Abstract
This study explored whether sufentanil, a lipophilic opioid, can protect against myocardial ischemia-reperfusion (I/R) injury via regulating Sestrin 2 (Sesn2) expression. The expression of Sesn2 was measured in the blood of patients with myocardial I/R after sufentanil administration. H9c2 cells were applied to establish I/R injury by oxygen-glucose deprivation and reoxygenation (OGD/R). After treatment with 5, 10 and 20 μmol/L sufentanil, the expression of Sesn2 was examined. Then, shRNA-Sesn2 was transfected into H9c2 cells. The concentrations of creatine kinase (CK), CK isoenzymes (CK-MB), cardiac troponin I (cTnI), inflammation-related factors and oxidative-related factors were sequentially determined by kits. Western blotting was exploited to determine the expression of AMP-activated protein kinase (AMPK) pathway proteins. The results revealed that the expression of Sesn2 was increased in patients after administration with sufentanil. OGD/R intervention upregulated the expression of Sesn2, which continued to increase in a dose-dependent manner following treatment with sufentanil. Moreover, sufentanil attenuated the concentrations of CK, CK-MB and cTnl induced by OGD/R in H9c2 cells. This effect was reversed after Sesn2 silencing. Meanwhile, the levels of inflammatory factors and oxidative stress were notably decreased when cells were treated with sufentanil, and these inhibitory effects were alleviated after transfection with shRNA-Sesn2. Furthermore, sufentanil enhanced the phosphorylation of AMPK and its downstream acetyl CoA carboxylase (ACC), whereas Sesn2 silencing inhibited the expression of the above proteins. These findings demonstrated that sufentanil could attenuate inflammation and oxidative stress in myocardial I/R injury via upregulating Sesn2 expression and activating the AMPK pathway.
Introduction
Myocardial ischemia/reperfusion (I/R) injury is a common cardiovascular disease accompanied with high morbidity and mortality globally, which derives from acute myocardial infarction and coronary artery disease [Citation1, Citation2]. Myocardial I/R injury arises from temporary interruption of blood supply followed by the recovery of perfusion and concomitant reoxygenation, which exacerbates the dysfunction and leads to structural damage [Citation3]. Mounting evidence supported that myocardial I/R is a long-term myocardial injury secondary to the excessive release of inflammatory mediators and formation of reactive oxygen species (ROS), which together contribute to the occurrence and development of myocardial I/R injury [Citation4–6]. Although many efforts have been made to explore the rescue mechanisms of I/R myocardium, there is still no effective therapy for preventing and treating I/R-induced cardiac injury. Hence, further studies to develop new strategies and targets to effectively relieve myocardial I/R injury are of paramount importance and necessity.
The clinical drug that is commonly used for ischemic heart disease is sufentanil, a lipophilic opioid. It has been well reported that sufentanil treatment is able to protect the rat myocardium from I/R injury [Citation7]. In a rabbit model of myocardial I/R, intrathecal sufentanil exhibited a cardioprotective effect [Citation8]. Sestrin 2 (Sesn2), a member of the Sestrin family, is a stress-responsive protein which can be induced by hypoxia [Citation9]. Previous studies have highlighted the importance of Sesn2 in protecting cardiomyocytes from I/R injury [Citation10]. However, whether sufentanil can protect against myocardial I/R injury via regulating Sesn2 expression and downstream pathways remains unknown.
In this study, the expression of Sesn2 was examined in patients with myocardial I/R injury after administration with sufentanil. In addition, cardiac muscle H9c2 cells were cultured under oxygen and glucose deprivation followed by reperfusion (OGD/R) condition to establish myocardial I/R injury in vitro. We aimed to explore whether sufentanil can protect against myocardial I/R injury via activating Sesn2 expression and regulating downstream pathways. All of our efforts are expected to elucidate the mechanisms underlying the effect of sufentanil on myocardial I/R injury and provide a potential candidate agent against myocardial I/R injury.
Subjects and methods
Clinical samples collection
This study involved 20 healthy persons (aged 59.5 ± 7.1 years) without myocardial I/R injury and 20 cases (aged 61.3 ± 9.3 years) with myocardial I/R injury, who were all recruited from Affiliated Wujin Hospital of Jiangsu University from November 2017 to April 2018. Blood samples were obtained from the above cases before any treatment. Then, patients with myocardial I/R injury received sufentanil administration (patient + sufentanil), and blood samples were acquired again. Participants were excluded from this research if they had a history of malignancy, liver or kidney disease. Samples were immediately frozen in liquid nitrogen at −80 °C for storage.
