Abstract
This study investigated the expression of inflammatory factors in tumour tissues, monocytes and blood of non-small cell lung cancer (NSCLC) patients taking tetrandrine (Tet) during low-dose radiotherapy. Sixty NSCLC patients underwent surgical resection of lung cancer after low-dose radiotherapy. Thirty patients did not take Tet (control group) and the other thirty patients took Tet during radiotherapy (experimental group). Lung cancer tissues were collected from patients, and tumour-adjacent tissues were collected as controls. Peripheral blood was collected on the morning of radiotherapy for mononuclear cell separation. Quantitative real-time polymerase chain reaction was used to determine mRNA expression, and Western blotting was used to measure protein expression. Enzyme-linked immunosorbent assay was used to determine the inflammatory factor contents in liquid samples. Cell proliferation was determined via MTT assay at 24, 48 and 72 h after transfection in A549 cells. Tet reduced COX-2 mRNA in serum and blood monocytes and decreased COX-2 protein expression in monocytes from patients with radiotherapy. It decreased the release of inflammatory factors to blood after radiotherapy. Combined treatment with low-dose radiotherapy and Tet tablets reduced the expression of Ki-67 protein in tumour tissues, and decreased the release of inflammatory factors from NSCLC tissues after radiotherapy. Combined treatment with low-dose radiotherapy and Tet tablets reduced the proliferation of cancer cells possibly through reducing inflammatory factors released by tumour tissues. The study demonstrates that Tet administration during low-dose radiotherapy reduces the release of inflammatory factors by NSCLC tissues, and decreases tumour proliferation. Tet plays an auxiliary role in NSCLC radiotherapy.
Introduction
Lung cancer has the highest morbidity and mortality among all malignant tumours in the world, accounting for 13% of new lung cancer cases every year [Citation1–3]. In China, about 600,000 people die of lung cancer every year [Citation4, Citation5]. Chronic inflammation is widely believed to be one of the physiological causes of tumours [Citation6]. It is discovered that up-regulation of COX-2 expression plays an important regulatory role in the occurrence and development of lung cancer, and occurrence of non-small cell lung cancer (NSCLC) is accompanied by a gradual increase in COX-2 expression [Citation7]. In addition, various cytokines are involved in the progression of cancers [Citation8]. As a bibenzyl isoquinoline alkaloid calcium antagonist, tetrandrine (Tet) decreases peripheral vascular resistance, lowers blood pressure [Citation9] and increases cardiac output, acting as a muscle relaxant, analgesic, antipyretic and anti-inflammatory agent [Citation10]. It has certain effects on various cancer cells, and is often combined with low-dose radiotherapy for lung cancer treatment [Citation11]. Tet can also be used in the treatment of simple silicosis and coal silicosis, early mild hypertension, rheumatism, arthralgia and neuralgia [Citation12–14]. However, the effect of Tet in combined use with low-dose radiotherapy has not been fully clarified.
In the present study, we examine the relevant indicators in tumour tissues and blood from patients treated with radiotherapy in combination with Tet.
Subjects and methods
Patients and samples
Sixty NSCLC patients who received treatment at our hospital between June 2014 and June 2018 were included in the study. All patients underwent surgical resection of lung cancer after low-dose radiotherapy. Thirty patients who did not take Tet during radiotherapy were included in the control group (18 males and 12 females; age range, 35–65 years; median age, 51.3 years), and the other 30 patients who took Tet during radiotherapy were included in the experimental group (19 males and 11 females; age range, 33–65 years; median age, 50.2 years). Lung cancer tissues were collected from all subjects, and tumour-adjacent tissues were also collected as control. The tissues (1 cm × 1 cm; 6 pieces) were mixed with 1 mL RPMI-1640 medium and incubated at 37 °C for 24 h before collecting supernatant by centrifugation at 3000 g for 15 min. Peripheral blood was collected from all patients on the morning of radiotherapy. Mononuclear cells were separated and collected using Human Monocyte Isolation Kit (P9260; Solarbio, Beijing, China). Serum was separated after centrifugation at 1000 g for 10 min. All patients had their first onset of the tumour.
Ethics statement
All procedures performed in the present study were approved by the Ethics Committee of Xuyi County Hospital of Traditional Chinese Medicine. Written informed consent was obtained from all patients or their families.
