Abstract
Vitiligo is caused by the disappearance of melanocyte function in the skin, but the mechanism has not been fully elucidated. This study aimed to investigate the expression and role of miR-217 in dysfunctional human primary melanocytes caused by oxidative stress, so as to explore its potential role in vitiligo. Hydrogen peroxide (H2O2) was used to induce the oxidative damage of melanocytes. The levels of sirtuin 1 (SIRT1) and miR-217 were measured by quantitative reverse transcription-polymerase chain reaction (qRT-PCR) and or Western blot assay. TargetScan and dual luciferase reporter gene assay were used to confirm the relationship between SIRT1 and miR-217. Cell viability and cell apoptosis were analysed by 3-(4,5-dimethyl-2-thiazolyl)−2,5-diphenyl-2-H-tetrazolium bromide (MTT) assay and flow cytometry assay, respectively. Reactive oxygen species (ROS) production, superoxide dismutase (SOD) and catalase (CAT) activities were determined using specific assay kits. SIRT1 was down-regulated, while miR-217 was up-regulated in H2O2-induced human primary melanocytes. SIRT1 was a target gene of miR-217. Inhibition of miR-217 increased cell viability and inhibited cell apoptosis in H2O2-treated melanocytes. Besides, inhibition of miR-217 enhanced the antioxidant activity of SOD and CAT and reduced the accumulation of intracellular ROS. Notably, all these effects were reversed by SIRT1-siRNA. The data indicated that SIRT1 was down-regulated, while miR-217 was up-regulated in dysfunctional human primary melanocytes caused by oxidative stress, and miR-217 inhibition relieved oxidative stress-induced melanocyte damage via targeting SIRT1, indicating that miR-217 might a potential target for vitiligo treatment.
Introduction
Vitiligo is a pigmentation disorder in which the loss of epidermal melanocytes is the main pathological feature [Citation1]. Patients with vitiligo often suffer from mental illness, and vitiligo seriously affects the quality of life of patients [Citation2]. Vitiligo has complex autoimmunity [Citation3], cytotoxicity metabolite [Citation4], and neural and genetic predisposition [Citation5,Citation6] cause, but the precise mechanisms underlying vitiligo pathogenesis has remained elusive.
Oxidative stress plays a crucial role in the entire pathological process of vitiligo [Citation7,Citation8]. According to the pro-oxidative state produced during melanin synthesis, melanocytes are particularly susceptible to oxidative stress [Citation9]. When intracellular metabolic disorder and intrinsic antioxidant defences impaired, hydrogen peroxide (H2O2) and other reactive oxygen species (ROS) accumulate in melanocytes, which leads to DNA damage and lipids and proteins peroxidation, thereby destroying melanocytes homeostasis [Citation10]. However, the mechanism of oxidative stress-induced melanocyte death remains poorly understood in vitiligo.
MicroRNAs (miRNAs) are a class of highly conserved, non-coding RNA molecules that are ∼22 nucleotides in length. miRNAs contribute to the cellular regulatory processes via inhibiting their specific mRNA target expression [Citation11]. It is known that miRNAs can regulate a variety of the cellular processes, including cell proliferation, apoptosis, differentiation and immune responses [Citation12,Citation13]. miRNAs dysregulation is associated with a variety of physiological and developmental cellular processes in human, including melanocytes and immune cells [Citation14]. Dysregulation of miRNA expression has been shown in the serum of the patients with vitiligo such as miR-224-3p, miR-4712-3p and miR-3940-5p [Citation15]. It has been reported that miRNAs mediate the pathogenic role of ROS in oxidative stress-related diseases. However, the exact role of miRNAs and their target genes in the pathogenesis of vitiligo remains poorly understood.
SIRT1 is the most widely studied human sirtuin, a cluster of highly conserved protein. SIRT1 is involved in the regulation of skin structure and function-related cellular pathways including inflammation, cancer and cutaneous infections [Citation16,Citation17]. SIRT1 also acts as a regulator of cellular stress responses [Citation18]. Recent studies have shown that miR-217 is closely associated with SIRT1 [Citation19]. miR-217 has been well studied in various types of cancer including liver cancer, gastric cancer, lung adenocarcinoma and acute myeloid leukemia [Citation20–23]. miR-217 has also been found to modulate human skin fibroblast senescence by directly targeting DNA methyltransferase 1 [Citation24]. Besides, study has revealed that miR-217 was down-regulated in psoriasis, and it could promote keratinocyte differentiation via targeting grainyhead-like 2 (GRHL2) [Citation25]. However, the expression and role of miR-217 in pathogenesis of vitiligo is still unknown.
