?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
This study was aimed at investigating the possibility of biological removal of crude oil with the use of native bacterial strains, isolated from the shorelines of the Caspian Sea. For this purpose, based on the growth rate, hemolytic and emulsification activity, oil spreading assay and liquid surface tension reduction, 115 bacterial strains were isolated from seven selected stations across the shorelines of the Caspian Sea. Among them, 15 isolates were selected for further analyses. Three isolates (J3, J5 and J12) showed the highest efficiency for crude oil removal. Based on the sequence of 16S rRNA gene, the isolates were identified as Bacillus cereus (J3), Staphylococcus haemolyticus (J5) and Pseudomonas aeruginosa (J12). The results showed that isolate J3 had a better ability to degrade short (C9–C13) and medium length (C13–C25) in comparison with the long length (C32–C35) of n-alkanes in the crude oil residues. The total petroleum hydrocarbon (TPH) reduction by the B. cereus (J3) isolate was about 88.8 (mg g−1). It can be concluded that about 60% of n-alkanes in crude oil residues were removed. Also, the TPH reduction for S. haemolyticus (J5) and P. aeruginosa (J12) isolates were about 123.6 and 113.2 (mg g−1), respectively, so these strains could reduce the TPH about 55% and 50%, respectively. The TPH reduction in consortia containing B. cereus, S. haemolyticus and P. aeruginosa was about 18.3 (mg g−1), 83% of crude oil residues (1%, v/v) in 22 days.
Introduction
One type of contamination of marine environments results from the leakage of crude oil during tankers’ transportation [Citation1,Citation2]. This pollution by oil spills is a major hazard to the marine environments [Citation3,Citation4]. Crude oil contains four fractions, including saturated hydrocarbons, aromatic, resin and asphaltene [Citation5]. Also, the complex mixture of paraffinic, aromatic hydrocarbons, as well as nitrogen, oxygen, sulfur-containing compounds and a variety of metal-containing organic and inorganic compounds are the main constituents of crude oil [Citation6,Citation7]. Crude oil compounds are potentially poisonous for different eukaryotic and prokaryotic organisms [Citation8]. Different chemical, physical and biological methods have been applied for the removal of crude oil residues in marine environments [Citation9–11]. In comparison with the traditional methods, the microbial bioremediation of crude oil is a more efficient way; this is because of its economy and no secondary contaminations [Citation12–15]. In the last decades, many crude oil degrading bacteria have been isolated from oil-polluted locations [Citation12,Citation16]. These bacteria have the ability to use oil hydrocarbons as their carbon and energy sources [Citation17]. Moreover, many bacteria are capable of emulsifying oil hydrocarbons, producing surface active agents such as biosurfactants, which increase the adherence of cells to the substrate and biodegradation. Rhamnolipids, surfactin, emulsan and alasan, produced by Pseudomonasa eruginosa, Bacillus subtilis, Acinetobacter venetianus RAG1 and Acinetobacter radioresistens, are the best-known biosurfactants, respectively [Citation18]. Biosurfactants decrease the amount of surface tension by being accumulated at the junction of immiscible fluids, this way increasing the surface area of insoluble compounds, which leads to increased bio availability and the following biodegradation of the hydrocarbons [Citation19]. The degradation rates of hydrocarbons by bacteria are limited in contaminated marine waters due to the poor nitrogen and phosphorus content of such environments [Citation20]. Compared with single kind of bacterium, oil-degrading bacteria in consortia have a higher biodegradation efficiency [Citation21,Citation22]. This is because a single microbe may not be able to degrade a wide range of complex compounds of crude oil residues [Citation23,Citation24].
The Caspian Sea is the largest internal water in the world, accounting for 40 to 44 percent of the world’s total coastal waters. It is bigger than the great American lakes and Lake Victoria in Africa concerning its surface area. Distinct from other lakes, the water of the Caspian Sea is not fresh but brackish [Citation25]. In recent years, in comparison with other marine environments such as the Persian Gulf, the amount of the oil pollution of the Caspian Sea has increased [Citation26,Citation27].
There is a number of studies concerning the use of bacterial consortia for oil degradation. Examples are Saravanan and Vijayakumar [Citation28] and Malik and Ahmed [Citation29]. But to the best of the authors’ knowledge, there is little information on using bacterial consortia for crude oil degradation, isolated from the shorelines of the Caspian Sea. To this end, this study aims at isolating and characterization of native bacterial strains from shoreline soils, sea waters and oil spills on the Caspian Sea and analyzing the biosurfactants’ activities and potential for crude oil removal through using single and consortia of the isolated strains.
Materials and methods
Chemicals
N-hexane, dichloromethane, methanol and mineral salt have been purchased from Merck (Darmstadt, Germany). The crude oil used in this study was obtained from the Tehran oil refinery (Iranian light crude oil) with a specific gravity of 0.845 g/cm3 at 25 °C.
Sampling, medium, isolation, and culture conditions
In order to isolate the crude oil removal bacteria, sediments, shoreline waters and tarball samples were collected from seven stations of the Caspian Sea (Anzali, Sangachin, and Kiahshahr; 49°, 28, E; 37°, 28, N). Three seawater samples, two sediments and three tarball samples were collected from each station. Using a sterile knife, sediment samples were extracted from 2 to 15 cm depth of soil’s surface. Seawater samples were collected from a depth of 15 cm in 50-mL sterile Backman tubes, and four tarballs (two grams each) were separated and collected in 50-mL sterile Backman tubes. The physicochemical properties of each sample were measured using the Horiba U-54 Multi-Parameter Water Quality Meter apparatus, which on average, had a temperature of 29 °C, a pH of 8.2 and an electrical conductivity of 48.5 DS/m. All samples were kept in ice and transported to the laboratory. The medium used for bacterial isolation was basal mineral salt medium (MSM) with 1% (v/v) crude oil as the sole carbon source. The composition of the basal MSM used in this study was as follows (g/L−1): 2.0NaNO3, 0.8 NaCl, 0.8 KCl, 0.1 CaCl2.2H2O, 2.0 KH2PO4, 2.0 Na2HPO4.12 H2O, 0.2MgSO4, 0.001FeSO4.7H2O; and 2 mL trace element stock solution composed of (g/L−1): 0.08 FeCl3.6H2O, 0.75ZnSO4.H2O, 0.08 CoCl2.6H2O, 0.075 CuSO4.5H2O, 0.75 MgSO4. H2O, 0.15 H3BO3, 0.05 Na2MoO4. 2H2O [Citation28]. The initial pH of the MSM medium was adjusted at 7.0.