Low-dose radiotherapy
According to NCCN Guidelines for NSCLC [Citation15], radiotherapy was performed using TrueBeam Radiotherapy System (Varian Medical Systems, Palo Alto, CA, USA). The total dose of preoperative radiotherapy was 45–54 Gy, the split size of which was 1.8–2 Gy, and the treatment lasted for 5 weeks. The two groups had the same basic treatments, including hydration and diuretic therapy, routine liver protection, antiemetic, nutritional support and symptomatic treatment. General symptomatic treatments included intake of high-quality protein, low-salt and low-sodium diet, adequate energy and vitamins, as well as proper exercises. During the period of radiotherapy, the experimental group took Tet tablets (20 mg/pill; H20063332; Sunshine Pharmaceutical, Beihai, China) according to the recommended dosage in the instructions, whereas the control group did not take the drug.
Quantitative real-time polymerase chain reaction (qRT-PCR)
Trizol (10606ES60; Yeasen, Shanghai, China) was used to extract the total RNA from cells. Briefly, the cells were lysed with Trizol before extraction of RNA using the phenol chloroform method. Using gel electrophoresis, the integrity of RNA bands was checked. RNA purity was evaluated by measuring the 260/280 ratio using a spectrophotometer (Nanodrop ND2000, Thermo Scientific, Waltham, MA, USA). Then, cDNA was obtained by reverse transcription (ChamQ SYBR qPCR Master Mix; Vazyme, Nanjing, China) from 1 μg RNA and was stored at −20 °C. Reverse transcription of mRNA was performed using TIANScript II cDNA First Strand Synthesis Kit (Tiangen, Beijing, China).
SuperReal PreMix (SYBR Green) qRT-PCR kit (Tiangen, Beijing, China) was used to detect the mRNA expression of COX-2, using β-actin as the internal reference. The primer sequences for COX-2 were 5′-CAGCCATACAGCAAATCCTTG-3′ (forward) and 5′-CAAATGTGATCTGGATGTCAAC-3′ (reverse). The primer sequences for β-actin were 5′-CACCAGGGCGTGATGGT-3′ (forward) and 5′-CTCAAACATGATCTGGGTCAT-3′ (reverse). The reaction system (20 μL) was composed of 10 μL SYBR Premix EXTaq, 0.5 μL upstream primer, 0.5 μL downstream primer, 2 μL cDNA and 7 μL ddH2O. The PCR programme was initial denaturation at 95 °C for 3 min; denaturation at 94 °C for 15 s, annealing at 58 °C for 30 s and elongation at 72 °C for 1 min (30 cycles) (iQ5; Bio-Rad, Hercules, CA, USA). The 2-ΔΔCq method [Citation16] was used to calculate the relative expression of COX-2 mRNA against β-actin. Each sample was tested in triplicate.
Western blotting
Cells (1 × 106) were trypsinized, collected and lysed with precooled Radio-Immunoprecipitation Assay (RIPA) lysis buffer (600 μL; 50 mmol/L Tris-base, 1 mmol/L ethylenediaminetetraacetic acid (EDTA), 150 mmol/L NaCl, 0.1% sodium dodecyl sulphate, 1% TritonX-100, 1% sodium deoxycholate; Beyotime Institute of Biotechnology, Shanghai, China) for 30 min on ice. The mixture was centrifuged at 11,000 g and 4 °C for 10 min. The supernatant was used to determine the protein concentration by a bicinchoninic acid (BCA) protein concentration determination kit (RTP7102, Real-Times Biotechnology Co., Ltd., Beijing, China). The samples were then mixed with 5× sodium dodecyl sulphate loading buffer before denaturation in boiling water bath for 10 min. Afterwards, the samples (20 µg) were subjected to 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis at 100 V. The resolved proteins were transferred to polyvinylidene difluoride membranes on ice (100 V, 2 h) and blocked with 5% skimmed milk at room temperature for 1 h. Then, the membranes were incubated with rabbit anti-human COX-2 (1:1000; ab52237; Abcam, Cambridge, UK), anti-Ki67 (1:1000; ab16667; Abcam, Cambridge, UK) or β-actin (1:5000; ab129348; Abcam, Cambridge, UK) monoclonal primary antibodies at 4 °C overnight. After extensive washing with phosphate-buffered saline with Tween 20 (3 times of 15 min), the membranes were incubated with goat anti-rabbit horseradish peroxidase-conjugated secondary antibody (1:3,000; Abcam, Cambridge, UK) for 1 h at room temperature before washing with phosphate-buffered saline with Tween 20 (3 times of 15 min). Then, the membrane was developed with an enhanced chemiluminescence detection kit (ab65623; Abcam, Cambridge, UK) for imaging. Image lab v3.0 software (Bio-Rad, Hercules, CA, USA) was used to acquire and analyze the imaging signals. The relative contents of target proteins were expressed against β-actin.