Ethics statement
The research protocol was approved by the Ethics Committee of the Affiliated Wujin Hospital of Jiangsu University and was executed according to the principles of the Declaration of Helsinki. All study participants provided written informed consent forms.
Cell culture and drug treatment
The cardiomyocytes cell line H9c2 cells were acquired from the Cell Bank of the Chinese Science Institute (Shanghai, China) and maintained in Dulbecco’s Modified Eagle’s Medium (DMEM; HyClone, Logan, UT) containing 10% fetal bovine serum (FBS; Invitrogen; Thermo Fisher Scientific, Inc.). Cells were cultured in a humidified incubator under 5% CO2 at 37 °C. Various gradient concentrations (5, 10 and 20 μmol/L) of sufentanil (Yichang Humanwell Pharmaceutical Co. Yichang, China) were used for pretreatment of cells for 24 h.
Construction of myocardial I/R injury model
To mimic I/R injury of cardiomyocytes, H9c2 cells were cultured under oxygen and glucose deprivation followed by reperfusion condition. H9c2 cells were cultured in serum-free DMEM (glucose-free) in a sealed container with 95% N2 and 5% CO2 for anaerobic conditions at 37 °C. After 2 h of OGD, cells were transferred to glucose-containing DMEM with 10% FBS under normoxic environment for specified time (2 h, 4 h, 6 h, 8 h, 10 h, 12 h, 24 h, 36 h) to undergo reoxygenation. Cells cultured in normoxic conditions were used as a control. Cells were arbitrarily allocated into the following five groups: control group, OGD/R group, OGD/R group plus treated with 5, 10 and 20 μmol/L sufentanil groups.
Cell transfection
To explore the effect of Sesn2 in myocardial I/R injury, small hairpin RNA (shRNA)-Sesn2-1, shRNA-Sesn2-2 and its negative control (shRNA-NC) supplied by RiboBio Co., Ltd. (Guangzhou, China) were transfected into H9c2 cells. At 24 h before transfection, cells were plated into the wells of six-well plates at 2 × 105 cells/well until 70% confluence. Lipofectamine 2000 (Invitrogen) was exploited for performing the transfection experiments according to the manufacturer’s protocols. The transfection efficiency was examined by Western blotting and quantitative reverse transcription polymerase chain reaction (RT-qPCR).
Test for creatine kinase (CK), CK isoenzymes (CK-MB) and cardiac troponin I (cTnI)
Culture supernatant was obtained by centrifugation at 4 °C(1000 g for 10 min) after appropriate treatment. Subsequently, the levels of CK, CK-MB and cTnI were detected chemical colorimetry detection kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, china) in accordance with the manufacturer’s protocol.
Measurement of inflammatory factors
Culture media was collected at the end of the reoxygenation stage. The concentrations of inflammatory cytokines including tumor necrosis factor-alpha (TNF-α), interleukin (IL)−6 and IL-1β were measured by enzyme-linked immunosorbent assay (ELISA) following the manufacturer’s protocols (R&D Systems, Abingdon, UK).
Measurement of oxidative stress-related factors
Following 12 h of reoxygenation, the contents of ROS (superoxide radical) and malondialdehyde (MDA) as well as the activities of lactate dehydrogenase (LDH) and superoxide dismutase (SOD) in culture media were evaluated by commercial kits according to colorimetric methods. The kits were all provided by Nanjing Jiancheng Bioengineering Institute (Nanjing, China). All operations were performed in strict accordance with the manufacturer’s instructions.
Quantitative reverse transcription polymerase chain reaction (RT-qPCR) analysis
After treatment, TRIzol reagent (Invitrogen) was applied to extract total RNA from myocardial cells. The complementary DNA (cDNA) was synthesized using a PrimeScript RT reagent (Takara, Japan). An SYBR-Green PCR kit (Takara) was employed to conduct two-step qPCR according to the manufacturer’s protocol on the ABI 7500 PCR System (Applied Biosystems). The primers used in this study were as follows: Sesn2, forward 5′-AGAGGGCACAGGAAAGAA-3′ and reverse 5′-TCAAGCATAAAGGACCAAA-3′; GAPDH, forward 5′-TATGATGATATCAAGAGGGTAGT-3′ and reverse 5′-TGTATCCA AACTCATTGTCATAC-3′. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was considered as an internal reference gene. Relative expression was calculated using the 2−ΔΔCt method.