The aliquots of each sample, sediment, tarball (2 g) and sea water samples (5 mL) were added into 250 mL Erlenmeyer flasks containing 100 mL MSM medium with 1% (v/v) crude oil as carbon source. The flasks were incubated for 7 days at 30 °C on a rotary shaker (150 RPM, Ectron INFORS AG, Switzerland). Than the 15 mL aliquot was transferred to fresh MSM medium, and the incubation was performed for another cycle. This procedure was repeated for three times; then inoculums from the flasks were streaked out, and phenotypically different colonies that were isolated and stocked in slant cultures for further analyses [Citation30].
Screening for biosurfactant activity by the isolates
Screening tests including the hemolytic activity and the oil spreading method were performed for biosurfactant production by the isolates. The isolates with the best biosurfactant activities were picked up for the complementary screening tests including the emulsification activity (E24) and liquid surface tension measurement [Citation31].
Hemolytic assay
For the hemolytic activity of isolates, 50 μL of bacterial culture were streaked out on 5% sheep blood agar plates (BA) and incubated at 37 °C for 48 to 72 h. The diameter of clear zones around the colonies and the type of hemolysis were calculated to measure the biosurfactant production by the isolates [Citation32].
Oil spreading assay
The oil spreading experiment was performed using the method described by Morikawa et al. [Citation33]. Twenty microliter of crude oil was added to 20 mL of distilled water in a petri dish. Then 10 μL of cell-free culture broth in MSM medium was added to the oil surface. If biosurfactant is present in cell free culture broth, the oil will be replaced with an oil free clear zone, which indicates biosurfactant activity. The diameters of clear zones visualized under visible light were measured and calculated after 30 s. The negative control was distilled water without surfactant in which no oil spreading was observed, and Tween 20 was used as the positive control.
Liquid surface tension
Liquid surface tension was measured by the pendant drop method using a tensiometer (Kruss K20 Easy Dyne, Hamburg, Germany) at 45 °C for 10 days of growth (this occurred according to the manufacturer’s instructions). The surface tension was expressed in units of mN m−1.
Emulsification activity
The emulsification activity (E24) was determined using Cooper and Goldenberg’s (1987) method [Citation34]. For this purpose, 5 mL culture broths in MSM medium, mixed with 5 mL of hydrocarbon (crude oil) in 30 mL screw-capped tubes, were shaken for 2 min at 200 rpm and placed in undistributed conditions for 24 h. The E24 index is obtained as the percentage of the height of the emulsified layer (in millimeters) divided by the total height of the liquid column (in millimeters).
Phenotypic, biochemical, and molecular characterization of the isolates
To identify and characterize the isolates, phenotypic characterization including Gram staining, colony size and morphology, formation of major pigment, biochemical tests such as oxidation/fermentation, catalase and oxidase measures, hemolysis, production of acid from carbohydrates, hydrolysis of citrate and gelatin and other biochemical tests were carried out according to the Bergey’s Manual of Systematic Bacteriology [Citation35]. For taxonomic characterization of isolates, the 16S rRNA analysis was performed. The genomic DNA was extracted from single colonies of the bacterial strains, using Qiagen DNeasy blood and tissue kit (QIAGEN N.V, Germany) (following the manufacturer’s instructions). The bacterial 16S rRNA loci were amplified using the forward 27f: 5′- GAGTTTGATCCTGGCTCAG -3′ and reverse 1505r: 5′- GATACGGCTACCTTGTTACGA -3′ primers. The PCR program was performed in a Techne FTC51S5D thermocycler (Staffordshire, UK) for 35 cycles under the following temperature profile: 95 °C for 3 min (1 cycle); 93 °C for 45 s, 58 °C for 1 min and 72 °C for 90 s (35 cycles); and 72 °C for 10 min at the end of the final cycle. The PCR product was purified using a GF-1 PCR Cleanup kit (Vivantis technologies, Malaysia). The purified products were sequenced using the following primers: 27f: 5′- GAGTTTGATCCTGGCTCAG -3′ and 1505r: 5′- GATACGGCTACCTTGTTACGA -3, 27f: 5′- GAGTTTGATCCTGGCTCAG -3′ and 16r339: 5′- ACTGCTGCCTCCCGTAGGAG -3′, 16f358: 5′- CTCCTACGGGAGGCAGCAG-3′, 1505r: 5′- GATACGGCTACCTTGTTACGA -3 704f: 5′- GTAGCGGTGAAATGCGTAGA- 3′ and 1505r: 5′- GATACGGCTACCTTGTTACGA- 3′.
The Sanger’s dideoxy chain-termination sequencing method was applied using an ABI 3730XL DNA analyzer (Thermo Fisher Scientific Inc. USA). The similarities between 16S rRNA sequences were compared in the National Center for Biotechnology Information (NCBI), Ribosomal Data Base and EzTaxon databases. Phylogenic dendrograms were generated through neighbor joining method with bootstrap test (1,000 replicates) using MEGA X (Molecular Evolutionary Genetic Analysis software version 10.1.). The tree was drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. The evolutionary distances were calculated using the Maximum Composite Likelihood method [Citation36,Citation37]. In NJ analyses, closely related strains were used, and the data for constructing the phylogenic trees were collected from the NCBI database.