Enzyme-linked immunosorbent assay (ELISA)
COX-2 ELISA kit (KB1567; Boyao Biotechnology, Shanghai, China), human tumour necrosis factor alpha (TNF-α) ELISA kit (ab181421; Abcam, Cambridge, UK), human interleukin 1β (IL-1β) ELISA kit (ab100562; Abcam, Cambridge, UK) and human interleukin 6 (IL-6) ELISA kit (ab46027; Abcam, Cambridge, UK) were used to determine the concentrations of respective proteins. In microplates, standards (50 μL) and samples (10 μL serum and 40 μL diluent) were added into predefined wells, whereas blank wells were left empty. In the wells for standards and samples, horseradish peroxidase-labeled conjugates (100 μL) were added before sealing the plates for incubation at 37 °C for 1 h. After washing the plates 5 times, substrates A (50 μL) and B (50 μL) were added into each well. After incubation at 37 °C for 15 min, stop solution (50 μL) was added into each well, and the absorbance of each well was measured at 450 nm within 15 min.
Cells
A549 cells (Cell Bank, Chinese Academy of Sciences, Shanghai, China) were cultured in DMEM medium (Hyclone, Logan, UT, USA) supplemented with 10% foetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA) at 5% CO2 and 37 °C.
MTT assay
A549 cells (Cell Bank, Chinese Academy of Sciences, Shanghai, China) were seeded into 96-well plates at a density of 2 × 103 cells per well. Each condition was tested in triplicate wells. At 24, 48 and 72 h after treatment with Tet, 20 µL MTT (5 g/L; JRDC000003, JRDUN Biotechnology, Shanghai, China) was added to each well, followed by incubation for 4 h at 37˚C. After aspiration of medium, dimethyl sulfoxide (150 µL per well) was added to dissolve purple crystals. Then, the absorbance of each well was measured at 490 nm with a microplate reader (Bio-Rad, Hercules, CA, USA) and cell proliferation curves were plotted.
Statistical analysis
The results were analyzed using SPSS 18.0 statistical software (IBM, Armonk, NY, USA). The data were expressed as mean values with standard deviations (±SD). Data were tested for normality. Multigroup measurement data were analyzed using one-way analysis of variance (ANOVA). In case of homogeneity of variance, Least Significant Difference and Student-Newman-Keuls methods were used; in case of heterogeneity of variance, Tamhane’s T2 or Dunnett’s T3 method was used. Comparison between two groups was carried out using Student’s t-test. p < 0.05 indicated statistically significant differences.
Results and discussion
COX-2 mRNA levels in the serum and blood monocytes are lower in NSCLC patients who take Tet along with radiotherapy
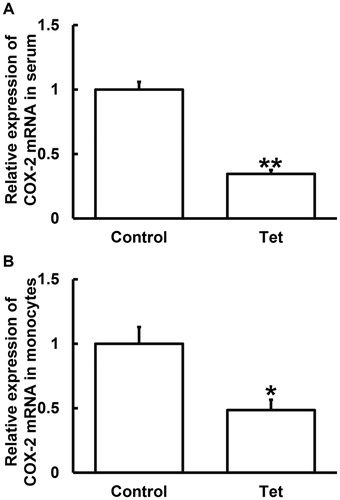
According to histological classification, lung cancer can be classified as NSCLC and small cell lung cancer (SCLC), and NSCLC accounts for 80–85% of all cases, with a five-year survival rate of 16% [Citation17]. The onset of lung cancer is insidious, and most patients are in advanced stage when they are diagnosed. Radiotherapy is one of the main treatments for lung cancer. It is reported that more than 70% of lung cancer patients need radiotherapy in the course of treatment, suggesting the important value of radiotherapy in the treatment of lung cancer [Citation18]. Changes of COX-2 expression are closely related to the occurrence and development of many kinds of tumours. Physical, chemical and biological stimuli may induce the production of COX-2, which catalyzes the synthesis of prostaglandin, a participant in inflammatory processes. COX-2 is not only an essential enzyme for prostaglandin synthesis, but also a key rate-limiting enzyme in the initial step of prostaglandin synthesis [Citation19]. To measure the expression of COX-2 mRNA, qRT-PCR was carried out. The data showed that COX-2 mRNA levels in serum and blood monocytes from NSCLC patients who took Tet tablets were significantly lower than those from NSCLC patients who did not take Tet tablets (p < 0.05) (). The result suggests that Tet is associated with reduction in the COX-2 mRNA levels in the serum and blood monocytes from NSCLC patients who undertake radiotherapy.