Western blotting
Total proteins in H9c2 cells were isolated using RIPA lysis buffer (Sigma, St Louis, MO). The BCA Protein Assay Kit (Beyotime, Shanghai, China) was executed for examining the concentration of protein in each group. Then, an equal concentration of protein lysates was separated in sodium dodecyl sulfate polyacrylamide (SDS/PAGE) gels, followed by transfer to polyvinylidene fluoride (PVDF) membranes. The membranes were sequentially washed with PBS and incubated with secondary antibodies (Stanta Cruz Biotechnology, CA, USA). The bonded proteins were visualized with the Odyssey Infrared Imaging System (LI-COR Inc., Lincoln, NE, USA). The intensity of bands was quantified using Image J software. GAPDH served as the internal control.
Statistical analysis
All data were represented as mean values with standard deviation (±SD) and statistical analysis was performed by SPSS software 19.0 (SPSS Inc, Chicago, IL). The two-tailed Student’s t test was adopted for comparisons between two groups. Comparisons involving multiple samples were conducted by one-way analysis of variance (ANOVA) followed by Turkey’s post hoc test. Differences were considered statistically significant at a level of p < 0.05. .
Results and discussion
Sesn2 expression was upregulated in blood of patients with myocardial I/R injury after sufentanil administration and in H9c2 cells induced by OGD/R
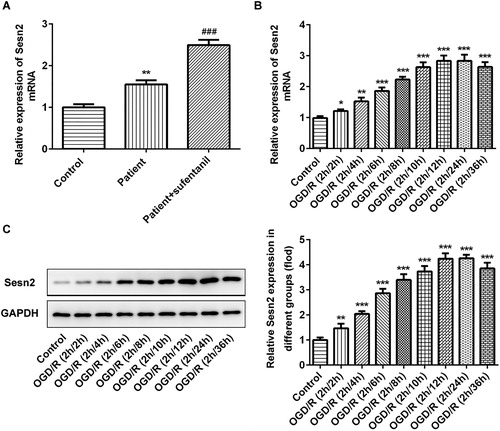
To explore the role of Sesn2 in myocardial I/R injury, we firstly detected the levels of Sesn2 in blood samples from patients with myocardial I/R injury after administration with sufentanil, and blood samples of patients who received no treatment as the negative control. As shown in , the expression level of Sesn2 was higher in patients compared with the healthy controls. After treatment with sufentanil, the level of Sesn2 was even higher. Then, OGD/R was exploited for inducing myocardial I/R injury in vitro, and we found that the expression of Sesn2 was significantly upregulated after treatment with OGD/R. The Sesn2 expression peaked at 2 h of OGD and 12 h of reoxygenation (). Therefore, we chose OGD/R (2 h/12 h) to perform the subsequent experiments.
Figure 1. Upregulated expression of Sesn2 in the blood of patients with myocardial I/R injury after administration with sufentanil and in H9c2 cells induced by OGD/R. (A) Expression of Sesn2 mRNA detected by RT-qPCR in clinical blood samples. **p < 0.01 vs. healthy control; ###p < 0.001 vs. patients not receiving sufentanil. (B,C) Expression of Sesn2 mRNA and protein evaluated by RT-qPCR (B) and Western blotting (C), respectively. *p < 0.05, **p < 0.01, ***p < 0.001 vs. control.
Note: Sesn2, Sestrin 2; I/R, ischemia reperfusion; OGD/R, oxygen-glucose deprivation and reoxygenation.