Therefore, the aim of this study was to investigate the expression and role of miR-217 in dysfunctional melanocytes caused by oxidative stress and elucidated its underlying mechanisms, so as to explore the potential role of miR-217 in vitiligo.
Materials and methods
Cell culture and treatment
Normal human primary epidermal melanocytes were obtained from American Type Culture Collection (cat. no. ATCC® PCS-200-013; Manassas, VA, USA). Cells were cultured in Medium 254 (Gibco, Grand Island, NY, USA) containing Human Melanocyte Growth Supplement (Gibco) at 37 °C in an atmosphere containing 5% CO2.
Vitiligo in vitro cell model conduction
For vitiligo in vitro cell model conduction, normal human melanocytes were treated with 250 μmol/L H2O2 (Sigma-Aldrich, St. Louis, MO, USA) for 4 h to induce oxidative stress [Citation26].
Cell transfection
Normal human melanocytes were transfected with inhibitor control (the negative control of miR-217 inhibitor; 5’-GCCUCCGGCUUCGCACCUCU-3’; Shanghai GenePharma Co., Ltd., Shanghai, China), miR-217 inhibitor (antagonist of miR-217; 5’-UACUGCAUCAGGAACUGAUUGGA-3’; Shanghai GenePharma Co., Ltd.), control-siRNA (cat. no. sc-36869; Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA), SIRT1-siRNA (cat. no. sc-40986; Santa Cruz Biotechnology, Inc.) or miR-217 inhibitor + SIRT1-siRNA using Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc. USA) according to the manufacturer’s protocol. 48 h after cell transfection, transfection efficiency was detected by qRT-PCR.
MTT assay
Cell viability was determined by MTT assay. Normal human melanocytes were seeded in 96-well plates. Cells were transfected with inhibitor control, miR-217 inhibitor or miR-217 inhibitor + SIRT1-siRNA for 48 h; then, the cells were treated with 250 μmol/L H2O2 for 4 h. Subsequently, 20 μL MTT (5 mg/mL, Sigma-Aldrich) was added into each well and further cultured for 4 h. Then, 150 μL dimethyl sulfoxide/well (DMSO, Sigma-Aldrich) was added and incubated for 30 min at 37 °C. Absorbance was measured at 490 nm using a micro-plate spectrophotometer (Bio-Rad Laboratories, Hercules, CA, USA).
Flow cytometry assay
Cell apoptosis was analysed using flow cytometry. Normal human melanocytes were seeded in six-well plates and transfected with inhibitor control, miR-217 inhibitor or miR-217 inhibitor + SIRT1-siRNA for 48 h. Then, the cells were exposed to 250 μmol/L H2O2 for another 4 h, and then, the cells were harvested. Subsequently, the cells were stained with annexin V-fluorescein isothiocyanate and propidium iodide (PI) single-staining solution (Beyotime, Shanghai, China) for 5 min at room temperature without light. Finally, the apoptosis of cells was analysed by flow cytometer (FACSCalibur, Becton Dickinson, San Jose, CA, USA).
Measurement of intracellular reactive oxygen species (ROS)
Melanocytes (5 × 104 cells/per well) were seeded into six-well plates. Cells were transfected with inhibitor control, miR-217 inhibitor or miR-217 inhibitor + SIRT1-siRNA for 48 h; then, the cells were treated with 250 μmol/L H2O2 for 4 h. Subsequently, Reactive Oxygen Species Assay Kit (Cat no. S0033; Beyotime, Shanghai, China) was used to detect the intracellular ROS levels of melanocytes according to the manufacturer’s protocol.
Superoxide dismutase and catalase assays
Melanocytes (5 × 104 cells/per well) were transfected with inhibitor control, miR-217 inhibitor or miR-217 inhibitor + SIRT1-siRNA for 48 h, and then, the cells were treated with 250 μmol/L H2O2 for 4 h. Subsequently, the levels of antioxidant enzymes superoxide dismutase (SOD) and catalase (CAT) in the supernatant of melanocytes were detected using the Total Superoxide Dismutase Assay Kit (Cat no. S0101; Beyotime, Shanghai, China) and Catalase Assay Kit (Cat no. S0051; Beyotime, Shanghai, China), respectively, according to the manuscript’s protocol.