Biological removal of crude oil using the single and consortia of the bacterial isolates
For biological removal of crude oil by the isolates, 8 groups of treatments were constructed in 100 mL Erlenmeyer flasks containing 50 mL of MSM medium and 1% v/v crude oil. Experimental groups were as follows: C - control without inoculum; E1 - inoculated with J3 isolate; E2 - inoculated with J5 isolate; E3 - inoculated with J12 isolate; E4 - inoculated with co-culture of J3 and J5 isolates; E5 - inoculated with co-culture of J3 and J12 isolates; E6 - inoculated with co-culture of J5 and J12 isolates; E7- inoculated with consortia of E3, E5 and E12 isolates. The flasks were inoculated with (10% v/v) single or consortia of the isolates and incubated in a shaker incubator (Ectron INFORS AG, Switzerland) at 180 rpm at 30 °C for 22 days. The total residual hydrocarbons in the treatments were determined using gas chromatography with Mass spectroscopy (GC-MS). The cell biomass were separated by centrifugation, and crude oil removal assay was carried out by dissolving crude oil in dichloromethane (DCM); then the residual crude oil was extracted and treated with anhydrous sodium sulfate (Na2SO4) to remove the residual water. Extracts were concentrated by separating funnel, and analysis was performed using the Agilent 7890 N gas-chromatograph, fitted with a splitless injector, a fused-silica capillary column (HP-5MS, 30 m × 0.32 mm × 0.25 μm), equipped column of medium polarity (30 × 0.25 mm × 0. 1 µm) and flame ionization detector. Helium was used as a carrier gas at a flow rate of 1 mL/min. The sample (2 μL) was injected at the flow rate of 12 mL. The injector and interface temperatures were held at 220 and 350 ˚C, respectively. The Alkanes standard mixture (Alkane-Mix 10,500 µg/mL in Toluene, Dr. Ehrenstorfer. DRE-YA03010010TO) was used as the standard for determining normal alkanes. The removal efficiency of n-alkanes (C13–C36) was calculated using the equation below:
In this equation; is the average value of n-alkanes area in the isolate, and
is the average value of area of n-alkanes in control.
Statistical analysis
In order to see the significant differences among the treatments at p < 0.05, analysis of variance [Citation38] was performed to compare the significant differences by Tukey’s test. All analyses were performed using the SPSS software version 19.0.
Results and discussion
Isolation and characterization of the isolate
In this study, 115 bacterial strains were isolated from seven selected stations across the shorelines of the Caspian Sea. Based on microscopic and macroscopic characteristics, 38 Gram-positive bacilli, 32-Gram positive cocci, 25-Gram negative bacteria and 20-Gram negative cocci were identified. According to hemolytic, oil spreading, liquid surface tension and emulsification activity tests (), amongst 115 isolates, fifteen isolates showed higher biosurfactant activities. Isolates J3, J5 and J12 showed the highest hemolytic, oil spreading, emulsification, and surface reduction activities (). The experiments confirmed that three isolates were able to grow in crude oil by using petroleum hydrocarbon as the soul carbon source. Based on the obtained results, isolates J3, J5 and J12 were selected for further analyses.
Table 1. Measurement of hemolytic activity, oil spreading, emulsification activity (E24%) and decrease in surface tension (mN m−1).
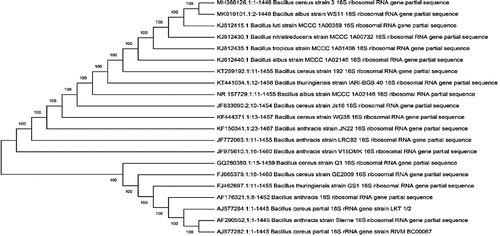
At first, morphological, physiological, and biochemical tests were used to characterize the isolates. Then the isolates were identified taxonomically based on 16S rRNA gene amplification and sequencing. For J3 isolate, a sequence of 1446 nucleotides was obtained showing 100% similar to the Bacillus cereus sensu lato group. The similarity of nucleotide sequence for 16S rRNA gene in J3 isolates with four Bacillus strains are shown in . For detailed characterization, Multi Locus Sequencing typing (MLST) is recommended.
Table 2. The similarity of Bacillus cereus J3 to other related strains in the Bacillus cereus group.
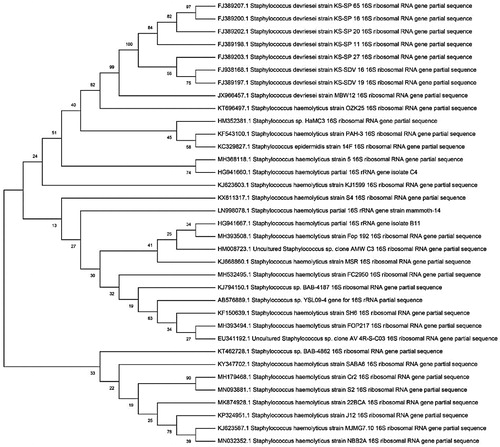
For the J5 isolate, a sequence of 1485 nucleotides was obtained, and BLAST sequence comparison showed 99/86% similarity to Staphylococcus haemolyticus MTCC 3383T. For J12 isolate, a sequence of 1456 nucleotides was obtained. The BLAST sequence comparison showed 100% similarity to Pseudomonas aeruginosa JCM 5962T. All nucleotide sequences of 16S rRNA for three isolated bacteria were deposited in the GenBank database. The GenBank IDs of isolates are MH368126 (for J3), MH368118 (for J5) and MH368439 (for J12), respectively. In order to identify the homology analysis of the isolates, the neighbor joining method was used to construct phylogenetic trees. The results are shown in . The phylogenetic trees of three isolates based on 16S rRNA gene sequences were constructed against different related Bacillus spp., Staphylococcus spp., and Pseudomonas spp. as shown in .