Sufentanil treatment promoted the expression of Sesn2 in OGD/R-induced H9c2 cells
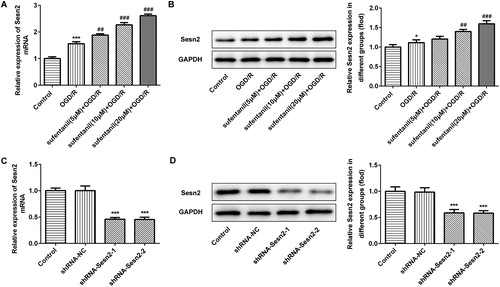
To investigate the effect of sufentanil on Sesn2 in H9c2 cells induced by OGD/R, RT-qPCR and Western blotting were employed to test the expression of Sesn2. As presented in , compared with the control, Sesn2 mRNA levels were notably increased in H9c2 cells after induction by OGD/R. Sufentanil promoted the expression of Sesn2 in a dose-dependent manner in comparison with the OGD/R group. The Sesn2 protein expression levels followed a similar trend (). Then, shRNA-Sesn2 were transfected into cells. Decreased mRNA and protein expression levels of Sesn2 were observed after transfection with shRNA-Sesn2-1 and shRNA-Sesn2-2 (). H9c2 cells transfected with shRNA-Sesn2-2 were used in the next steps.
Figure 2. Effect of sufentanil treatment on the expression of Sesn2 in OGD/R-induced H9c2 cells. (A,B) Expression of Sesn2 determined by RT-qPCR (A) and Western blotting (B) after treatment with different doses of sufentanil. *p < 0.05, ***p < 0.001 vs. control; ##p < 0.01, ###p < 0.001 vs. OGD/R. (C,D) Expression of Sesn2 mRNA and protein determined by RT-qPCR (C) and Western blotting (D) after transfection with shRNA-Sesn2-1 or shRNA-Sesn2-2. ***p < 0.001 vs. shRNA-NC.
Note: Sesn2, Sestrin 2; OGD/R, oxygen-glucose deprivation and reoxygenation.
Sufentanil alleviated myocardial injury and inflammation induced by OGD/R in H9c2 cells via regulating Sesn2 expression
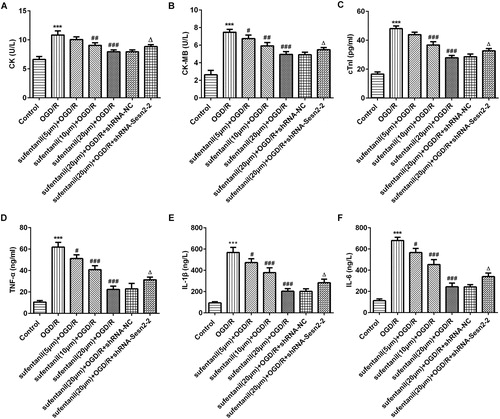
A growing body of evidence suggests that CK, CK-MB and cTnI, markers reflecting myocardial injury, are widely applied to detect the extent of myocardial injury [Citation11, Citation12]. As presented in , the levels of CK, CK-MB and cTnl were dramatically increased in the OGD/R group compared with the control group. After treatment with sufentanil, the levels of the above factors significantly decreased in a dose-dependent manner, whereas Sesn2 silencing attenuated the inhibitory effects of sufentanil on CK, CK-MB and cTnl. These results were in accordance with a previous study [Citation13]. Numerous studies unveil that a large amount of inflammatory cytokines are released during myocardial I/R. These cytokines play a crucial role in the pathogenesis of myocardial IR injury and lead to severe cardiac damage [Citation14, Citation15]. Emerging evidence demonstrates that sufentanil treatment is able to protect against hepatic I/R injury via inhibiting inflammation [Citation16]. In addition, Sesn2 could inhibit pro-inflammatory signalling in macrophages [Citation17]. The results are shown in . We demonstrated that sufentanil intervention reduced the levels of TNF-α, IL-1β and IL-6 induced by OGD/R. These effects were reversed after transfection with shRNA-Sesn2. These findings suggested that sufentanil alleviated the inflammation response induced by OGD/R in H9c2 cells via regulating Sesn2 expression.
Figure 3. Effect of sufentanil on myocardial injury and inflammation response induced by OGD/R in H9c2 cells and reversal of the effect after Sesn2 silencing. (A,B,C) Concentrations of myocardial injury factors CK (A), CK-MB (B) and cTnI (C) measured by kits. (D,E,F) Levels of inflammatory cytokines TNF-α (D), IL-1β (E) and IL-6 (F) detected by ELISA. ***p < 0.001 vs. control; #p < 0.05, ##p < 0.01, ###p < 0.001 vs. OGD/R; △p < 0.05 vs. sufentanil (20 μmol/L) + OGD/R + shRNA-NC.