Quantitative reverse transcription-polymerase chain reaction (qRT-PCR)
Total RNA from cells was extracted using TRIzol® regent (Invitrogen; Thermo Fisher Scientific Inc.) according to the manufacturer’s protocol. The total RNA was reverse-transcribed into cDNA using the AMV Reverse Transcriptase (Promega Corporation, Madison, WI, USA) following the manufacturer’s protocol. PCR analysis was performed using SYBR Premix Ex Taq GC kit (Takara, Japan) on an ABI PRISM 7900 HT sequence-detection system. The primer sequences used were as follows: miR-217, forward 5′-TACTGCATCAGGAACTGACTGGA-3′, reverse 5′-GTGCAGGGTCCGAGGT-3′; SIRT1 forward, 5′-GAAACCCTCAATTTCTGTTCTGCT-3′, reverse 5′-AATGCGATGCTGACTTCCTTCT-3′;GAPDH forward 5′-CTTCACCACCATGGAGAAGGC-3′, reverse 5′-GGCATGGACTGTGGTCATGAG-3′; U6 forward: 5′-CTCGCTTCGGCAGCACATAT-3′, reverse 5′-TTGCGTGTCATCCTTGCG-3′. GAPDH and U6 were used as the internal control for mRNA and miRNA expression, respectively. The relative gene expression level quantification was analysed using the 2–ΔΔCt method [Citation27].
Western blot assay
After treatment, total cell protein was extracted using lysis buffer (Beijing Solarbio Science & Technology Co., Ltd., Beijing, China). The protein concentration was detected by Bicinchoninic acid assay kit (Pierce; Thermo Fisher Scientific, Inc.). Equal amounts of protein (25 µg/lane) were then separated by 10% SDS-PAGE and transferred to polyvinylidene difluoride membranes (PVDF; Roche, Basel, Switzerland). Following blocking with 5% non-fat milk at room temperature for 1 h, membranes were incubated with primary antibodies: Bcl-2 (Cat no. sc-7382; dilution rate: 1:500; Santa Cruz Biotechnology), Bax (Cat no. sc-7480; dilution rate: 1:500; Santa Cruz Biotechnology), SRIT1 (Cat no. sc-74465; dilution rate: 1:200; Santa Cruz Biotechnology), phosphorylated-p65 (p-p65; Cat no. 13346; dilution rate: 1:1000, Cell Signaling Technology, Danvers, MA, USA), p65 (Cat no. 6956; dilution rate: 1:1000, Cell Signaling Technology) and β-actin (Cat no. 3700; dilution rate: 1:1000; Cell Signaling Technology) overnight at 4˚C. Subsequently, the membranes were incubated with anti-mouse IgG, HRP linked antibody (cat. no. 7076; dilution rate: 1:2,000; Cell Signaling Technology, Inc.) at room temperature for 2 h. The bands were detected by electrochemiluminescence ECL kit (Thermo Scientific, USA) and Image J 2.0 software (Bio-Rad Laboratories Inc., USA) was used to quantify the intensity of band.
Dual luciferase reporter assays
Bioinformatics software TargetScan 7.2 (http://www.targetscan.org/vert_72/) was used to predict the binding sites between SIRT1 and miR-217, and the results showed the direct binding sites between SIRT1 and miR-217. The 3’-UTR of SIRT1 containing a miR-217 binding sites amplified from normal human melanocytes cDNA or the mutant UTR was cloned into pGL3-promoter vector (Promega, Madison, WI, USA). 293 T cells (5 × 104 cells per well) were cultured in 24-well plates and co-transfected with PGL3-SIRT1-3’UTR-wild-type (SIRT1-WT) and PGL3-SIRT1-3’UTR-mutant (SIRT1-MUT) plasmid and miR-217 mimic or mimic control using Lipofectamine 3000 (Invitrogen; Thermo Fisher Scientific, Inc.) according to the manufacturer’s protocol. After 48 h, relative luciferase activities were detected using a dual luciferase reporter assay kit (Promega) following the manufacturer’s instruct. Renilla luciferase activity was used as the control.