Figure 1. Phylogenic tree based on 16S rRNA gene sequence data for isolated Bacillus cereus strain (J3).
Biosurfactant production, emulsification activity and liquid surface tension
The results of hemolytic activity, oil spreading method, emulsification activity (E24) and liquid surface tension by fifteen isolates were shown in . Amongst the isolates, B. cereus (J3), S. haemolyticus (J5) and P. aeruginosa (J12) had the best activity in a reduction of surface tension about 42.85, 38.48 and 37.34 mN/m, respectively. Reduction of surface tension (below) than 40 mN/m is considered as the biosurfactant production index [Citation39]. In addition, the surface tension was often reduced to values below 30 mN/m as a result of biosurfactant synthesis [Citation40]. The growth rate and percentage of crude oil removal of fifteen isolates were analyzed using the spectrophotometry-based method. The results were presented in . The isolates B. cereus (J3), S. haemolyticus (J5) and P. aeruginosa (J12) showed the highest oil removal capacity that is about 66%, 53%, and 43%, respectively. The isolates J7, 14 and 15 belonged to genus Bacillus and showed the lowest oil removal, that is about 7.7%, 14.8%, and 8%, respectively.
Table 3. Growth rate and percentage of crude oil removal by fifteen isolates.
Biological removal of crude oil using single and bacterial consortia
The ratio of single and bacterial consortia designed for biological removal experiments is shown in . As shown in the table, biological removal of crude oil residues at 1% concentration differs amongst single and different consortia. Combinations of B. cereus (J3) isolate with other two isolates could remove crude oil more efficiently, that is about 72.9% (E4) and 78.9% (E5) of crude oil residues were removed, respectively.
Table 4. Crude oil removal and total petroleum hydrocarbons (TPH) analysis of single and bacterial consortium.
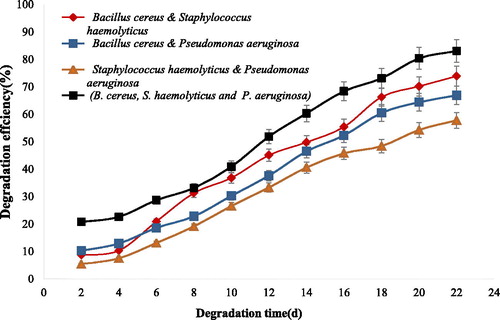
Maximum crude oil removal and total petroleum reduction were obtained using consortia containing B. cereus (J3), S. haemolyticus (J5) and P. aeruginosa (J12) E7 experiment, with more than 83% of crude oil degraded after 22 days. Total petroleum hydrocarbon (TPH) reduction reached to 18.3 mg g−1, that is about 83% of crude oil degraded as shown in . Degradation percentage of n-alkanes by B. cereus and consortia containing three strains are presented in . The results showed that B. cereus has ability to degrade short (C9–C13) and medium length (C13–C25) n-alkanes more efficiently than that of the long length (C32–C35) of n-alkanes.
Table 5. Comparison of the removal percentage of n-alkanes by the Bacillus cereus isolates (J3) and consortia E7 (B. cereus, S. haemolyticus and P. aeruginosa).
Gas chromatograms of the crude oil treated with B. cereus and consortium including B. cereus, S. haemolyticus and P. aeruginosa (E7) is shown in . It can be concluded that compared to the control () the degradation of short and medium chains of normal alkanes by free B. cereus (J3) is more efficient than those of other strains (). In consortia containing isolates of B. cereus (J3), S. haemolyticus (J5) and P. aeruginosa (J12), the degradation of short, medium and long chains of n-alkanes increased significantly (). The degradation efficiency of each single and all combinations of three isolates are presented in and . As seen in , the degradation efficiency by B. cereus (J3) is higher than those of S. haemolyticus (J5) and P. aeruginosa (J12) isolates, that is, more than 55% of 1% (v/v) of crude oil residues was degraded by B. cereus (J3). The degradation efficiency by consortia containing three isolates was more than 80%, as shown in .
Figure 4. GC-Mass chromatograms of crude oil samples before and after degradation. (a) The crude oil control; (b) degradation by Bacillus cereus (J3) isolate; (c) Degradation by B. cereus (J3), S. haemolyticus (J5) and P. aeruginosa (J12) consortium.
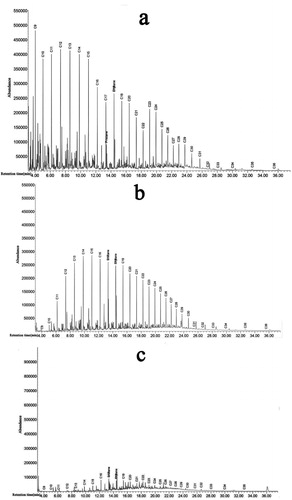
Figure 5. Degradation efficiency of crude oil by Bacillus cereus (J3), Staphylococcus haemolyticus (J5) and Pseudomonas aeruginosa isolates. Note: Data are means ± SD of triplicate determinations.
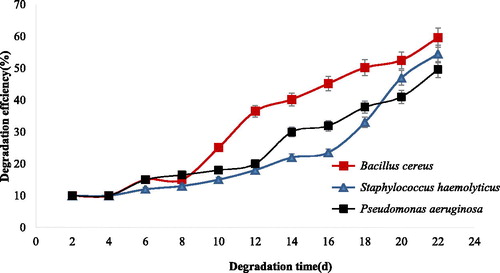
Figure 6. Degradation efficiency of crude oil by consortia of isolated bacteria. Note: Each color shows combinations of each treatment. Data are means ± SD of triplicate determinations.