Note: Sesn2, Sestrin 2; OGD/R, oxygen-glucose deprivation and reoxygenation; CK, creatine kinase; CK-MB, CK isoenzymes; cTnI, cardiac troponin I; TNF-α, tumor necrosis factor-alpha; IL, interleukin.
Sufentanil relieved oxidative stress induced by OGD/R in H9c2 cells via regulating Sesn2 expression
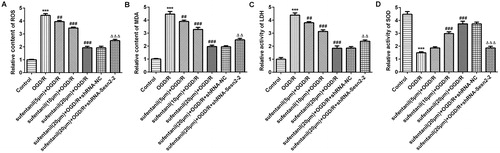
Oxidative stress has been considered as a crucial feature of the pathogenesis of myocardial I/R injury [Citation18]. During the reperfusion period, the generation of ROS leads to multiple cellular damage, which is an important risk factor in the occurrence and development of cardiovascular ischemic diseases [Citation19, Citation20]. It has been well reported that sufentanil can decrease the content of MDA, which is the end product of lipid peroxidation and a biomarker for oxidative stress [Citation7, Citation21]. SOD serves as an important anti-oxidase, which participates in protecting against oxidative stress during myocardial I/R injury [Citation22, Citation23]. In addition, previous studies have highlighted the importance of Sesn2 in oxidative stress in I/R injury. For instance, silencing of Sesn2 could increase oxidative stress and aggravate cerebral I/R injury [Citation24]. Sesn2 knockdown promoted lipopolysaccharide-induced oxidative stress in H9c2 cells and heart tissues of mice [Citation25]. In the present study, we observed that sufentanil remarkably reduced the levels of ROS, MDA and LDH in a dose-dependent way whereas it enhanced the activity of SOD. These effects were reversed after Sesn2 silencing (). Altogether, these results suggest that sufentanil has a strong protective effect against oxidative stress induced by OGD/R in H9c2 cells via regulating Sesn2 expression.
Figure 4. Effect of sufentanil on oxidative stress induced by OGD/R in H9c2 cells and attenuation of the inhibitory effect following Sesn2 silencing. Contents of ROS (A) and MDA (B) and activities of LDH (C) and SOD (D) detected by commercial kits. ***p < 0.001 vs. control; ##p < 0.01, ###p < 0.001 vs. OGD/R; △△p < 0.01, △△△p < 0.001 vs. sufentanil (20 μmol/L) + OGD/R + shRNA-NC.
Note: Sesn2, Sestrin 2; OGD/R, oxygen-glucose deprivation and reoxygenation; ROS, reactive oxygen species; MDA, malondialdehyde; LDH, lactate dehydrogenase; SOD, superoxide dismutase.
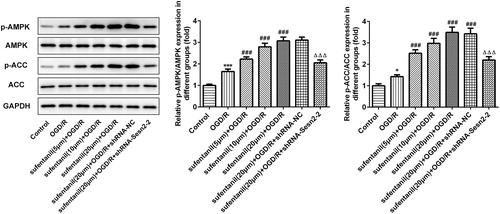
Sufentanil protected against myocardial I/R injury in H9c2 via activating Sesn2 expression and AMPK signalling pathway
To investigate the potential mechanisms underlying the effects of sufentanil on myocardial I/R injury, we examined the expression of the AMPK signalling pathway. It has been well documented that the AMPK pathway is closely implicated in I/R injury [Citation26]. For instance, ghrelin pretreatment protects against I/R-induced H9c2 cell injury via the AMPK pathway [Citation27]. Activation of AMPK can suppress inflammatory response and oxidative stress during OGD/R in H9c2 cells [Citation28]. Importantly, accumulating evidence shows that Sesn2 can activate the AMPK pathway and further protect against a variety of conditions [Citation29, Citation30]. In this study, we found that the expression levels of p-AMPK and p-ACC were upregulated in the OGD/R group compared with the control group, whereas sufentanil treatment further enhanced the expression levels of the studied proteins (). Sesn2 silencing inhibited the activation of the AMPK signalling pathway. These findings suggest that sufentanil protects against myocardial I/R injury via activating Sesn2 expression and the AMPK signalling pathway.