Statistical analysis
Data were presented as the mean ± standard deviation (SD). The significance of differences between groups was evaluated using Student’s t-test or one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test. p < .05 was considered to indicate a statistically significant difference.
Results and discussion
SIRT1 is an anti-ageing molecule, as a member of mammalian NAD+-dependent deacetylase family. Previous study has reported that SIRT1 can inhibit inflammation and oxidative stress, and activation of SIRT1 leads to p53-mediated apoptosis decreasing in UV-exposed keratinocytes [Citation28]. Recent findings showed that H2O2 induced down-regulation of SIRT1 in keratinocytes, and SIRT1 reduced oxidative stress and cell apoptosis in perilesional vitiligo keratinocytes [Citation29]. These results indicated that SIRT1 may be a potential target to prevent melanocyte damage induced by oxidative stress. So in this study, we firstly explored the expression of SIRT1 in H2O2-treated primary human melanocytes and our results showed that SIRT1 was down-regulated in H2O2-treated primary human melanocytes.
Decreased SIRT1 expression in H2O2-treated primary human melanocytes
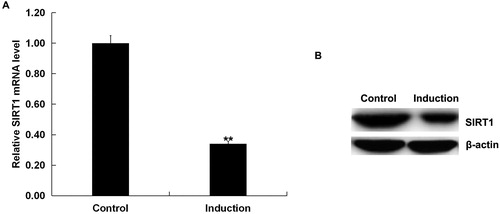
We first assessed the expression of SIRT1 in the H2O2-treated primary human melanocytes (Induction group). Cells without any treatment were considered as the control (Control group). As depicted in , compared to the control group, the mRNA and protein expression of SIRT1 significantly down-regulated in H2O2-treated primary human melanocytes.
Figure 1. Expression of SIRT1 in the H2O2-treated primary human melanocytes.
The expression of SIRT1 in the H2O2-treated primary human melanocytes was detected using qRT-PCR and Western blot assay. Induction: cells were treated with 250 μmol/L H2O2 for 4 h. Control: cells without any treatment. Data were expressed as the mean ± SD. **p < .01 vs. control.
SIRT1 was a target gene of miR-217
Recently, several miRNAs, such as miR-25, miR-155 and miR-211, have been reported to be involved in vitiligo. miR-217, which has been well studied in various types of cancer [Citation20–23], has also been found to play critical roles in skin related disease [Citation24,Citation25]. Besides, several studies have confirmed the relationship between miR-217 and SIRT1 [Citation19,Citation30–32]. Thus, we hypothesized that miR-217 might play important roles in vitiligo through regulating the expression of SIRT1.
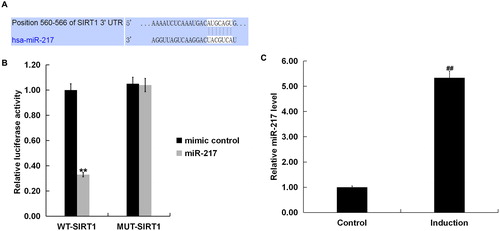
Bioinformatics software predicted SIRT1 contained theoretical miR-217 binding site in its 3’UTR (). To confirm SIRT1 was a target gene of miR-217, a luciferase reporter assay was performed. As shown in , co-expression of miR-217 significantly inhibited the luciferase reporter activity of the wild-type SIRT1 3’-UTR but not of the mutant SIRT1 3’-UTR, confirming that miR-217 directly targeted the 3’ UTR region in SIRT1. We further validated that miR-217 expression was significantly up-regulated in H2O2-treated primary human melanocytes ().
Figure 2. SIRT1 as a target of miR-217.
(A) Interaction between miR-217 and 3’UTR of SIRT1 was predicted using microRNA target site prediction software (TargetScan); (B) dual luciferase reporter assay was performed to confirm the binding sites between SIRT1 and miR-217. “MUT-SIRT1” indicates the SIRT1 3’ UTR with a mutation in the miR-217 binding site. UTR, untranslated region. (C) The expression of miR-217 in the H2O2-treated primary human melanocytes was detected using qRT-PCR. Induction: cells were treated with 250 μM H2O2 for 4 h. Control: cells without any treatment.