Large amounts of petroleum enter into the environment from anthropogenic and natural sources every year, and environmental pollution due to oil and its derivatives has become a global concern [Citation3]. Hence, the development of bio-cleansing methods that can be used in oil-polluted environments are important. Amongst the available techniques, biological methods are widely used to reduce the hazardous impacts of petroleum compounds on contaminated sites. Bioremediation, mainly produced by native bacteria, has been regarded as the most promising way to clean up marine crude oil pollution. According to the principle of adaptation, bacteria with the potential of biological removal are found in places where they are in contact with contamination of petroleum. In the present study, 115 isolates with potential of biological removal of crude oil were isolated from sediments, shoreline waters, and tarballs, from seven stations of the Caspian Sea. Amongst the isolates, according to hemolytic, oil spreading, liquid surface tension, and emulsification activity (E24) tests, 15 isolates showed higher biosurfactant activities. Based on suitable growth and removal of hydrocarbons on 1% (v/v) of crude oil, three isolates were selected for further analyses. A taxonomic study of three isolates were performed, and based on phylogenetic analyses using 16S rRNA gene for isolate J3, the 16S rRNA gene sequence analysis showed that it belongs to the Bacillus cereus group. The similarity of J3 isolate to four species in Bacillus cereus including Bacillus albus, Bacillus tropics, Bacillus nitratireducens and Bacillus luti is 100%. From a taxonomic standpoint, the multilocus sequence typing (MLST) is recommended for detailed identification. Nine Bacillus strains were isolated by Liu et al. [Citation41] from samples including sediments and seawaters in China. In this study, we isolated B. cereus (J3) from soil samples polluted with crude oil across the shorelines of the Caspian Sea. The isolated strain had a suitable potential to remove hydrocarbons from crude oil. It seems that similar to our study, Bacillus strains isolated by Liu et al. [Citation41] had a parallel behavior in marine environments in China. Six surface-active agents produced by isolates, belonging to the Bacillus genera, were isolated from marine sediments of Tamil Nadu coastal areas in India by Gnanamani et al. [Citation42]. They showed that the removal of crude oil from aqueous phase is more than 90% within 60-120 min of exposure to the partially purified surface–active agents, produced by the isolated bacteria. All six strains grown in the presence of sucrose at 1% concentration could reduce the surface tension on an average of 26-36 mN/m. In a study by Christova et al. [Citation37], a Bacillus cereus (BN66) strain was isolated by selective enrichment from soils polluted by hydrocarbons near a gas station in Sofia, Bulgaria, in which the B. cereus strain (BN66) could degrade the n-alkanes about 93% in 2 days. Out of the tested aromatics, benzene, toluene and biphenyl were degraded to 84%, 90% and 70%; however, the degradation of naphthalene, anthracene and phenanthrene were 95%, 58% and 52%. Thus, the isolated strain, found by Christova et al. [Citation37], is useful for enhancing the biodegradation of oil. The B. cereus (J3) isolate in the current study could degrade short length n-alkanes about 95%. This result was compatible with the results of Christova et al. [Citation37]. Also, in the current study, B. cereus (J3) isolate could reduce the surface tension to its lowest point of 38 mN/m, which is similar to the results obtained by Gnanamani et al. [Citation42]. In another study, a B. subtilis strain was used for microbial oil enhanced recovery [Citation37]. In this study, B. subtilis 32811 was able to produce biosurfactants of cyclic lipopeptides during the fermentation process. Emulsification of three crude oils from different regions by the strain showed that the fermentation broth had good emulsifying and degrading abilities on the light and medium crude oils [Citation37]. Bacillus and other bacteria have also shown the degradation of oil in soils and composts contaminated with petroleum hydrocarbons [Citation43]. The results obtained in the current study are compatible with the results of Gnanamani et al. [Citation42] and Ubani et al. [Citation44]. Isolate J5 belongs to the genus Staphylococcus and 16S rRNA sequencing, identified as Staphylococcus haemolyticus. Finding strains of the genus Staphylococcus across the shorelines of Caspian Sea is not very surprising. This happens due to the entering of wastewaters from urban or rural areas. In the current study, isolate J12 was identified as Pseudomonas aeruginosa. The Pseudomonas strains as crude-oil-degrading-bacteria were isolated from the Persian Gulf and Caspian Sea by Hassanshahian et al. [Citation3]. Also, different biosurfactant producing bacteria such as Bacillus megaterium and Pseudomonas aeruginosa were isolated from water samples in Toticorin port on the southeast coasts of India [Citation45]. Seventeen strains of biosurfactant producing bacteria were isolated by Batista et al. [Citation19]. Eight isolates reduced the growth medium surface tension below 40 mN m−1, and 5 isolates exhibited this capacity in cell-free extracts [Citation19]. The biosurfactant production was different among the isolates, initiating synthesis during the exponential growth phase, and therefore a stationary phase was reached. Increasing the temperature from 25 °C to 35 °C augmented the biosurfactant production in two isolates, while pH (6.5–7.6) had no effect on any of the isolates. The results obtained in this study showed that there is a direct relationship between biosurfactant production and oil residues removal by the isolates. The isolated B. cereus (J3), showed the best activity in oil removal and biosurfactant production, compared to S. haemolyticus (J5) and P. aeruginosa (J12). In the current study, three strains performed well in crude oil biodegradation, with more than 80% of degradation efficiencies with 1% (v/v) crude oil. As shown in , more than any other isolates, the B. cereus isolate (J3) could reduce the total petroleum hydrocarbons in crude oil (different alkanes). Alkanes are parts of the aliphatic compounds in petroleum hydrocarbons. Normal alkanes, according to the proximity of chemical compounds, are classified into six categories as follows: group 1 (≤C13), group 2 (C13–C16), group 3 (C17–C2), group 4 (C22–C25), group 5 (C25–C29), and group 6 (C29–C36). The first group accounts for about 42% of total crude oil, while the sixth group from the smallest volume accounts for about 3% in total crude oil. In the current study, pythan and pristan were used as the indicators of oil pollution in different environments and analyzed as the main constituents of crude oil . The concentrations of these non-toxicogenic isopropenoids were about 3.18% and 1.1% of total crude oil, respectively.