Figure 1. Relative expression of COX-2 mRNA in serum (A) and blood monocytes (B) from NSCLC patients undertaking radiotherapy. Note: Control, patients (n = 30) who did not take Tet during radiotherapy; Tet, patients (n = 30) who took Tet during radiotherapy. The expression of mRNA was determined by qRT-PCR using β-actin as internal control. The relative expression of COX-2 against β-actin in Tet group was normalized to the relative expression of COX-2 against β-actin in control group. *p < 0.05 and **p < 0.01 compared with control group.
COX-2 protein expression in blood monocytes is lower in NSCLC patients who take Tet along with radiotherapy
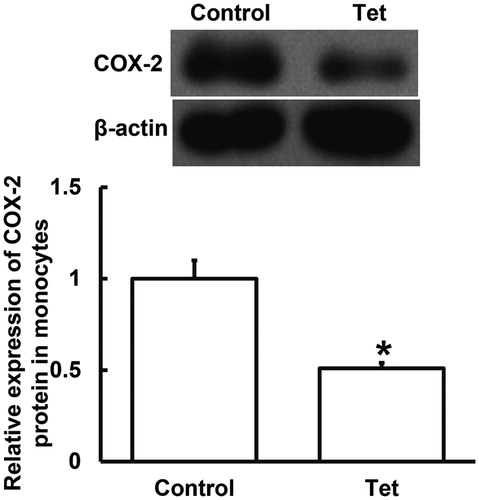
To determine the expression of COX-2 protein, Western blotting was performed. The data showed that COX-2 protein expression in blood monocytes from NSCLC patients who took Tet tablets was significantly smaller than that from NSCLC patients who did not take Tet tablets (p < 0.05) (). The result indicates that Tet intake is associated with a decrease in COX-2 protein expression in blood monocytes from NSCLC patients who undertake radiotherapy. Based on our results that the expression of COX-2 in serum and monocytes of patients taking Tet is lower, we may speculate that the intervention by Tet may reduce inflammation in NSCLC patients by regulating COX-2.
Figure 2. Relative expression of COX-2 protein in blood monocytes from NSCLC patients undertaking radiotherapy. Note: Control, patients (n = 30) who did not take Tet during radiotherapy; Tet, patients (n = 30) who took Tet during radiotherapy. Expression of protein was measured by Western blotting using β-actin as internal control. *p < 0.05 compared with control group.
Tet intake is associated with decreased release of inflammatory factors to the blood of NSCLC patients who undertake radiotherapy
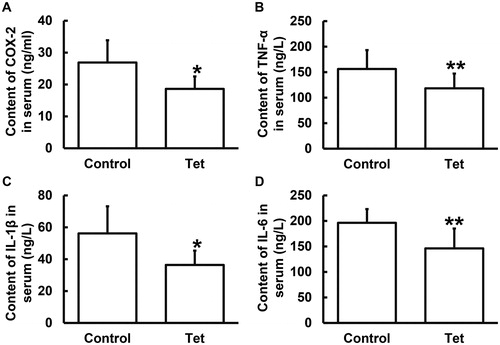
Tet is a natural non-selective Ca2+ channel blocker. Similar to the effect of verapamil, a slow channel blocker, Tet inhibits the action and release of allergic mediators, reduces myocardial contractility, dilates peripheral blood vessels and relaxes muscles. Tet may play a role in anti-inflammation. It is generally believed that one of the physiological causes of tumours is chronic inflammation, and even some scientists believe that most tumours are caused by chronic inflammation [Citation20]. The control of inflammatory factors in tumours is also a new direction of prevention and treatment of tumours [Citation21]. In addition, TNF-α, IL-1β and IL-6 are important factors in inflammation [Citation22]. TNF-α, IL-1β and IL-6 in tumour microenvironment can promote the proliferation and metastasis of tumours, and seriously affect the prognosis of patients [Citation23]. To examine the contents of inflammation-associated factors such as COX-2, TNF-α, IL-1β and IL-6 in serum from NSCLC patients who undertook radiotherapy, ELISA was used. The data showed that the contents of COX-2, TNF-α, IL-1β and IL-6 in serum from NSCLC patients who took Tet tablets were significantly lower (p < 0.05) than those from NSCLC patients who did not take Tet tablets (). These results suggest that Tet is associated with decreased release of inflammatory factors to the blood of NSCLC patients who undertake radiotherapy.