Note: Data were expressed as the mean ± SD. ##p < .01 vs. Control. **p < .01 vs. mimic control.
Down-regulation of miR-217 protected primary human melanocytes from H2O2-induced cytotoxicity and apoptosis
We then investigated the effect of miR-217 down-regulation on the growth of H2O2-treated primary human melanocytes and oxidative stress. Antioxidant enzymes SOD and CAT play a vital role in protecting melanocytes from oxidative damage [Citation33].
To further investigate the biological function of miR-217 on melanocytes under oxidative stress and whether it was mediated by SIRT1, normal human melanocytes were transfected with inhibitor control, miR-217 inhibitor, control-siRNA, SIRT1-siRNA or miR217 inhibitor + SIRT1-siRNA for 48 h, and then, cells were treated with 250 μmol/L H2O2 for 4 h. Transfection efficiency was detected by qRT-PCR. As shown in , miR-217 inhibitor transfection caused a significant decrease in miR-217 expression () in human melanocytes, and SIRT1-siRNA transfection caused a significant decrease in SIRT1-mRNA expression in human melanocytes (). In addition, miR-217 inhibitor transfection significantly increased SIRT1 expression, which could be reversed by SIRT1-siRNA (). Cell viability was detected using MTT assay. As shown in , miR-217 inhibition significantly increased cell viability in H2O2-treated primary human melanocytes, which was reversed by SIRT1-siRNA transfection.
Figure 3. Negative regulation of SIRT1 by miR 217.
(A) The expression of miR-217 in the primary human melanocytes transfected with inhibitor control or miR-217 inhibitor detected using qRT-PCR; (B) the mRNA expression of SIRT1 in the primary human melanocytes transfected with control-siRNA or SIRT1-siRNA detected using qRT-PCR; (C and D) the mRNA and protein expression of SIRT1 in the primary human melanocytes transfected with inhibitor control, miR-217 inhibitor or miR-217 inhibitor + SIRT1-siRNA detected using qRT-PCR and Western blotting.
Note: Data were expressed as the mean ± SD. **p < .01 vs. control. ##p < .01 vs. inhibitor.
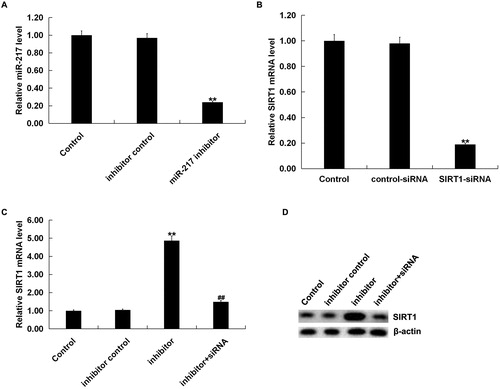
Figure 4. Effect of miR-217 inhibitor on H2O2-treated primary human melanocytes.
The primary human melanocytes transfected with inhibitor control, miR-217 inhibitor or miR-217 inhibitor + SIRT1-siRNA for 48 h and subsequently treated with 250 μmol/L H2O2 for 4 h. MTT assay (A) and flow cytometry (B and C) for detection of cell viability and cell apoptosis. Detection of the protein level of Bax and Bcl-2 by Western blot assay (D).
Note: Data were expressed as the mean ± SD. **p < .01 vs. control; #,##p < .05, .01 vs. induction; &, && p < .05, .01 vs. inhibitor.
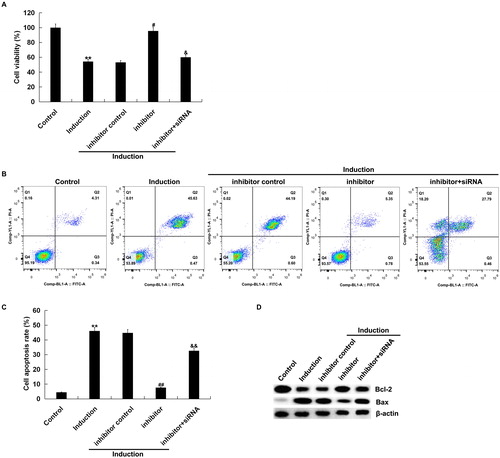
We also found that the number of apoptotic cells in H2O2-treated primary human melanocytes was significantly decreased by miR-217 inhibitor, as compared to the control group, which was reversed by SIRT1-siRNA transfection (). In addition, miR-217 inhibition increased the level of anti-apoptotic protein Bcl-2, but down-regulated the pro-apoptotic protein Bax, and all these changes were also reversed by SIRT1-siRNA transfection ().