The results of the current study showed that the B. cereus (J3) strain had a better degradation ability for n-alkanes with short (C9-C13) and medium (C13–C25) lengths (rate of degradation > of 95%) than that with long lengths (C25–C36). Biological removal of crude oil biodegradation by B. cereus strain for short, medium and long lengths were about 58%, 66% and 26%, respectively. B. cereus could reduce the total petroleum hydrocarbon (TPH) about 88.8 (mg g−1), that is, more than 60% of crude oil removals in comparison with the control. Also, the total petroleum hydrocarbon reduction (TPH) for S. haemolyticus and P. aeruginosa isolates were about 123.6 and 113.2. (mg g−1), respectively. So these strains could reduce TPH about 55% and 50%, respectively.
Consortium E7 (B. cereus: S. haemolyticus: P. aeruginosa) had a better ability to degrade long length (C25–C36), short (C9-C13) and medium chains (C13-C24), compared to isolate J3, respectively. The biological removal of crude oil by consortium E7 for short, medium and long lengths were about 60%, 76% and 69%, respectively. It seems that the S. haemolyticus (J5) and (J12) could remove long length n-alkanes more efficiently. In consortium E7, the total petroleum hydrocarbon (TPH) was about 18.3 (mg g−1), that is about 83% reduction observed in comparison with the control 223.5 (mg g−1). All combinations showed cooperation actions in biological crude oil removal, and degradation efficiencies were lower with the single ones. The combinations of three strains showed the best cooperation actions got 83% removal efficiencies with 1% (v/v) crude oil, as shown in . Since bacterial consortium may not be efficient in natural environments, some microcosm studies have been used by Roy et al. [Citation15], Wang et al. [Citation46] and Llirós et al [Citation47] to remove crude oil residues. Sathishkumar et al. [Citation48] reported that microbial consortia are more efficient in comparison with the individual bacteria. They concluded that in microbial consortia, there are different enzymes for various compounds of crude oil degradation, and the degradation is faster with high performance. A consortium of three bacteria, including Acinetobacter faecalis WD2, Staphyloccus sp. DD3 and Neisseria elongate TDA4, were constructed for bioremediation of crude petroleum-oil by Mukerd et al. [Citation49]. Varjani et al. [Citation48] reported about 30% crude oil (3% v/v) biodegradation by a halotolerant bacterial consortium that occurred in a 12-day period [Citation50]. There was 51.9% crude oil (2% v/v) degradation after 7 days of incubation according to Adam [Citation51]. A study by Chen et al. [Citation52] showed about 68% of biodegradation efficiency of crude oil (2% v/v) after 1 week of cultivation. Diesel degradation abilities by Staphylococcus sp., Pseudomonas sp. and Bacillus sp. were studied by Paul et al. [Citation53]. The results showed that the strains were able to degrade the diesel efficiently. In our study, about 83% oil removal was observed in 22 days, which is higher than the above studies.
In a study by Hanafy et al. [Citation54], 23 crude-oil-degrading bacteria were isolated from oil-contaminated sites near the Red Sea. Based on the growth rate of crude oil and hydrocarbon degradation ability, four strains were selected for further analyses. Among the four isolates, strains S5 (Pseudomonas sp., 95%) and 4b (Nitratireductor sp., 70%) were the most effective in degrading crude oil residues [Citation54]. Biodegradation of phenol has been investigated using a bacterial consortium containing two bacterial isolates; one of them used for the first time in phenol biodegradation. This consortium was isolated from the activated sludge and identified as Providencia stuartii PL4 and Pseudomonas aeruginosa PDM (accession numbers KY848366 and MF445102, respectively). After optimizing the biodegradation conditions, this consortium was able to completely degrade phenol up to 1500 mg within 58 h [Citation55]. A study by Wang et al. [Citation56] showed that compared with individual bacteria, bacterial consortium based on the Penglai oil spill accident (China) had higher oil degradation efficiency. This also demonstrated that this indigenous consortium had the potential for bioremediating crude oil dispersed in the marine ecosystem. In recent years, the level of oil pollution of the Caspian Sea has increased, and the ecosystem of the Caspian Sea with cold climate in different seasons allows the medium and long-chain alkanes to remain in the crude oil residues [Citation3,Citation27]; therefore, the biological removal of these alkanes are important. The consortium containing B. cereus, S. haemolyticus and P. aeruginosa could remove short, medium and length alkanes more efficiently. It seems that the isolated strains are able to express three groups of alkane hydroxylases genes including group (I), and (III) alkane hydroxylases, which are responsible for the degradation of short, medium and long-chain n-alkanes, respectively.
Conclusions
In the current study, the biological removal of crude oil by native strains, isolated from Caspian Sea, was studied. Three oil-degrading bacterial strains (B. cereus J3, S. haemolyticus J5 and P. aeruginosa J12) were isolated from the shorelines of the Caspian Sea, and the combinations of the isolates showed more than 83% of crude oil (1%, v/v) degradation in 22 days. In comparison with the recent similar studies in the research literature, the results regarding the degradation of n-alkanes by the isolates in this study were similar to the other recent studies. Also, 83% oil removal that was observed in 22 days is higher than that of some the recent studies.
Acknowledgments
The authors would like to thank the Director of Persian Type Culture Collection (PTCC) Dr. Farzaneh Aziz Mohseni for her valuable assistance in the identification of the isolates.
Disclosure statement
No potential conflict of interest is reported by the authors.
References
- Dave D, Ghaly AE. Remediation technologies for marine oil spills: a critical review and comparative analysis. Am J Environ Sci. 2011;7(5):423–440.