Figure 5. Protective effect of sufentanil against myocardial I/R injury in H9c2 via activating Sesn2 expression and AMPK signaling pathway. The expression levels of AMPK signaling pathway proteins were examined by Western blotting. *p < 0.05, ***p < 0.001 vs. control; ###p < 0.001 vs. OGD/R; △△△p < 0.001 vs. sufentanil (20 μmol/L) + OGD/R + shRNA-NC.
Note: Sesn2, Sestrin 2; OGD/R, oxygen-glucose deprivation and reoxygenation.
Conclusions
The results from this study indicate that sufentanil could attenuate inflammation and oxidative stress in myocardial I/R injury via upregulating Sesn2 expression and sequentially activating the AMPK signalling pathway. These results shed light on the mechanisms underlying the action of sufentanil. Our findings might be useful for the development of novel targeted therapies for myocardial I/R injury.
Ethics approval and consent to participate
The present study was approved by the Ethics Committee of the Affiliated Wujin Hospital of Jiangsu University (Changzhou, China) and was executed according to the principles of the Declaration of Helsinki. All study participants provided written informed consent forms.
Disclosure statement
The authors declare that they have no competing interests.
References
- Al-Herz W, Babiker F. Acute intravenous infusion of immunoglobulins protects against myocardial ischemia-reperfusion injury through inhibition of caspase-3. Cell Physiol Biochem. 2017;42(6):2295–2306.
- Hausenloy DJ, Yellon DM. Myocardial ischemia-reperfusion injury: a neglected therapeutic target. J Clin Invest. 2013;123(1):92–100.
- Zhou QL, Teng F, Zhang YS, et al. FPR1 gene silencing suppresses cardiomyocyte apoptosis and ventricular remodeling in rats with ischemia/reperfusion injury through the inhibition of MAPK signaling pathway. Exp Cell Res. 2018;370(2):506–518.
- Turer AT, Hill JA. Pathogenesis of myocardial ischemia-reperfusion injury and rationale for therapy. Am J Cardiol. 2010;106(3):360–368.
- Sheibani M, Faghir-Ghanesefat H, Dehpour S, et al. Sumatriptan protects against myocardial ischaemia-reperfusion injury by inhibition of inflammation in rat model. Inflammopharmacology. 2019;27(5):1071–1080.
- Wang YF, Wang Y, Wang XC, et al. Tilianin-loaded reactive oxygen species-scavenging nano-micelles protect H9c2 cardiomyocyte against hypoxia/reoxygenation-induced injury. J Cardiovasc Pharmacol. 2018;72(1):32–39.
- Tao H, Nuo M, Min S. Sufentanil protects the rat myocardium against ischemia-reperfusion injury via activation of the ERK1/2 pathway. Cytotechnology. 2018;70(1):169–176.
- Tire Y, Sarkilar G, Esen H, et al. The effect of intrathecal sufentanil preconditioning against myocardial ischemia-reperfusion injury. BLL. 2018;119(04):240–244.
- Peng M, Yin N, Li MO. Sestrins function as guanine nucleotide dissociation inhibitors for Rag GTPases to control mTORC1 signaling. Cell. 2014;159(1):122–133.
- Dong B, Xue RC, Sun Y, et al. Sestrin 2 attenuates neonatal rat cardiomyocyte hypertrophy induced by phenylephrine via inhibiting ERK1/2. Mol Cell Biochem. 2017;433(1-2):113–123.
- Tong GX, Wang YM, Xu CK, et al. Long non-coding RNA FOXD3-AS1 aggravates ischemia/reperfusion injury of cardiomyocytes through promoting autophagy. Am J Transl Res. 2019;11(9):5634–5644.
- Zhou Y, Liu L, Gao C, et al. Puerarin pre-conditioning on the expression levels of CK-MB, cTnI and inflammatory factors in patients undergoing cardiac valve replacement. Exp Ther Med. 2019;17(4):2598–2602.
- Zuo YM, Cheng XQ, Gu EW, et al. Effect of aortic root infusion of sufentanil on ischemia-reperfusion injury in patients undergoing mitral valve replacement. J Cardiothorac Vasc Anesth. 2014;28(6):1474–1478.