Down-regulation of miR-217 promoted the antioxidant capacity of H2O2-treated primary human melanocytes
The accumulation of ROS, mainly consisting of H2O2, superoxide anions and hydroxyl radicals, in melanocytes induces apoptosis and might therefore be involved in melanocyte destruction [Citation34,Citation35]. Thus, ROS production and the activity of SOD and CAT were analysed in this study to explore the effect of miR-217 down-regulation on H2O2-induced primary human melanocyte injury.
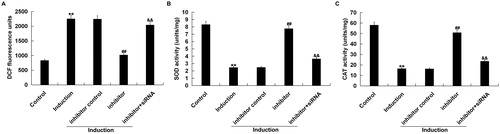
We found that ROS level significantly up-regulated in H2O2-treated primary human melanocytes, as compared to the control group. miR-217 inhibitor transfection significantly decreased the ROS level in H2O2-treated primary human melanocytes, which was reversed by SIRT1-siRNA (). miR-217 inhibitor transfection also alleviated the inhibition of SOD and CAT activities in H2O2-treated primary human melanocytes, which was also reversed by SIRT1-siRNA ().
Figure 5. Antioxidant effect exerted by miR-217 inhibitor on H2O2-treated melanocytes.
The primary human melanocytes transfected with inhibitor control, miR-217 inhibitor or miR-217 inhibitor + SIRT1-siRNA for 48 h and subsequently treated with 250 μmol/L H2O2 for 4 h. (A) The fluorescence intensity corresponding to the ROS levels. (B and C) Superoxide dismutase (SOD) and catalase (CAT) activities.
Note: Data were expressed as the mean ± SD. **p < .01 vs. control; ##p < .01 vs. induction; &&p < .01 vs. inhibitor.
Down-regulation of miR-217 inhibited NF-κB activation in H2O2-treated melanocytes
NF-κB signalling plays a key role in several cellular responses, including cellular survival under stress [Citation36]. NF-κB signalling can be enhanced by activators like cytokines and ROS. Oxidative stress stimulates NF-κB and causes translocation of NF-κB to the nucleus [Citation37]. In addition, there are studies shown that SIRT1 affects NF-κB signalling pathway [Citation38–39]. So we next detect whether miR-217 affects NF-κB signalling pathway through regulating SIRT1 and then plays important roles in H2O2-treated melanocytes.
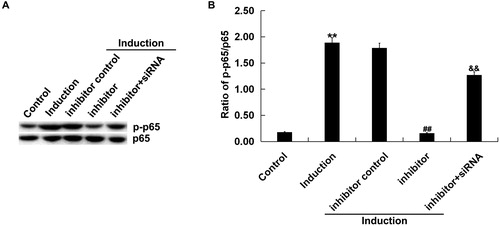
As shown in , the protein level of p-p65 was notably increased in melanocytes treated with H2O2 alone compared with the untreated cells. miR-217 inhibitor reduced p-p65 protein expression, and this reduction was reversed by SIRT1-siRNA. Besides, miR-217 inhibitor transfection significantly decreased the ratio of p-p65/p65 in H2O2-treated primary human melanocytes, and this decrease was significantly reversed by SIRT1-siRNA ().
Figure 6. Effect of miR-217 inhibitor on p-p65 expression in H2O2-treated primary human melanocytes.
The primary human melanocytes transfected with inhibitor control, miR-217 inhibitor or miR-217 inhibitor + SIRT1-siRNA for 48 h and subsequently treated with 250 μmol/L H2O2 for 4 h. Detection of p65 and p-p65 by Western blotting (A), and calculation of the ratio of p-p65/p65 (B).
Note: Data were expressed as the mean ± SD. **p < .01 vs. control; ##p < .01 vs. Induction; &&p < .01 vs. inhibitor.
Our results showed that miR-217/SIRT1 axis plays an important role in melanocyte dysfunction, so it is expected to be a potential target for the treatment of vitiligo caused by melanocyte dysfunction loss. This study throws light on the role of miR-217 in vitiligo. However, the aetiology of vitiligo is very complicated. To determine the role and value of miR-217/SIRT1 axis in vitiligo, a lot of in-depth research is needed. We will continue to study this topic in the future.