- Ghanavati H, Emtiazi G, Hassanshahian M. Synergism effects of phenol-degrading yeast and ammonia-oxidizing bacteria for nitrification in coke wastewater of Esfahan Steel Company. Waste Manage Res. 2008;26(2):203–208.
- Hassanshahian M, Emtiazi G, Cappello S. Isolation and characterization of crude-oil-degrading bacteria from the Persian Gulf and the Caspian Sea. Mar Pollut Bull. 2012;64(1):7–12.
- Tanzadeh J, Ghasemi MF. A review on bioremediation of bulk oil in sea waters and shoreline. Chem Biol Interface. 2016;6(5):282–289.
- Wu B, Lan T, Lu D, et al. Ecological and enzymatic responses to petroleum contamination. Environ Sci Process Impacts. 2014;16(6):1501–1509.
- Bachmann RT, Johnson AC, Edyvean RG. Biotechnology in the petroleum industry: an overview. Int Biodeterior Biodegrad. 2014;86:225–237.
- Haritash A, Kaushik C. Biodegradation aspects of polycyclic aromatic hydrocarbons (PAHs): a review. J Hazard Mater. 2009;169(1-3):1–15.
- Abbasian F, Lockington R, Megharaj M, et al. The biodiversity changes in the microbial population of soils contaminated with crude oil. Curr Microbiol. 2016;72(6):663–670.
- Bacosa HP, Suto K, Inoue C. Degradation potential and microbial community structure of heavy oil-enriched microbial consortia from mangrove sediments in Okinawa, Japan. J Environ Sci Health A. 2013;48(8):835–846.
- Nwadiogbu J, Ajiwe V, Okoye P. Removal of crude oil from aqueous medium by sorption on hydrophobic corncobs: equilibrium and kinetic studies. J Taibah Univ Sci. 2016;10(1):56–63.
- Seddighi M, Hejazi SM. Water–oil separation performance of technical textiles used for marine pollution disasters. Mar Pollut Bull. 2015;96(1-2):286–293.
- Chandankere R, Yao J, Cai M, et al. Properties and characterization of biosurfactant in crude oil biodegradation by bacterium Bacillus methylotrophicus USTBa. Fuel. 2014;122:140–148.
- Ferradji FZ, Mnif S, Badis A, et al. Naphthalene and crude oil degradation by biosurfactant producing Streptomyces spp. isolated from Mitidja plain soil (North of Algeria). Int Biodeterior Biodegrad. 2014;86:300–308.
- Kuyukina MS, Ivshina IB, Kamenskikh TN, et al. Survival of cryogel-immobilized Rhodococcus strains in crude oil-contaminated soil and their impact on biodegradation efficiency. Int Biodeterior Biodegrad. 2013;84:118–125.
- Roy AS, Baruah R, Borah M, et al. Bioremediation potential of native hydrocarbon degrading bacterial strains in crude oil contaminated soil under microcosm study. Int Biodeterior Biodegrad. 2014;94:79–89.
- Pasumarthi R, Chandrasekaran S, Mutnuri S. Biodegradation of crude oil by Pseudomonas aeruginosa and Escherichia fergusonii isolated from the Goan coast. Mar Pollut Bull. 2013;76(1-2):276–282.
- Bao M, Pi Y, Wang L, et al. Lipopeptide biosurfactant production bacteria Acinetobacter sp. D3-2 and its biodegradation of crude oil. Environ Sci Process Impacts. 2014;16(4):897–903.
- Uzoigwe C, Burgess JG, Ennis CJ, et al. Bioemulsifiers are not biosurfactants and require different screening approaches. Front Microbiol. 2015;6:245–2019. [
- Batista S, Mounteer A, Amorim F, et al. Isolation and characterization of biosurfactant/bioemulsifier-producing bacteria from petroleum contaminated sites. Bioresour Technol. 2006;97(6):868–875.
- Das N, Chandran P. Microbial degradation of petroleum hydrocarbon contaminants: an overview. Biotechnol Res Int. 2011;2011:1–13. [
- Bao M-t, Wang L-n, Sun P-y, et al. Biodegradation of crude oil using an efficient microbial consortium in a simulated marine environment. Mar Pollut Bull. 2012;64(6):1177–1185.
- Chen Y, Li C, Zhou Z, et al. Enhanced biodegradation of alkane hydrocarbons and crude oil by mixed strains and bacterial community analysis. Appl Biochem Biotechnol. 2014;172(7):3433–3447.
- Balba M, Al-Awadhi N, Al-Daher R. Bioremediation of oil-contaminated soil: microbiological methods for feasibility assessment and field evaluation. J Microbiol Methods. 1998;32(2):155–164.
- Suja F, Rahim F, Taha MR, et al. Effects of local microbial bioaugmentation and biostimulation on the bioremediation of total petroleum hydrocarbons (TPH) in crude oil contaminated soil based on laboratory and field observations. Int Biodeterior Biodegrad. 2014;90:115–122.
- Faezi M, Javanbakht S, Mohseni FA, et al. Isolation and characterization of bacterial strains from shoreline waters of Caspian Sea with novel antibacterial activity. Int J Mol Clin Microbiol. 2018;8(2):982–992.
- Hassanshahian M, Emtiazi G, Caruso G, et al. Bioremediation (bioaugmentation/biostimulation) trials of oil polluted seawater: a mesocosm simulation study. Mar Environ Res. 2014;95:28–38.
- Radwan S, Al-Hasan R, Ali N, et al. Oil-consuming microbial consortia floating in the Arabian Gulf. Int Biodeterior Biodegrad. 2005;56(1):28–33.
- Saravanan V, Vijayakumar S. Isolation and screening of biosurfactant producing microorganisms from oil contaminated soil. J Acad Indus Res. 2012;1(5):264–268.