- Yang B, Yan P, Yang GZ, et al. Triptolide reduces ischemia/reperfusion injury in rats and H9C2 cells via inhibition of NF‑κB, ROS and the ERK1/2 pathway. Int J Mol Med. 2018;41(6):3127–3136.
- Yu LM, Li Q, Yu B, et al. Berberine attenuates myocardial ischemia/reperfusion injury by reducing oxidative stress and inflammation response: role of silent information regulator 1. Oxid Med Cell Longev. 2016;16–2019.
- Lian YH, Fang J, Zhou HD, Jiang HF, et al. Sufentanil preconditioning protects against hepatic ischemia-reperfusion injury by suppressing inflammation. Med Sci Monit. 2019;25:2265–2273.
- Yang JH, Kim KM, Kim MG, et al. Role of sestrin2 in the regulation of proinflammatory signaling in macrophages. Free Radic Biol Med. 2015;78:156–167.
- Chu QW, Zhang YM, Zhong SP, et al. N-n-butyl haloperidol iodide ameliorates oxidative stress in mitochondria induced by hypoxia/reoxygenation through the mitochondrial c-Jun N-terminal kinase/Sab/Src/reactive oxygen species pathway in H9c2 Cells. Oxid Med Cell Longev. 2019;14.
- Lakshmi SV, Padmaja G, Kuppusamy P, et al. Oxidative stress in cardiovascular disease. Indian J Biochem Biophys. 2009;46(6):421–440.
- Zhao D, Li Q, Huang Q, et al. Cardioprotective effect of propofol against oxygen glucose deprivation and reperfusion injury in H9c2 cells. Oxid Med Cell Longev. 2015;2015:1–8.
- Nielsen F, Mikkelsen BB, Nielsen JB, et al. Plasma malondialdehyde as biomarker for oxidative stress: reference interval and effects of life-style factors. Clin Chem. 1997;43(7):1209–1214.
- Yang P, Zhou YP, Xia Q, et al. Astragaloside IV regulates the PI3K/Akt/HO-1 signaling pathway and inhibits H9c2 cardiomyocyte injury induced by hypoxia-reoxygenation. Biol Pharm Bull. 2019;42(5):721–727.
- Wang Z, Yu JG, Wu JB, et al. Scutellarin protects cardiomyocyte ischemia-reperfusion injury by reducing apoptosis and oxidative stress. Life Sci. 2016;157:200–207.
- Li LY, Xiao LN, Hou YH, He Q, et al. Sestrin2 Silencing Exacerbates Cerebral Ischemia/Reperfusion Injury by Decreasing Mitochondrial Biogenesis through the AMPK/PGC-1 alpha Pathway in Rats. Sci Rep. 2016;6:11.
- Hwang HJ, Kim JW, Chung HS, et al. Knockdown of Sestrin2 increases lipopolysaccharide-induced oxidative stress, apoptosis, and fibrotic reactions in H9c2 cells and heart tissues of mice via an AMPK-dependent mechanism. Mediat Inflamm. 2019;2019:1–2.
- Tian L, Cao WJ, Yue RJ, et al. Pretreatment with Tilianin improves mitochondrial energy metabolism and oxidative stress in rats with myocardial ischemia/reperfusion injury via AMPK/SIRT1/PGC-1 alpha signaling pathway. J Pharmacol Sci. 2019;139(4):352–360.
- Chen YB, Wang HG, Zhang Y, et al. Pretreatment of ghrelin protects H9c2 cells against hypoxia/reoxygenation-induced cell death via PI3K/AKT and AMPK pathways. Artif Cell Nanomed Biotechnol. 2019;47(1):2179–2187.
- Chen X, Li X, Zhang WY, et al. Activation of AMPK inhibits inflammatory response during hypoxia and reoxygenation through modulating JNK-mediated NF-kappa B pathway. Metab-Clin Exp. 2018;83:256–270.
- Sun X, Han F, Li X, et al. Empagliflozin improves obesity-related heart failure with preserved ejection fraction by Sestrin2-mediated AMPK activation and antioxidant system in obese mice. Diabetologia. 2019;62:S101–S.
- Hwang HJ, Jung TW, Choi JH, et al. Knockdown of sestrin2 increases pro-inflammatory reactions and ER stress in the endothelium via an AMPK dependent mechanism. Biochim Biophys Acta-Mol Basis Dis. 2017;1863(6):1436–1444.