Conclusion
In conclusion, our results demonstrated that miR-217 was up-regulated in H2O2-treated primary human melanocytes. Inhibition of miR-217 protected melanocytes from H2O2-induced oxidative damage via targeting SIRT1. These results indicated that miR-217 is a promising therapeutic target for the treatment of vitiligo caused by melanocyte dysfunction.
Disclosure statement
Authors declare no conflict of interest.
Additional information
Funding
References
- Boniface K, Seneschal J. Vitiligo as a skin memory disease: the need for early intervention with immunomodulating agents and a maintenance therapy to target resident memory T cells. Exp Dermatol. 2019;28(6):656–661.
- Silverberg JI, Silverberg NB. Association between vitiligo extent and distribution and quality-of-life impairment. JAMA Dermatol. 2013;149(2):159–164.
- Gey A, Diallo A, Seneschal J, et al. Autoimmune thyroid disease in vitiligo: multivariate analysis indicates intricate pathomechanisms. Br J Dermatol. 2013;168(4):756–761.
- Schallreuter KU, Wood JM, Ziegler I, et al. Defective tetrahydrobiopterin and catecholamine biosynthesis in the depigmentation disorder vitiligo. Biochim Biophys Acta. 1994;1226:181–192.
- Jin Y, Birlea SA, Fain PR, et al. Genome-wide association analyses identify 13 new susceptibility loci for generalized vitiligo. Nat Genet. 2012;44(6):676–680.
- Quan C, Ren YQ, Xiang LH, et al. Genome-wide association study for vitiligo identifies susceptibility loci at 6q27 and the MHC. Nat Genet. 2010;42(7):614–618.
- Laddha NC, Dwivedi M, Mansuri MS, et al. Vitiligo: interplay between oxidative stress and immune system. Exp Dermatol. 2013;22(4):245–250.
- Jimbow K, Chen H, Park JS, et al. Increased sensitivity of melanocytes to oxidative stress and abnormal expression of tyrosinase-related protein in vitiligo. Br J Dermatol. 2001;144(1):55–65.
- He Y, Li S, Zhang W, et al. Dysregulated autophagy increased melanocyte sensitivity to H2O2-induced oxidative stress in vitiligo. Sci Rep. 2017;7(1):42394.
- Colucci R, Dragoni F, Moretti S. Oxidative stress and immune system in vitiligo and thyroid diseases. Oxid Med Cell Longev. 2015;2015:1–7.
- Mansuri MS, Singh M, Begum R. miRNA signatures and transcriptional regulation of their target genes in vitiligo. J Dermatol Sci.2016;84(1):50–58.
- Ruksha TG, Komina AV, Palkina NV. MicroRNA in skin diseases. Eur J Dermatol. 2017;27:343–352.
- Mehta A, Baltimore D. MicroRNAs as regulatory elements in immune system logic. Nat Rev Immunol. 2016;16(5):279–294.
- Shi Q, Zhang W, Guo S, et al. Oxidative stress-induced overexpression of miR-25: the mechanism underlying the degeneration of melanocytes in vitiligo. Cell Death Differ. 2016;23(3):496–508.
- Wang Y, Wang K, Liang J, et al. Differential expression analysis of miRNA in peripheral blood mononuclear cells of patients with non-segmental vitiligo. J Dermatol. 2015;42(2):193–197.
- Garcia-Peterson LM, Wilking-Busch MJ, Ndiaye MA, et al. Sirtuins in skin and skin cancers. Skin Pharmacol Physiol. 2017;30(4):216–224.
- Qiang L, Sample A, Liu H, et al. Epidermal SIRT1 regulates inflammation, cell migration, and wound healing. Sci Rep. 2017;7(1):14110.
- Carlomosti F, D'Agostino M, Beji S, et al. Oxidative stress-induced miR-200c disrupts the regulatory loop among SIRT1, FOXO1, and eNOS. Antioxid Redox Signal. 2017;27(6):328–344.
- Shao Y, Lv C, Wu C, et al. Mir-217 promotes inflammation and fibrosis in high glucose cultured rat glomerular mesangial cells via Sirt1/HIF-1alpha signaling pathway. Diabetes Metab Res Rev. 2016;32(6):534–543.
