et al. Gene expression elucidates functional impact of polygenic risk for schizophrenia. Nat Neurosci. 2016;19(11):1442–1453.
- Kotzadimitriou D, Nissen W, Paizs M, et al. Neuregulin 1 type I overexpression is associated with reduced NMDA receptor–mediated synaptic signaling in hippocampal interneurons expressing PV or CCK. eNeuro. 2018;5(2):ENEURO.0418-17.2018.
- Suazo V, Lubeiro A, Jurado-Barba R, et al. Elevated midline-parietal gamma band noise power in schizophrenia but not in bipolar patients. Eur Arch Psychiatry Clin Neurosci. 2016;266(8):743–753.
- Harrison PJ. Recent genetic findings in schizophrenia and their therapeutic relevance. J Psychopharmacol. 2015;29(2):85–96.
- Wang C, Aleksic B, Ozaki N. Glia-related genes and their contribution to schizophrenia. Psychiatry Clin Neurosci. 2015;69(8):448–461.
- Tosato S, Zanoni M, Bonetto C, et al. No association between NRG1 and ErbB4 genes and psychopathological symptoms of schizophrenia. Neuromol Med. 2014;16(4):742–751.
- Agim ZS, Esendal M, Briollais L, et al. Discovery, validation and characterization of Erbb4 and Nrg1 haplotypes using data from three genome-wide association studies of schizophrenia. PLoS One. 2013;8(1):e53042.
- Sun Y, Ikrar T, Davis MF, et al. Neuregulin-1/ErbB4 signaling regulates visual cortical plasticity. Neuron. 2016;92(1):160–173.
- Perez-Garcia CG. ErbB4 in laminated brain structures: a neurodevelopmental approach to schizophrenia. Front Cell Neurosci. 2015;9:472.
- Sun Z, Jiang T, Wu Y, et al. Low field magnetic stimulation ameliorates schizophrenia-like behavior and up-regulates neuregulin-1 expression in a mouse model of cuprizone-induced demyelination. Front Psychiatry. 2018;9:675.
- Law AJ. NRG1/ErbB4 and the PI3K/AKT pathway: novel therapeutic options for schizophrenia. Schizophr Res. 2014;160(1–3):e10–e11.
- Yoshimi A, Suda A, Hayano F, et al. Effects of NRG1 genotypes on orbitofrontal sulcogyral patterns in Japanese patients diagnosed with schizophrenia. Psychiatry Clin Neurosci. 2016;70(7):261–268.
- Tian J, Geng F, Gao F, et al. Down-regulation of Neuregulin1/ErbB4 signaling in the hippocampus is critical for learning and memory. Mol Neurobiol. 2017;54(6):3976–3987.
- Zhang C, Ni P, Liu Y, et al. GABAergic abnormalities associated with sensorimotor cortico-striatal community structural deficits in ErbB4 knockout mice and first-episode treatment-naïve patients with schizophrenia. Neurosci Bull. 2019;36(2):97–109.
- Jones KA, Menniti FS, Sivarao DV. Translational psychiatry–light at the end of the tunnel. Ann NY Acad Sci. 2015;1344(1):1–11.
- Hou XJ, Ni KM, Yang JM, et al. Neuregulin 1/ErbB4 enhances synchronized oscillations of prefrontal cortex neurons via inhibitory synapses. Neuroscience. 2014;261:107–117.
- Liu S, Huang S, Chen F, et al. Genomic analyses from non-invasive prenatal testing reveal genetic associations, patterns of viral infections, and Chinese population History. Cell. 2018;175(2):347–359.
- Yang JM, Shen CJ, Chen XJ, et al. Erbb4 deficits in chandelier cells of the medial prefrontal cortex confer cognitive dysfunctions: implications for schizophrenia. Cereb Cortex. 2019;29(10):4334–4346.
- Wang H, Liu F, Chen W, et al. Genetic recovery of ErbB4 in adulthood partially restores brain functions in null mice. Proc Natl Acad Sci USA. 2018;115(51):13105–13110.
- Wang F, Wang H, Liu X, et al. Pharmacological postconditioning with Neuregulin-1 mimics the cardioprotective effects of ischaemic postconditioning via ErbB4-dependent activation of reperfusion injury salvage kinase pathway. Mol Med. 2018;24(1):39.
- Cho Y, Ryu S, Huh I, et al. Effects of genetic variations in NRG1 on cognitive domains in patients with schizophrenia and healthy individuals. Psychiatr Genet. 2015;25(4):147–154.
- Moran PM, O'Tuathaigh CMP, Papaleo F, et al. Dopaminergic function in relation to genes associated with risk for schizophrenia: translational mutant mouse models. Prog Brain Res. 2014;211:79–112.
- Iannitelli A, Quartini A, Tirassa P, et al. Schizophrenia and neurogenesis: a stem cell approach. Neurosci Biobehav Rev. 2017;80:414–442.
- Yang JM, Zhang J, Chen XJ, et al. Development of GABA circuitry of fast-spiking basket interneurons in the medial prefrontal cortex of erbb4-mutant mice. J Neurosci. 2013;33(50):19724–19733.