Figure 3. Contents of COX-2 (ng/mL), TNF-α (ng/L), IL-1β (ng/L) and IL-6 (ng/L) in serum from NSCLC patients undertaking radiotherapy. Note: Control, patients (n = 30) who did not take Tet during radiotherapy; Tet, patients (n = 30) who took Tet during radiotherapy. ELISA was used to determine protein content in serum. *p < 0.05 and **p < 0.01 compared with control group.
Combined treatment with low-dose radiotherapy and Tet tablets is associated with lower expression of proliferation-associated Ki-67 protein in tumour tissues from NSCLC patients
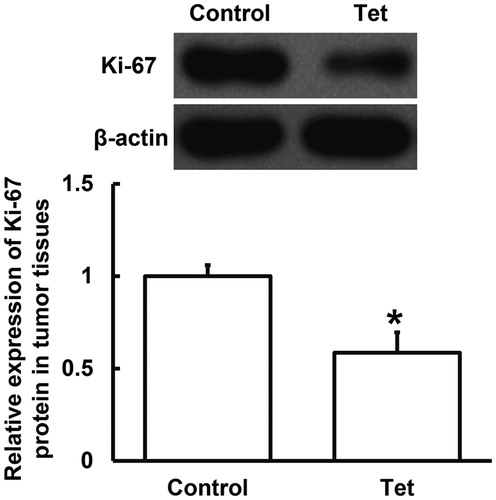
The main therapeutic mechanism of radiotherapy is to irradiate the patients’ tumour tissues and cells by high-dose radiation, so as to kill cancer cells and prevent them from proliferating and differentiating [Citation24]. It is reported that Tet can scavenge oxygen free radicals, protect pancreatic β-cell membrane, reduce Ca2+ overload, prevent superoxide accumulation in cells, and reduce apoptosis of pancreatic islet cells [Citation25, Citation26]. To measure the expression of Ki-67 protein in tumour tissues, Western blotting was employed. The data showed that Ki-67 protein expression in tumour tissues from NSCLC patients who had both radiotherapy and Tet tablets was significantly lower (p < 0.05) than that from NSCLC patients who only had radiotherapy (). This result indicates that combined treatment with low-dose radiotherapy and Tet tablets is associated with reduced expression of proliferation-associated Ki-67 protein in tumour tissues from NSCLC patients.
Figure 4. Relative expression of Ki-67 protein in tumour tissues from NSCLC patients undertaking radiotherapy. Note: Control, patients (n = 30) who did not take Tet during radiotherapy; Tet, patients (n = 30) who took Tet during radiotherapy. Expression of protein was measured by Western blotting using β-actin as internal control. *p < 0.05 compared with control group.
Tet is associated with decreased release of inflammatory factors from NSCLC tissues of patients who undertake radiotherapy
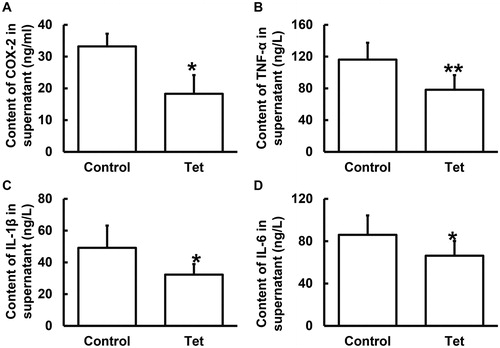
To determine the contents of COX-2, TNF-α, IL-1β and IL-6 in incubated tumour tissue supernatants, ELISA was carried out. The data showed that the contents of COX-2, TNF-α, IL-1β and IL-6 in the supernatants of incubated tumour tissue from NSCLC patients who took Tet tablets were significantly lower (p < 0.05) than those from NSCLC patients who did not take Tet tablets (). These results suggest that Tet is associated with a decrease in the release of inflammatory factors from NSCLC tissues of patients who undertake radiotherapy.