- Wang LP, Wang JP, Wang XP. HOTAIR contributes to the growth of liver cancer via targeting miR-217. Oncol Lett. 2018;15(5):7963–7972.
- Safaralizadeh R, Ajami N, Nemati M, et al. Dysregulation of miR-216a and miR-217 in gastric cancer and their clinical significance. J Gastrointest Canc. 2019;50(1):78–83.
- Liu AN, Qu HJ, Yu CY, et al. Knockdown of LINC01614 inhibits lung adenocarcinoma cell progression by upregulating miR-217 and downregulating FOXP1. J Cell Mol Med. 2018;22(9):4034–4044.
- Yan J, Wu G, Chen J, et al. Downregulated miR-217 expression predicts a poor outcome in acute myeloid leukemia. CBM. 2018;22(1):73–78.
- Wang B, Du R, Xiao X, et al. Microrna-217 modulates human skin fibroblast senescence by directly targeting DNA methyltransferase 1. Oncotarget 2017;8:33475–33486.
- Zhu H, Hou L, Liu J, et al. MiR-217 is down-regulated in psoriasis and promotes keratinocyte differentiation via targeting GRHL2. Biochem Biophys Res Commun. 2016;471(1):169–176.
- Lu W, Zhao Y, Kong Y, et al. Geniposide prevents H2O2-induced oxidative damage in melanocytes by activating the PI3K-Akt signalling pathway. Clin Exp Dermatol. 2018;43(6):667–674.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408.
- Chen ML, Li J, Xiao WR, et al. Protective effect of resveratrol against oxidative damage of UVA irradiated HaCaT cells. Zhong Nan Da Xue Xue Bao Yi Xue Ban. 2006;31(5):635–639.
- Cao C, Lu S, Kivlin R, et al. SIRT1 confers protection against UVB- and H2O2-induced cell death via modulation of p53 and JNK in cultured skin keratinocytes. J Cell Mol Med. 2009;13(9b):3632–3643.
- Zhang S, Liu L, Wang R, et al. MicroRNA-217 promotes angiogenesis of human cytomegalovirus-infected endothelial cells through downregulation of SIRT1 and FOXO3A. PLoS One. 2013;8(12):e83620.
- Deng S, Zhu S, Wang B, et al. Chronic pancreatitis and pancreatic cancer demonstrate active epithelial-mesenchymal transition profile, regulated by miR-217-SIRT1 pathway. Cancer Lett. 2014;355(2):184–191.
- Yin H, Hu M, Zhang R, et al. MicroRNA-217 promotes ethanol-induced fat accumulation in hepatocytes by down-regulating SIRT1. J Biol Chem. 2012;287(13):9817–9826.
- Mehaney DA, Darwish HA, Hegazy RA, et al. Analysis of oxidative stress status, catalase and catechol-O-methyltransferase polymorphisms in Egyptian vitiligo patients. PLoS One. 2014;9(6):e99286.
- Zhang Y, Liu L, Jin L, et al. Oxidative stress-induced calreticulin expression and translocation: new insights into the destruction of melanocytes. J Invest Dermatol. 2014;134(1):183–191.
- Chang Y, Li S, Guo W, et al. Simvastatin Protects human melanocytes from H2O2-induced oxidative stress by activating Nrf2. J Invest Dermatol. 2017;137(6):1286–1296.
- Storz P, Toker A. NF-kappaB signaling–an alternate pathway for oxidative stress responses. Cell Cycle. 2003;2(1):9–10.
- Won JH, Im HT, Kim YH, et al. Anti-inflammatory effect of buddlejasaponin IV through the inhibition of iNOS and COX-2 expression in RAW 264.7 macrophages via the NF-kappaB inactivation. Br J Pharmacol. 2006;148(2):216–225.
- Zhang P, He L, Zhang J, et al. Preparation of novel berberine nano-colloids for improving wound healing of diabetic rats by acting Sirt1/NF-κB pathway. Colloids Surf B. 2019;14:110647.
- Xue P, Zhao J, Zheng A, et al. Chrysophanol alleviates myocardial injury in diabetic db/db mice by regulating the SIRT1/HMGB1/NF-κB signaling pathway. Exp Ther Med. 2019;18:4406–4412.