- Varjani SJ, Rana DP, Jain AK, et al. Synergistic ex-situ biodegradation of crude oil by halotolerant bacterial consortium of indigenous strains isolated from on shore sites of Gujarat. India Inte Biodeterior Biodegrad. 2015;103:116–124.
- Gautam K, Tyagi V. Microbial surfactants: a review. J Oleo Sci. 2006;55(4):155–166.
- Branch T. Biosurfactan production by Bacillus sp. isolated from petroleum contaminated soils of Sirri Island. Am J Appl Sci. 2012;9(1):1–6.
- Mulligan CN, Gibbs BF. Types, production and applications of biosurfactants. Proc Indian Natl Sci Acad Part B. 2004;70(1):31–56.
- Morikawa M, Hirata Y, Imanaka T. A study on the structure–function relationship of lipopeptide biosurfactants. Biochim Biophys Acta. 2000;1488(3):211–218.
- Cooper DG, Goldenberg BG. Surface-active agents from two Bacillus species. Appl Environ Microbiol. 1987;53(2):224–229.
- Holt JG, Krieg N, Sneath PH, et al. Bergey’s manual of determinative bacteriology. 9th ed. Baltimore (MD): William & Wilkins, 1994.
- Tamura K, Peterson D, Peterson N, et al. MEGA5: molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol Biol Evol. 2011;28(10):2731–2739.
- Xu H, Wang H, Jia W, et al. Application of Bacillus subtilis strain for microbial-enhanced oil recovery. Int J Green Energy. 2019;16(7):530–539.
- Christova N, Kabaivanova L, Nacheva L, et al. Biodegradation of crude oil hydrocarbons by a newly isolated biosurfactant producing strain. Biotechnol Biotechnol Equip. 2019;33(1):863–872.
- Agarwal P, Sharma D. Comparative studies on the bio-desulfurization of crude oil with other desulfurization techniques and deep desulfurization through integrated processes. Energy Fuels. 2010;24(1):518–524.
- Shavandi M, Mohebali G, Haddadi A, et al. Emulsification potential of a newly isolated biosurfactant-producing bacterium, Rhodococcus sp. strain TA6. Colloids Surf B. 2011;82(2):477–482.
- Liu Y, Du J, Lai Q, et al. Proposal of nine novel species of the Bacillus cereus group. Int J Syst Evol Microbiol. 2017;67(8):2499–2508.
- Gnanamani A, Kavitha V, Radhakrishnan N, et al. Bioremediation of crude oil contamination using microbial surface-active agents: isolation, production and characterization. J Bioremed Biodegrad. 2010;1:107.
- Kumar SN, Siji J, Ramya R, et al. Improvement of antimicrobial activity of compounds produced by Bacillus sp. associated with a Rhabditid sp.(entomopathogenic nematode) by changing carbon and nitrogen sources in fermentation media. J Microbiol Biotechnol Food Sci. 2012;1(6):1424–1438.
- Ubani O, Atagana HI, Thantsha MS, et al. Identification and characterisation of oil sludge degrading bacteria isolated from compost. Arch Environ Prot. 2016;42(2):67–77.
- Mishra S, Sarma PM, Lal B. Crude oil degradation efficiency of a recombinant Acinetobacter baumannii strain and its survival in crude oil-contaminated soil microcosm. FEMS Microbiol Lett. 2004;235(2):323–331.
- Wang H, Wang C, Lin M, et al. Phylogenetic diversity of bacterial communities associated with bioremediation of crude oil in microcosms. Int Biodeterior Biodegrad. 2013;85:400–406.
- Llirós M, Gaju N, de Oteyza TG, et al. Microcosm experiments of oil degradation by microbial mats. II. The changes in microbial species. Sci Total Environ. 2008;393(1):39–49.
- Sathishkumar M, Binupriya AR, Baik SH, et al. Biodegradation of crude oil by individual bacterial strains and a mixed bacterial consortium isolated from hydrocarbon contaminated areas. Clean Soil Air Water. 2008;36(1):92–96.
- Mukred A, Hamid AA, Hamzah A, et al. Enhancement of biodegradation of crude petroleum-oil in contaminated water by the addition of nitrogen sources. Pak J Biol Sci. 2008;11(17):2122–2127.
- Varjani SJ, Upasani VN. Biodegradation of petroleum hydrocarbons by oleophilic strain of Pseudomonas aeruginosa NCIM 5514. Bioresour Technol. 2016;222:195–201.
- Li X, Zhao L, Adam M. Biodegradation of marine crude oil pollution using a salt-tolerant bacterial consortium isolated from Bohai Bay, China. Mar Pollut Bull. 2016;105(1):43–50.
- Chen Q, Li J, Liu M, et al. Study on the biodegradation of crude oil by free and immobilized bacterial consortium in marine environment. PLoS One. 2017;12(3):e0174445.
- Paul BK, George MM, Nisha P. Biosurfactant production and diesel degradation by bacterial consortium isolated from crude oil polluted soil. Int J Pharm Chem Biol Sci. 2016;6(3):326–331.
- Hanafy AA-EME, Anwar Y, Mohamed SA, et al. Isolation and identification of bacterial consortia responsible for degrading oil spills from the coastal area of Yanbu, Saudi Arabia. Biotechnol Biotechnol Equip. 2016;30(1):69–74.
- Youssef M, El-Shatoury EH, Ali SS, et al. Enhancement of phenol degradation by free and immobilized mixed culture of Providencia stuartii PL4 and Pseudomonas aeruginosa PDM isolated from activated sludge. Biorem J. 2019;23(2):53–19.
- Wang C, Liu X, Guo J, et al. Biodegradation of marine oil spill residues using aboriginal bacterial consortium based on Penglai 19-3 oil spill accident, China. Ecotoxicol Environ Saf. 2018;159:20–27.