Figure 5. Contents of COX-2 (A), TNF-α (B), IL-1β (C) and IL-6 (D) in supernatants of incubated tumour tissues from NSCLC patients undertaking radiotherapy. Note: Control, patients (n = 30) who did not take Tet during radiotherapy; Tet, patients (n = 30) who took Tet during radiotherapy. ELISA was used to determine protein contents in supernatants. *p < 0.05 and **p < 0.01 compared with control group.
Combined treatment with low-dose radiotherapy and Tet tablets is associated with reduced proliferation of lung cancer cells in vitro
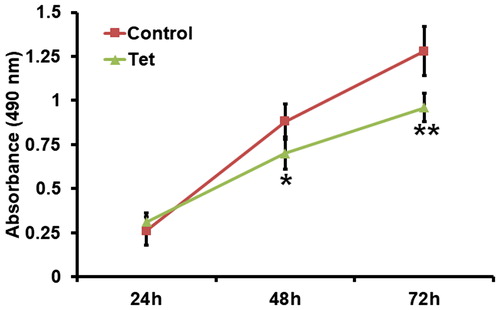
To test the effect of NSCLC tissue culture medium on the proliferation of lung cancer A549 cells, MTT assay was used. The data showed that the absorbance of A549 cells treated with NSCLC tissue culture medium from patients who took Tet tablets was significantly lower (p < 0.05) than that from patients who did not take Tet tablets (). We speculate that combined treatment with low-dose radiotherapy and Tet tablets reduce the proliferation of lung cancer cells possibly through reducing inflammatory factors released by tumour tissues.
Figure 6. Proliferation of A549 cells treated with supernatant of incubated tumour tissues from NSCLC patients undertaking radiotherapy. Note: Control, patients (n = 30) who did not take Tet during radiotherapy; Tet, patients (n = 30) who took Tet during radiotherapy. MTT assay was used to determine cell proliferation. *p < 0.05 and **p < 0.01 compared with control group.
Conclusions
The present study demonstrates that tetrandrine can alleviate inflammation and the release of inflammatory factors in lung cancer tissues and can delay the growth of lung cancer to a certain extent during low-dose radiotherapy of NSCLC. Tetrandrine may play an active role in this process.
Author contributions
The final version of the manuscript has been read and approved by all authors, and each author believes that the manuscript represents honest work. CL and XC collaborated to design the study. CL and HC performed experiments. CL and XC analyzed the data. All authors collaborated to interpret results and develop the manuscript.
Ethical approval and consent to participate
All procedures performed in the current study were approved by the Ethics Committee of Xuyi County Hospital of Traditional Chinese Medicine. Written informed consent was obtained from all patients or their families.
Consent for publication
Written informed consents for publication of any associated data and accompanying images were obtained from all patients or their parents, guardians or next of kin.
Acknowledgements
The authors wish to thank Zhande He of Xuyi County Hospital of Traditional Chinese Medicine.
Disclosure statement
The authors declare that they have no competing interests.
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108.
- Xu YJ, Du Y, Fan Y. Long noncoding RNAs in lung cancer: what we know in 2015. Clin Transl Oncol. 2016;18(7):660–665.
- Kang CG, Lee HJ, Kim SH, et al. Zerumbone suppresses osteopontin-induced cell invasion through inhibiting the FAK/AKT/ROCK pathway in human non-small cell lung cancer A549 cells. J Nat Prod. 2016;79(1):156–160.
- She J, Yang P, Hong Q, et al. Lung cancer in China: challenges and interventions. Chest 2013;143(4):1117–1126.
- Liu Q, Zhou Q. The challenges of lung cancer in China. J Can Res Ther. 2013;9(Suppl 2):S65–S66.
- Eiro N, Vizoso FJ. Inflammation and cancer. World J Gastrointest Surg. 2012;4(3):62–72.
- Butkiewicz D, Krześniak M, Drosik A, et al. The VEGFR2, COX-2 and MMP-2 polymorphisms are associated with clinical outcome of patients with inoperable non-small cell lung cancer. Int J Cancer. 2015;137(10):2332–2342.
- Boutsikou E, Domvri K, Hardavella G, et al. Tumour necrosis factor, interferon-gamma and interleukins as predictive markers of antiprogrammed cell-death protein-1 treatment in advanced non-small cell lung cancer: a pragmatic approach in clinical practice. Ther Adv Med Oncol. 2018;10:175883591876823.
- Xu XH, Gan YC, Xu GB, et al. Tetrandrine citrate eliminates imatinib-resistant chronic myeloid leukemia cells in vitro and in vivo by inhibiting Bcr-Abl/beta-catenin axis. J Zhejiang Univ Sci B. 2012;13(11):867–874.
- Cho HS, Chang SH, Chung YS, et al. Synergistic effect of ERK inhibition on tetrandrine-induced apoptosis in A549 human lung carcinoma cells. J Vet Sci. 2009;10(1):23–28.
- Ye LY, Hu S, Xu HE, et al. The effect of tetrandrine combined with cisplatin on proliferation and apoptosis of A549/DDP cells and A549 cells. Cancer cell Int. 2017;17(1):40.
- Chen Y, Tsai YH, Tseng SH. The potential of tetrandrine as a protective agent for ischemic stroke. Molecules 2011;16(9):8020–8032.
- Xie W, Du L. Diabetes is an inflammatory disease: evidence from traditional Chinese medicines. Diabetes Obes Metab. 2011;13(4):289–301.
- Idec-Sadkowska I, Andrzejak R, Antonowicz-Juchniewicz J, et al. [Trials of casual treatment of silicosis]. Med Pr. 2006;57(3):271–280.
- Ettinger DS, Wood DE, Aisner DL, et al. Non-small cell lung cancer, version 5.2017, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2017;15(4):504–535.
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods 2001;25(4):402–408.
- Chen G, Umelo IA, Lv S, et al. miR-146a inhibits cell growth, cell migration and induces apoptosis in non-small cell lung cancer cells. PLoS One. 2013;8(3):e60317.
- Zhao Y, Chen L, Zhang S, et al. Predictive factors for acute radiation pneumonitis in postoperative intensity modulated radiation therapy and volumetric modulated arc therapy of esophageal cancer. Thorac Cancer. 2015;6(1):49–57.
- Baker S, Dahele M, Lagerwaard FJ, et al. A critical review of recent developments in radiotherapy for non-small cell lung cancer. Radiat Oncol. 2016;11(1):115.
- Gao J, Wang D, Liu D, et al. Tumor necrosis factor-related apoptosis-inducing ligand induces the expression of proinflammatory cytokines in macrophages and re-educates tumor-associated macrophages to an antitumor phenotype. Mol Biol Cell. 2015;26(18):3178–3189.
- Kuninaka S, Yano T, Yokoyama H, et al. Direct influences of pro-inflammatory cytokines (IL-1beta, TNF-alpha, IL-6) on the proliferation and cell-surface antigen expression of cancer cells. Cytokine 2000;12(1):8–11.
- Serag El-Dien MM, Abdou AG, Asaad NY, et al. Intratumoral FOXP3+ regulatory T cells in diffuse large B-cell lymphoma. Appl Immunohistochem Mol Morphol. 2017;25(8):534–542.
- Liu JY, Feng CP, Li X, et al. Immunomodulatory and antioxidative activity of Cordyceps militaris polysaccharides in mice. Int J Biol Macromol. 2016;86:594–598.
- Alhouayek M, Muccioli GG. COX-2-derived endocannabinoid metabolites as novel inflammatory mediators. Trends Pharmacol Sci. 2014;35(6):284–292.
- Eapen MS, Hansbro PM, Larsson-Callerfelt AK, et al. Chronic obstructive pulmonary disease and lung cancer: underlying pathophysiology and new therapeutic modalities. Drugs 2018;78(16):1717–1740.
- Dai P, Li J, Ma XP, et al. Efficacy and safety of COX-2 inhibitors for advanced non-small-cell lung cancer with chemotherapy: a meta-analysis. Onco Targets Ther. 2018;11:721–730.
